Abstract
Background—The liver is a key organ in the metabolism of cholesterol in humans. It is the only organ by which substantial amounts of cholesterol are excreted from the body, either directly as free cholesterol into the bile or after conversion to bile acids. The major part of cholesterol synthesis in the body occurs in the liver. Cholesterol is also taken up by the liver from plasma lipoproteins. The relative contributions of newly synthesised cholesterol and plasma lipoprotein cholesterol to bile acid synthesis and biliary cholesterol secretion, respectively, are not known in detail. Aims—To determine how a rapid lowering of plasma low density lipoprotein (LDL) and very low density lipoprotein (VLDL) cholesterol influences the biliary secretion rates of cholesterol and bile acids in patients with cholesterol gallstones and complete biliary drainage. In this model with a completely interrupted enterohepatic circulation, the secretion of bile acids equals the new synthesis of bile acids in the liver. Patients—Eight patients with common bile duct stones of cholesterol type undergoing conventional cholecystectomy and choledocholithotomy. Methods—At operation a balloon occludable Foley catheter attached to a T tube was inserted into the bile duct with the balloon placed just past the distal limb of the T tube. The T tube was allowed to drain the bile externally. One week after the operation the Foley catheter balloon was inflated, creating complete biliary drainage. Twelve hours following the inflation plasma LDL apheresis was carried out for two hours. Bile was collected for 15 minute periods starting one hour before the apheresis and ending two hours after its termination. During the collection of bile, plasma lipids were analysed on several occasions. Results—The plasma level of LDL cholesterol decreased by 26% from (mean (SEM)) 2.19 (0.29) to 1.63 (0.17) mmol/l during the LDL apheresis while high density lipoprotein (HDL) cholesterol in plasma was unaffected. During LDL apheresis apolipoprotein B containing lipoproteins bind to the column, causing a significant decrease of not only plasma LDL but also of VLDL cholesterol. The secretion rate of bile acids decreased significantly by 31% from 131 (38) to 90 (16) µmol/15 minutes (p=0.045). The output of phospholipids also decreased by 19%. The biliary secretion rate of cholesterol was not, however, affected by the plasma LDL apheresis. Conclusions—The results suggest that, in patients with cholesterol gallstones and complete biliary drainage, lowering of plasma LDL and VLDL cholesterol reduces the biliary secretion rate—synthesis—of bile acids without affecting the biliary secretion rate of cholesterol.
Keywords: bile acids; biliary lipids; cholesterol; lipoproteins; plasma apheresis
Full Text
The Full Text of this article is available as a PDF (117.4 KB).
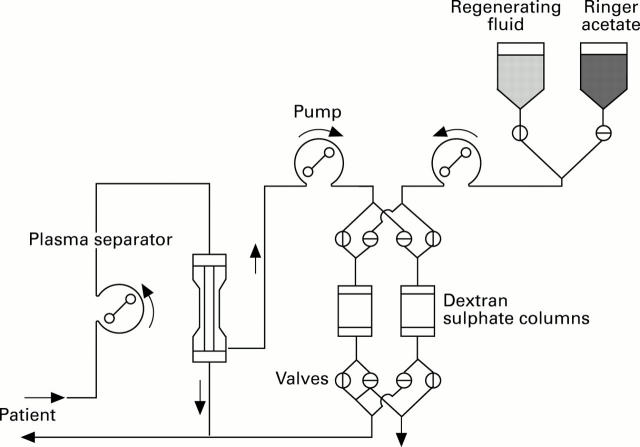
Figure 1 .
: Schematic set up of the LDL apheresis system.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund J. E., Reihnér E., Angelin B., Rudling M., Ewerth S., Björkhem I., Einarsson K. Hepatic metabolism of cholesterol in Crohn's disease. Effect of partial resection of ileum. Gastroenterology. 1991 Apr;100(4):1046–1053. doi: 10.1016/0016-5085(91)90281-o. [DOI] [PubMed] [Google Scholar]
- Angelin B., Einarsson K., Leijd B. Biliary lipid composition during treatment with different hypolipidaemic drugs. Eur J Clin Invest. 1979 Jun;9(3):185–190. doi: 10.1111/j.1365-2362.1979.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978 Nov;19(8):945–955. [PubMed] [Google Scholar]
- Carey M. C., Lamont J. T. Cholesterol gallstone formation. 1. Physical-chemistry of bile and biliary lipid secretion. Prog Liver Dis. 1992;10:139–163. [PubMed] [Google Scholar]
- Dietschy J. M., Turley S. D., Spady D. K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993 Oct;34(10):1637–1659. [PubMed] [Google Scholar]
- Einarsson K., Angelin B., Ewerth S., Nilsell K., Björkhem I. Bile acid synthesis in man: assay of hepatic microsomal cholesterol 7 alpha-hydroxylase activity by isotope dilution-mass spectrometry. J Lipid Res. 1986 Jan;27(1):82–88. [PubMed] [Google Scholar]
- Evans G. W., Phillips G., Mukherjee T. M., Snow M. R., Lawrence J. R., Thomas D. W. Identification of crystals deposited in brain and kidney after xylitol administration by biochemical, histochemical, and electron diffraction methods. J Clin Pathol. 1973 Jan;26(1):32–36. doi: 10.1136/jcp.26.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausa O., Skålhegg B. A. Quantitative determination of bile acids and their conjugates using thin-layer chromatography and a purified 3alpha-hydroxysteroid dehydrogenase. Scand J Gastroenterol. 1974;9(3):249–254. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- Heaton K. W. Disturbances of bile acid metabolism in intestinal disease. Clin Gastroenterol. 1977 Jan;6(1):69–89. [PubMed] [Google Scholar]
- Lamont J. T., Carey M. C. Cholesterol gallstone formation. 2. Pathobiology and pathomechanics. Prog Liver Dis. 1992;10:165–191. [PubMed] [Google Scholar]
- Lopes-Virella M. F., Stone P., Ellis S., Colwell J. A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977 May;23(5):882–884. [PubMed] [Google Scholar]
- Mabuchi H., Michishita I., Takeda M., Fujita H., Koizumi J., Takeda R., Takada S., Oonishi M. A new low density lipoprotein apheresis system using two dextran sulfate cellulose columns in an automated column regenerating unit (LDL continuous apheresis). Atherosclerosis. 1987 Nov;68(1-2):19–25. doi: 10.1016/0021-9150(87)90089-x. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Einarsson K., Angelin B., Nyberg B., Bergström K., Thulin L. Effects of somatostatin on hepatic bile formation. Gastroenterology. 1989 Jan;96(1):206–212. doi: 10.1016/0016-5085(89)90782-8. [DOI] [PubMed] [Google Scholar]
- Reihnér E., Angelin B., Rudling M., Ewerth S., Björkhem I., Einarsson K. Regulation of hepatic cholesterol metabolism in humans: stimulatory effects of cholestyramine on HMG-CoA reductase activity and low density lipoprotein receptor expression in gallstone patients. J Lipid Res. 1990 Dec;31(12):2219–2226. [PubMed] [Google Scholar]
- Roda A., Festi D., Sama C., Mazzella G., Alini R., Roda E., Barbara L. Enzymatic determination of cholesterol in bile. Clin Chim Acta. 1975 Nov 3;64(3):337–341. doi: 10.1016/0009-8981(75)90364-2. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Rudling M. J., Reihnér E., Einarsson K., Ewerth S., Angelin B. Low density lipoprotein receptor-binding activity in human tissues: quantitative importance of hepatic receptors and evidence for regulation of their expression in vivo. Proc Natl Acad Sci U S A. 1990 May;87(9):3469–3473. doi: 10.1073/pnas.87.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. C., Berman M., Vlahcevic Z. R., Halloran L. G., Gregory D. H., Swell L. Multicompartmental analysis of cholesterol metabolism in man. Characterization of the hepatic bile acid and biliary cholesterol precursor sites. J Clin Invest. 1978 Feb;61(2):408–423. doi: 10.1172/JCI108952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. C., Halloran L. G., Vlahcevic Z. R., Gregory D. H., Swell L. Preferential utilization of free cholesterol from high-density lipoproteins for biliary cholesterol secretion in man. Science. 1978 Apr 7;200(4337):62–64. doi: 10.1126/science.204996. [DOI] [PubMed] [Google Scholar]
- Schwartz C. C., Vlahcevic Z. R., Halloran L. G., Gregory D. H., Meek J. B., Swell L. Evidence for the existence of definitive hepatic cholesterol precursor compartments for bile acids and biliary cholesterol in man. Gastroenterology. 1975 Dec;69(6):1379–1382. [PubMed] [Google Scholar]
- Steinberg D. A docking receptor for HDL cholesterol esters. Science. 1996 Jan 26;271(5248):460–461. doi: 10.1126/science.271.5248.460. [DOI] [PubMed] [Google Scholar]