Abstract
Background—The exact mechanisms by which Helicobacter pylori infection results in gastric mucosal injury are unclear. Aims—To assess in vivo whether H pylori extracts could initiate an inflammatory response in the rat gastric mucosal microcirculation. Methods—Extracts of H pylori, Escherichia coli, or distilled water were administered topically to the gastric mucosa of anaesthetised animals. Fluorescence in vivo microscopy assessed macromolecular leakage of labelled albumin from mucosal vessels, leucocyte adherence/rolling, and platelet activity for 90minutes. Results—H pylori induced increases (p<0.001) in adherent platelet thrombi and circulating platelet emboli after five and 15minutes respectively. Adherent platelet thrombi (mean of four per field of view) remained significantly increased throughout the experiment, but circulating emboli (maximum of five at 30minutes) decreased with time. Leucocyte adherence did not occur although early transient rolling was observed. An 11% increase (p<0.02) in albumin leakage occurred after five minutes only. The induction of platelet aggregation was only observed following H pylori administration. Conclusion—This in vivo study demonstrated the ability of H pylori extracts to promote platelet aggregation within gastric mucosal microvessels. Recruitment of leucocytes was not observed. The results suggest that the early events associated with H pylori infection are platelet aggregation with perhaps subsequent leucocyte recruitment by activated platelets.
Keywords: gastric mucosal microcirculation; Helicobacter pylori; fluorescence in vivo microscopy; platelet aggregation; leucocytes
Full Text
The Full Text of this article is available as a PDF (123.8 KB).
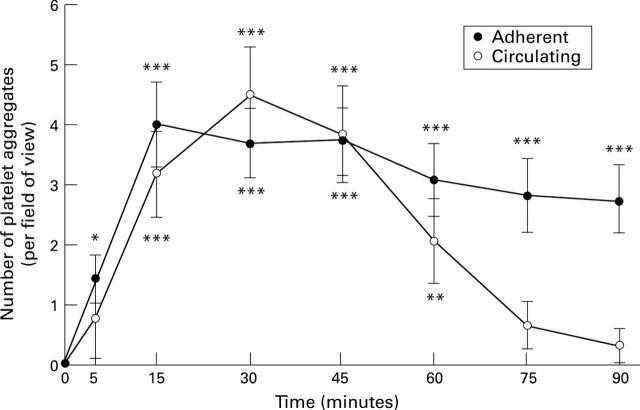
Figure 1 .
: Number of adherent and circulating platelet aggregates in superficial gastric mucosal vessels after exposure to H pylori extracts. Results represent means (SEM); n=6. *p<0.05, **p<0.01, ***p<0.001.
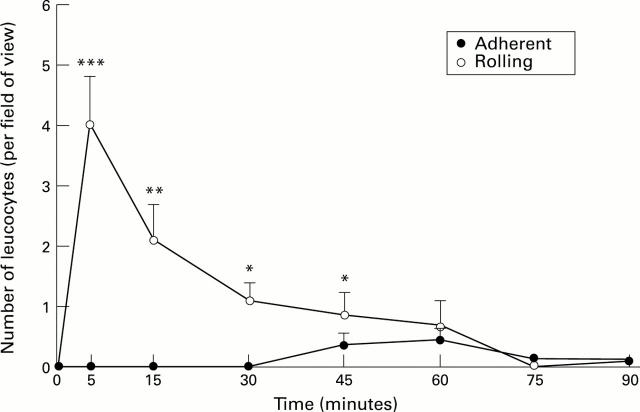
Figure 2 .
: Number of adherent and rolling leucocytes in superficial gastric mucosal vessels after exposure to H pylori extracts. Results represent means (SEM); n=6. *p<0.05, **p<0.01, ***p<0.001.
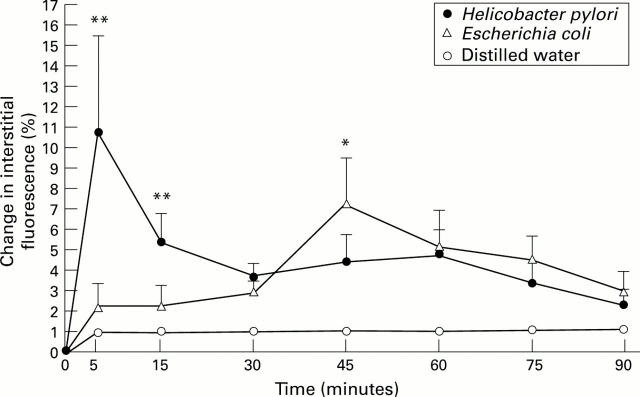
Figure 3 .
: Effect of H pylori extracts on macromolecular leakage of labelled albumin from superficial gastric mucosal post-capillary venules. Results represent means (SEM); n=6. **p<0.02, *p<0.05.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Cheung L. Y., Reese R. S., Moody F. G. Direct effect of endotoxin on the gastric mucosal microcirculation and electrical gradient. Surgery. 1976 May;79(5):564–568. [PubMed] [Google Scholar]
- Collins C. E., Rampton D. S. Platelet dysfunction: a new dimension in inflammatory bowel disease. Gut. 1995 Jan;36(1):5–8. doi: 10.1136/gut.36.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P. M., Territo M. C., Karnes W. E., Walsh J. H. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut. 1992 Aug;33(8):1020–1023. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F., Wallace J. L., MacNaughton W. K., Ibbotson G. C., Arroyo V., Rodés J. Endotoxin-induced ascites formation in the rat: partial mediation by platelet-activating factor. Hepatology. 1989 Nov;10(5):788–794. doi: 10.1002/hep.1840100507. [DOI] [PubMed] [Google Scholar]
- Kalia N., Brown N. J., Jacob S., Reed M. W., Bardhan K. D. Studies on gastric mucosal microcirculation. 1. The nature of regional variations induced by ethanol injury. Gut. 1997 Jan;40(1):31–35. doi: 10.1136/gut.40.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose I., Granger D. N., Evans D. J., Jr, Evans D. G., Graham D. Y., Miyasaka M., Anderson D. C., Wolf R. E., Cepinskas G., Kvietys P. R. Helicobacter pylori-induced microvascular protein leakage in rats: role of neutrophils, mast cells, and platelets. Gastroenterology. 1994 Jul;107(1):70–79. doi: 10.1016/0016-5085(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Twohig B., Danzell J., Specian R. D. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils and xanthine oxidase-derived radicals. Gastroenterology. 1990 Apr;98(4):909–920. doi: 10.1016/0016-5085(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Allen J. B., Wahl S. M., Blaser M. J., Smith P. D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992 Feb 1;175(2):517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai U. E., Perez-Perez G. I., Wahl L. M., Wahl S. M., Blaser M. J., Smith P. D. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991 Mar;87(3):894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. N., Joshua I. G., Anderson G. L. Quantitation of vasodilator-induced macromolecular leakage by in vivo fluorescent microscopy. Microvasc Res. 1982 Jul;24(1):56–67. doi: 10.1016/0026-2862(82)90042-5. [DOI] [PubMed] [Google Scholar]
- Nielsen H., Andersen L. P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992 Jun;33(6):738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymunde A., Deren J., Nachamkin I., Oppenheim D., Weinbaum G. Production of chemoattractant by Helicobacter pylori. Dig Dis Sci. 1993 Sep;38(9):1697–1701. doi: 10.1007/BF01303180. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Granger D. N., Evans D. J., Jr, Evans D. G., Graham D. Y., Anderson D. C., Wolf R. E., Kvietys P. R. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993 Nov;105(5):1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]