Abstract
This study describes lysostaphin's effect against methicillin-sensitive Staphylococcus aureus in suckling rats. Standard techniques determined minimal inhibitory and bactericidal concentrations, pharmacokinetics, and efficacy. The numbers of surviving rats after vancomycin, oxacillin, and lysostaphin treatment were comparable and were different from that of controls (P < 0.00001). Lysostaphin appears effective in the treatment of neonatal S. aureus infection.
Additional antibacterial agents for staphylococcal infection are needed (3, 9, 10, 12, 13, 21, 27). Lysostaphin, first identified in 1944 (20), is an endopeptidase which specifically cleaves the cell wall cross-linking pentaglycine bridges of Staphylococcus aureus (25) and is active against nondividing organisms (17, 19). We describe the effect of lysostaphin against S. aureus in a neonatal animal model.
A clinical methicillin-sensitive S. aureus (MSSA) serotype 5 strain (Hernandez) was stored in tryptic soy broth at −80°C and grown before each experiment. An MSSA strain allowed comparison of lysostaphin to oxacillin and vancomycin. Minimum bactericidal concentrations (MBCs) and MICs of vancomycin, oxacillin, and lysostaphin were determined (7, 18).
Fourteen-day timed-pregnancy Wistar rats (Charles River Laboratories) received antibiotic-free water and food ad libitum and delivered at 21 to 22 days of gestation. Pups remained with their dams throughout the experiment. The Baylor College of Medicine Institutional Animal Use Committee approved the research.
Three-day-old pups received 1 mg/kg of body weight/dose of lysostaphin at 1, 6, 24, and 30 h. Blood was obtained by cardiac puncture at 1, 2, 3, 7, 8, 23, 25, 26, 27, 31, 32, 72, and 96 h after the first dose. Three pups' blood was pooled for each data point, and each time point had two data points. Lysostaphin serum concentration was determined by enzyme-linked immunosorbent assay (26).
Plates were coated with 100 μl (1 μg/ml) of rabbit antilysostaphin for 2 h at room temperature and washed with phosphate-buffered saline plus 0.01% Tween 20, and 100 μl of standard or sera was added. After 60 min, plates were washed and incubated with biotinylated rabbit antilysostaphin and ExtrAvidin (catalog no. 81K4875; Sigma) for 30 min separately. Substrate tetramethylbenzidine (catalog no. TMBW0100-01; BioFx) was added. Absorbances were measured and converted to concentrations.
Three-day-old pups received 0.2 ml subcutaneously (1 × 107 CFU/ml) of S. aureus cephalad to the tail. The bacterial concentration was determined by optical density and confirmed by quantitative culture. In the first experiment, littermate pups were randomly assigned to either earlier treatment at 0.5, 6.5, 24, and 30 h after infection or later treatment at 6, 24, 30, and 48 h after infection. Each pup received 0.2 ml intraperitoneally of lysostaphin (1 mg/kg) or an equal volume of normal saline. In the second experiment, littermate pups randomly received 0.2 ml intraperitoneally of lysostaphin (1 mg/kg), vancomycin (15 mg/kg), oxacillin (50 mg/kg), or normal saline at 0.5, 6.5, 24, and 30 h after infection. Pups were evaluated daily for 7 days or until death for survival and weight. Tail vein quantitative blood cultures were obtained from each pup in randomly selected litters 24 h after infection.
Survival data were analyzed by a chi-square or Fisher's exact test. Blood culture data were analyzed with the Kruskal-Wallis test. Growth data were analyzed with a two-way analysis of variance. Statistical significance was defined as a P value of ≤0.05.
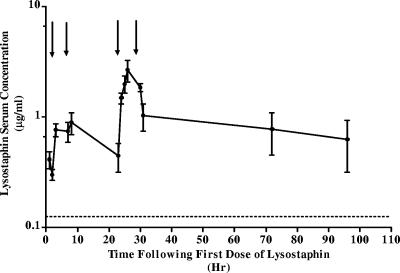
MICs and MBCs were 0.125 μg/ml and 0.25 μg/ml for oxacillin, 0.125 μg/ml and 0.25 μg/ml for vancomycin, and 0.031 μg/ml and 0.125 μg/ml for lysostaphin. To ensure adequate lysostaphin serum levels, our goal was to achieve trough levels greater than four times the MIC or 0.125 μg/ml. Serum lysostaphin levels (μg/ml) were greater than or equal to the target concentration from <1 to >96 h after the initial dose (Fig. 1). With the series of standard curves, the coefficient of variation was 6.9% and lower limit of detection was 0.92 ng/ml (three times the background activity). The kinetics of elimination appears to decrease from the first dose to the fourth dose. This may reflect the varied dosing interval or drug accumulation.
FIG. 1.
Serum levels of lysostaphin (mean concentrations [μg/ml], with error bars representing ranges) after the injection of 1 mg/kg/dose of lysostaphin intraperitoneally at 0 h (first dose) and then 6, 24, and 30 h later. Arrows indicate time of each dose. There are two data points for each time point. The lysostaphin target trough concentration was >0.125 μg/ml (dotted line).
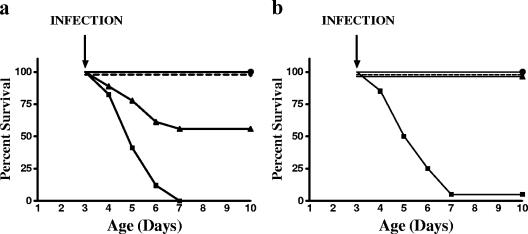
Lysostaphin treatment 30 min or 6 h after infection significantly improved survival (Fig. 2a). Treatment with lysostaphin, vancomycin, or oxacillin after infection resulted in similar rates of survival (Fig. 2b). Blood cultures confirmed bacteremia in all groups. Lysostaphin, vancomycin, or oxacillin treatment did not affect weight gain (data not shown).
FIG. 2.
(a) Survival curves for rat pups receiving lysostaphin treatment. •, noninfected pups treated with lysostaphin (n = 17); ⧫, S. aureus-infected pups with lysostaphin treatment beginning 30 min after infection (n = 22); ▴, S. aureus-infected pups with lysostaphin treatment beginning 6 h after infection (n = 19); ▪, S. aureus-infected and untreated pups (n = 17). Lysostaphin treatment significantly improved survival in infected rats treated 30 min (P < 0.00001) and 6 h (P < 0.0044) after S. aureus infection. Lysostaphin treatment without infection had no associated morbidity. (b) Survival curves for S. aureus-infected rat pups receiving lysostaphin (•) (n = 18), vancomycin (⧫) (n = 18), oxacillin (n = 20) (▴), or normal saline (▪) (n = 16) 30 min after infection are shown. Each antibiotic treatment group was significantly different from saline-treated infected pups (P < 0.00001).
This is the first use of lysostaphin in a neonatal animal model of infection. This is important because staphylococci are the most common cause of neonatal late-onset (>3 days after birth) sepsis, with coagulase-negative staphylococci (CONS) occurring in about 48% of infections and S. aureus organisms occurring in 7.8% of infections (23); the cause of persistent bacteremia in CONS (48%) and S. aureus (25%) neonatal infections (4); and associated with neonatal mortality rates of 9.1% (CONS) and 17.2% (S. aureus) and increased morbidities (such as hospitalization and high treatment costs) (23).
Lysostaphin use in humans is limited to a case report of a patient with methicillin-resistant Staphylococcus aureus-induced pneumonia and abscesses (22) and a study using lysostaphin spray that eradicates 80% of S. aureus from the nares of carriers (15). Other reports suggest that lysostaphin is effective in the treatment of bovine mastitis (19), eradication of S. aureus nasal colonization in rodents (11), and treatment of rabbit staphylococcal endophthalmitis (8) and endocarditis (6).
Lysostaphin appears as effective as vancomycin or oxacillin in the treatment of severe MSSA neonatal infection at levels consistently above the MIC. Our MIC is within the range reported for 17 methicillin-resistant Staphylococcus aureus strains in rabbits with aortic endocarditis (0.007 to 0.125 μg/ml) (6). More than 95% of the lysostaphin was reported cleared from adult CF-1 mice blood within one hour. However, the 40-mg/kg dose resulted in peak levels of only 0.02 μg/ml. The lysostaphin pharmacokinetics for adults appears different than that for neonates (26).
Adult rats receiving 30 mg/kg of vancomycin subcutaneously had serum levels of 36 μg/ml 1 h after administration (2), those receiving 100 mg/kg of vancomycin intravenously had levels of 179.9 μg/ml and 44.98 μg/ml 0.5 and 2 h after administration, respectively (16), and 25 mg/kg intramuscular of nafcillin resulted in serum levels of 20.6 μg/ml (1). Our pups received 15 mg/kg of vancomycin or 50 mg/kg of oxacillin intraperitoneally, and we speculate they had serum levels substantially greater than the MIC of 0.125 μg/ml.
In vitro lysostaphin was effective against over 50 S. aureus strains independent of phage type, antibiotic resistance, cell wall, or capsule condition (20, 25). Lysostaphin resistance has developed following chronic exposure to low subinhibitory levels in vitro (24) and among oxacillin-resistant S. aureus strains in vitro and in vivo (5). Resistance appears to be due to femA or femB mutations; however, these strains become hypersusceptible to beta-lactam antibiotics (5, 24). Thus, combining lysostaphin with a beta-lactam antibiotic should be investigated further (14). In addition, lysostaphin's ability to disrupt staphylococcal biofilms on plastic (28) may allow prevention or treatment of neonatal infection without removing the device.
Acknowledgments
This work was supported in part by Biosynexus Incorporated, who provided the lysostaphin, lysostaphin antibodies, and a grant to support these experiments.
Footnotes
Published ahead of print on 9 April 2007.
REFERENCES
- 1.Agapitova, I. V., V. I. Bobrov, V. I. Trubnikov, and V. P. Iakovlev. 1984. Penetration of ampicillin and oxacillin into the tissues of rats with aseptic inflammation. Antibiotiki 29:370-373. (In Russian.) [PubMed] [Google Scholar]
- 2.Cantoni, L., A. Wenger, M. P. Glauser, and J. Bille. 1989. Comparative efficacy of amoxicillin-clavulanate, cloxacillin, and vancomycin against methicillin-sensitive and methicillin-resistant Staphylococcus aureus endocarditis in rats. J. Infect. Dis. 159:989-993. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman, R. L., and R. G. Faix. 2003. Persistent bacteremia and outcome in late onset infection among infants in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 22:17-21. [DOI] [PubMed] [Google Scholar]
- 5.Climo, M. W., K. Ehlert, and G. L. Archer. 2001. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Dajcs, J. J., B. A. Thibodeaux, E. B. Hume, X. Zheng, G. D. Sloop, and R. J. O'Callaghan. 2001. Lysostaphin is effective in treating methicillin-resistant Staphylococcus aureus endophthalmitis in the rabbit. Curr. Eye Res. 22:451-457. [DOI] [PubMed] [Google Scholar]
- 9.Karchmer, T. B., L. J. Durbin, B. M. Simonton, and B. M. Farr. 2002. Cost-effectiveness of active surveillance cultures and contact/droplet precautions for control of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 51:126-132. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman, D., and K. D. Fairchild. 2004. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin. Microbiol. Rev. 17:638-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokai-Kun, J. F., S. M. Walsh, T. Chanturiya, and J. J. Mond. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 47:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp, B. J., D. E. Nix, and E. P. Armstrong. 2004. Clinical and economic analysis of methicillin-susceptible and -resistant Staphylococcus aureus infections. Ann. Pharmacother. 38:1377-1382. [DOI] [PubMed] [Google Scholar]
- 13.Lodise, T. P., and P. S. McKinnon. 2005. Clinical and economic impact of methicillin resistance in patients with Staphylococcus aureus bacteremia. Diagn. Microbiol. Infect. Dis. 52:113-122. [DOI] [PubMed] [Google Scholar]
- 14.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 15.Martin, R. R., and A. White. 1967. The selective activity of lysostaphin in vivo. J. Lab. Clin. Med. 70:1-8. [PubMed] [Google Scholar]
- 16.Mori, H., T. Nakajima, A. Nakayama, M. Yamori, F. Izushi, and Y. Gomita. 1998. Interaction between levofloxacin and vancomycin in rats—study of serum and organ levels. Chemotherapy 44:181-189. [DOI] [PubMed] [Google Scholar]
- 17.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCCLS/CLSI. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 6th ed. M7-A6. NCCLS, Wayne, PA.
- 19.Oldham, E. R., and M. J. Daley. 1991. Lysostaphin: use of a recombinant bactericidal enzyme as a mastitis therapeutic. J. Dairy Sci. 74:4175-4182. [DOI] [PubMed] [Google Scholar]
- 20.Schindler, C. A., and V. T. Schuhardt. 1964. Lysostaphin: a new bacteriolytic agent for the staphylococcus. Proc. Natl. Acad. Sci. USA 51:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schito, G. C. 2006. The importance of the development of antibiotic resistance in Staphylococcus aureus. Clin. Microbiol. Infect. 12(Suppl. 1):3-8. [DOI] [PubMed] [Google Scholar]
- 22.Stark, F. R., C. Thornsvard, E. P. Flannery, and M. S. Artenstein. 1974. Systemic lysostaphin in man-apparent antimicrobial activity in a neutropenic patient. N. Engl. J. Med. 291:239-240. [DOI] [PubMed] [Google Scholar]
- 23.Stoll, B. J., N. Hansen, A. A. Fanaroff, L. L. Wright, W. A. Carlo, R. A. Ehrenkranz, et al. 2002. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110:285-291. [DOI] [PubMed] [Google Scholar]
- 24.Stranden, A. M., K. Ehlert, H. Labischinski, and B. Berger-Bachi. 1997. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 179:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trayer, H. R., and C. E. Buckley III. 1970. Molecular properties of lysostaphin, a bacteriolytic agent specific for Staphylococcus aureus. J. Biol. Chem. 245:4842-4846. [PubMed] [Google Scholar]
- 26.Walsh, S., A. Shah, and J. Mond. 2003. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob. Agents Chemother. 47:554-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weisman, L. E. 2004. Coagulase-negative staphylococcal disease: emerging therapies for the neonatal and pediatric patient. Curr. Opin. Infect. Dis. 17:237-241. [DOI] [PubMed] [Google Scholar]
- 28.Wu, J. A., C. Kusuma, J. J. Mond, and J. F. Kokai-Kun. 2003. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob. Agents Chemother. 47:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]