Abstract
Our previous study demonstrated formation of T cell-dendritic cell (DC) clusters in inflamed dermis of intraorally autotransplanted skin flaps. Such T cell-DC clusters are supposed to be important for close interactions between T cells and DCs including the specific antigen presentation. Here we show the involvement of the macrophage-derived chemokine (MDC/CCL22) and its specific receptor CC chemokine receptor 4 (CCR4) in the formation of T cell-DC clusters. Reverse transcriptase-polymerase chain reaction analysis revealed high levels of mRNA expression for MDC and CCR4 in inflamed skin and neck lymph nodes (LNs), but not in normal skin. Immunohistochemically, MDC+ cells and CCR4+ cells were mainly located within the T cell-DC clusters both in the dermis of inflamed skin and the T cell area of LNs. MDC+ cells were identified to be DCs both in inflamed skin and LNs. The majority of CCR4+ cells were CD4+ T cells, accounting for approximately one-third of total CD4+ T cells in the inflamed skin. Our data suggest that the MDC-CCR4 system plays an important role in the formation of T cell-DC clusters both in inflamed skin and LNs.
Chemokines are small secreted proteins that play critical roles in the migration of various types of leukocytes. Some chemokines are also likely to be involved in the interactions between T cells and dendritic cells (DC). 1,2 Chemokines are now classified into the four subfamilies, CXC, CC, C, and CX3C, based on the sequence motif of conserved N-terminal cysteine residues. 1,2 Macrophage-derived chemokine (MDC/CCL22) is one of the CC chemokines produced by macrophages and DCs, 3 and its production by DCs is up-regulated during their maturation. 4,5 In vivo, MDC was shown to be expressed by epithelial cells of human thymic medulla, 6 by DCs within the T cell area of regional lymph nodes (LNs) from murine contact sensitized-skin, 4 and by dermal DCs in murine atopic dermatitis. 7 MDC acts via CCR4, 8 which is also the functional receptor for another CC chemokine, thymus and activation-regulated chemokine (TARC/CCL17). 9 CCR4 is first reported to be preferentially expressed by Th2 cells, 10-12 which are involved in humoral immunity and allergic responses. 7,13 Recently, the involvement of CCR4 in Th1 cells was also shown in inflamed skin tissues. 14 Thus, CCR4 may not be exclusive to Th2 cells but may also be expressed in Th0 cells and even some Th1 cells in certain activated conditions.
As one of the surgical procedures for advanced oral cancer, autologous skin flaps are routinely transplanted into the oral cavity to reconstruct a large tissue defect after the radical resection of cancer. 15 During the follow-up of patients, skin flaps are frequently infected with Candida albicans. 16 Histological investigation of the inflamed skin flaps revealed clusters of CD45RO+CD4+ T cells around Langerhans cell (LC)-like DCs in the dermis. 17 Such clusters of T cells and DCs (designated as T cell-DC clusters in the present article) 18 have a close resemblance to those observed in the T cell area of LNs. DCs in both regions are of either maturing or fully mature phenotypes. 17,19 Therefore, these T cell-DC clusters may be involved in specific antigen presentation and/or other pathophysiological interactions between T cells and DCs.
A number of chemokines have been shown to be expressed in the secondary lymphoid tissues, such as pulmonary and activation-regulated chemokine (PARC)/CCL18, 20,21 EBI1-ligand chemokine (ELC)/CCL19, 20,22 secondary lymphoid-tissue chemokine (SLC)/CCL21, 23 and MDC/CCL22. 3,4,5,20 All these chemokines are produced by DCs, 1 only except SLC, which is mainly produced by the endothelium of high endothelial venules and lymphatic vessels. 23 Therefore, we speculate that some chemokines may be involved in the formation of T cell-DC clusters. Here we show that DCs within the T cell-DC clusters of both inflamed skin flaps and T cell area of LNs are strongly positive for MDC and are surrounded by CCR4+CD4+ T cells. This suggests an important role of the MDC-CCR4 system in the interaction between DCs and activated memory CD4+ T cells in both inflamed skin and secondary lymphoid tissues. 4
Materials and Methods
Surgery and Tissue
A forearm skin flap was autotransplanted into the oral cavity to reconstruct an extensive defect in the oral cavity caused by curative resection of advanced oral cancer. The skin flap included the epidermis, dermis, subcutaneous adipose tissue, underlying muscle fascia, and vascular pedicle. Twenty-three biopsy specimens were obtained during the second surgery performed ∼2 years after the primary surgery for cosmetic reason (to revise the contour of skin flaps). Of these 23 cases, 17 cases were severely inflamed by C. albicans infection. 16 Neck LNs were obtained from five patients with oral cancer during the radical neck dissection. All LNs examined were devoid of cancer metastasis. The remnant normal forearm-skin tissues trimmed off at the initial surgery were used as a normal control in seven cases. The present study has been approved by the Ethics Committee of Tohoku University School of Dentistry. Specimens were collected after the informed consent has been obtained. For immunohistochemistry and immunoelectron microscopy, specimens were fixed in periodate-lysine-4% paraformaldehyde for 6 hours at 4°C. After washing in phosphate-buffered saline containing sucrose, fixed specimens were embedded in OCT compound (Miles, Elkhart, IN) and rapidly frozen. For immunofluorescence staining, parts of the specimens were frozen without prefixation. For reverse transcriptase-polymerase chain reaction (RT-PCR), samples were frozen and stored at −80°C until use.
RT-PCR Analysis
Total RNA was prepared from inflamed skin (two patients), normal skin (two patients), and LN (two patients) by using Trizol reagent (Life Technologies, Inc., Gaithersburg, MD). RNA was further purified by using RNeasy (Qiagen, Hilden, Germany). Total RNA (1 μg) was reverse-transcribed using oligo (dT) primer and SuperScript II reverse transcriptase (Life Technologies, Inc.). Resulting first-strand DNA (20 ng total RNA equivalent) and original total RNA (20 ng) were amplified in a final volume of 20 μl containing 10 pmol of each primer and 1 U of Ex-Taq polymerase (Takara Shuzo, Kyoto, Japan). The primers used were: +5′-AGGACAGAGCATGGCTCGCCTACAGA-3′ and −3′-TAATGGCAGGGAGGTAGGGCTCCTGA-3′ for MDC; +5′-AAGAAGAACAAGGCGGTGAAGATG-3′ and −5′-AGGCCCCTGCAGGTTTTGAAG-3′ for CCR4; +5′-GCCAAGGTCATCCATGACAACTTTGG-3′ and −5′-GCCTGCTTCACCACCTTCTTGATGTC-3′ for glyceraldehyde-3-phosphate dehydrogenase (G3PDH). PCR amplification was performed by denaturation at 94°C for 30 seconds (5 minutes for the first cycle), annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds (5 minutes for the last cycle) for 36 cycles for MDC and CCR4, and 28 cycles for G3PDH. Amplification products (10 μl each) were subjected to electrophoresis on 2% agarose and stained with ethidium bromide for visualization.
Antibodies and Immunohistochemistry
The specificity of murine monoclonal anti-CCR4 antibody (clone KM2160, IgG1) 10 and anti-vascular endothelial growth factor-receptor 3 antibody (VEGFR-3) (IgG1) 24 was described previously. Rabbit polyclonal anti-MDC antibody was obtained from PeproTech (Rocky Hill, NJ). Murine monoclonal anti-CD1a (IgG2a; DAKO, Glostrup, Denmark), anti-CD83 (IgG2b; Immunotech, Marseille, France), anti-CD4 (IgG1; Becton Dickinson, San Jose, CA) and anti-CD20 (IgG2a; DAKO) were also purchased. Phycoerythrin (PE)-conjugated murine monoclonal anti-CD1a (IgG1; Coulter, Miami, FL), anti-CD83 (IgG2b; Immunotech), and anti-CD4 (IgG1; Becton Dickinson) were used for double staining.
For immunohistochemistry, periodate-lysine-4% paraformaldehyde-prefixed frozen sections were used. After the application of primary antibody, either goat anti-mouse or goat anti-rabbit Envision plus system (DAKO) was applied. As negative control, murine isotype-matched monoclonal IgG1, IgG2a, and IgG2b (DAKO) were used. The absorption test of anti-human MDC polyclonal antibody was performed using recombinant human MDC consisting of 69 amino acid residues (PeproTech). MDC immunostaining was completely abolished using the antibody solution pre-absorbed by a 10-fold excess of MDC.
Double Immunohistochemistry
The following combinations of double enzyme-linked immunohistochemistry were performed with periodate-lysine-4% paraformaldehyde-prefixed frozen sections of eight representative cases as described previously: 17 MDC/CCR4, MDC/CD1a, MDC/CD83, MDC/VEGFR-3, CCR4/CD20, and CD1a/CD4. Cross-reactivity of anti-MDC antibody with the second-step primary antibodies was examined by processing four pairs, ie, anti-MDC antibody, (+)/second-step primary antibody (+), (+)/(−), (−)/(+), and (−)/(−). Cross-reactivity of anti-CCR4 antibody and anti-CD20 antibody, or anti-CD1a antibody and anti-CD4 antibody was also examined in the same way. No cross-reactivity was observed.
Double-Immunofluorescence Staining
Fresh frozen sections mounted on glass slides were fixed for 10 minutes in chilled acetone before use. The following combinations of double-immunofluorescence staining were performed in five representative cases as described previously: 17 CCR4/CD1a, CCR4/CD83, and CCR4/CD4.
Immunoelectron Microscopy for CCR4 and MDC
The pre-embedding immunoperoxidase method was adopted in four cases as described previously. 17
Double-Labeling Immunoelectron Microscopy for MDC/CD1a or MDC/CD83
Immunoperoxidase MDC staining was performed first by the same method as in light microscopic immunohistochemistry, followed by silver-enhanced immunogold labeling for CD1a or CD83. After diaminobenzidine reaction for MDC, specimens were incubated with anti-CD1a or anti-CD83 overnight. Specimens were incubated for 2 hours with anti-mouse Fab labeled with <0.8-nm gold particles (GP-Ultra Small; Aurion, Wageningen, Netherlands). Gold particles were treated with the silver-enhancing system (R-Gent, Aurion). Specimens were postfixed with 2.5% glutaraldehyde and then with 1% osmium tetroxide, and embedded in Epon.
Results
Expression of MDC and CCR4 mRNA
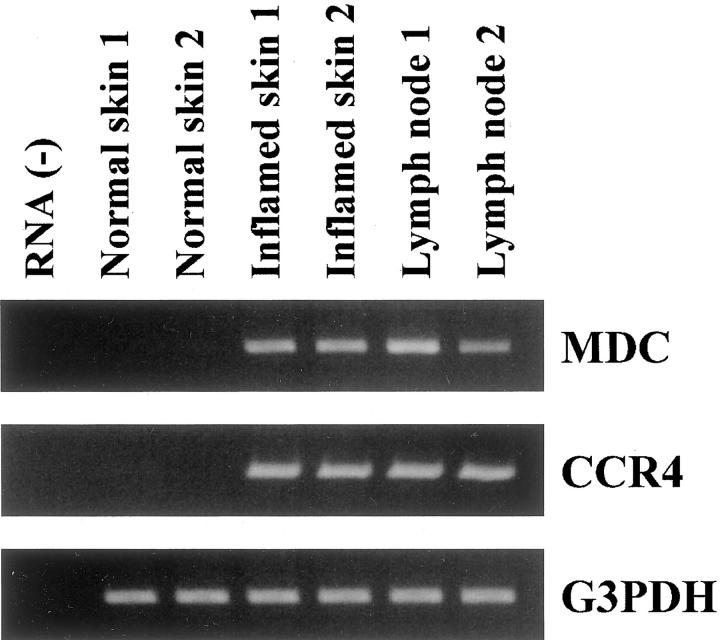
RT-PCR analysis revealed that MDC and CCR4 mRNAs were strongly up-regulated in inflamed skin samples and neck LNs in comparison to normal skin samples (Figure 1) ▶ .
Figure 1.
RT-PCR analysis for MDC, CCR4, and G3PDH in inflamed skin, normal skin, and LN. Expression of MDC and CCR4 mRNA is seen both in inflamed skin and LN, but not in normal skin. A representative result from two experiments is shown.
Immunohistochemical Localization of CCR4+ and MDC+ Cells
Normal Skin
We observed hardly any cells clearly positive for CCR4 or MDC in the epidermis or dermis of normal skin tissues (data not shown).
Inflamed Skin
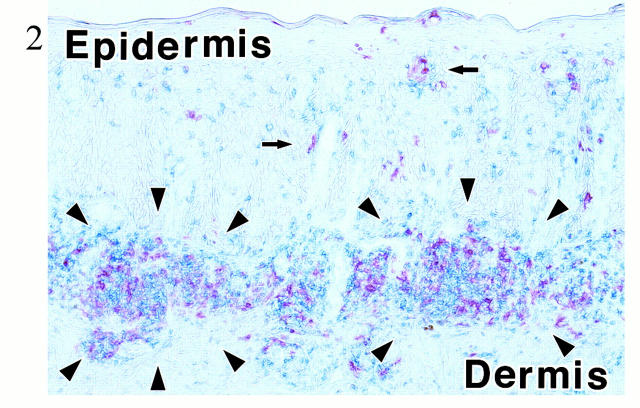
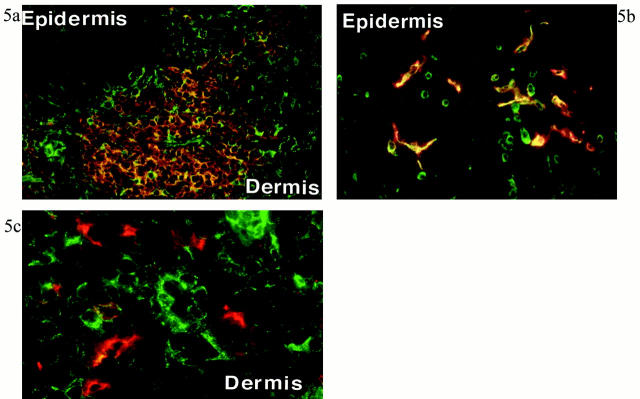
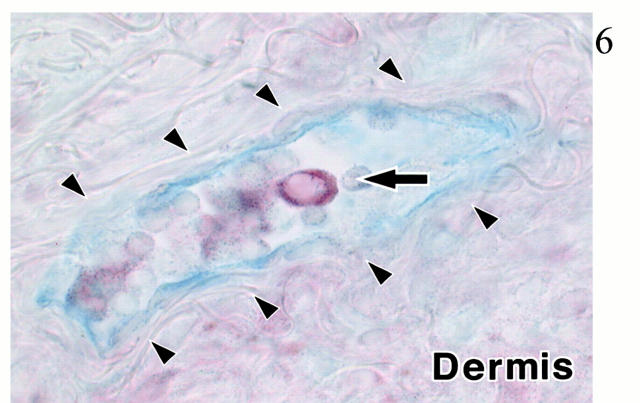
As shown in our previous article, 17 inflamed dermis is infiltrated by lymphocytes and DCs, forming T cell-DC clusters (Figure 2) ▶ . In these T cell-DC clusters, CCR4 was expressed in small round cells (probably lymphocytes; Figure 3A ▶ ), whereas larger cells expressed MDC (Figure 3B) ▶ . Double staining revealed that CCR4+ cells clustered around MDC+ cells (Figure 3C) ▶ . Figure 3C ▶ also suggested that vast majority of MDC+ cells did not co-express CCR4. Double staining identified MDC+ cells as LC-like DCs because of co-expression of CD1a or CD83 (Figure 4) ▶ . Most of CCR4+ cells were identified as CD4+ T lymphocytes, and such CCR4+ cells accounted for approximately one-third of CD4+ T lymphocytes present in the inflamed dermis (Figure 5a) ▶ . In the epidermis, ∼50% of epidermal LCs were found to express CCR4 in the inflamed skin (Figure 5b) ▶ . This is contrasted with the absence of CCR4 in MDC+ LC-like DCs in T cell-DC clusters in the dermis (Figure 5c) ▶ . MDC+ DCs were also present within the lumen of lymphatic vessels stained by anti-VEGFR-3 in the inflamed dermis (Figure 6) ▶ . Immunoelectron microscopy confirmed CCR4 expression on the plasma membrane of lymphocytes clustering around DCs (Figure 7A) ▶ . Double-immunoelectron microscopy revealed that DCs, identified by CD1a or CD83 expression on the plasma membrane (dotted silver-enhanced colloidal gold), also expressed MDC in the perinuclear space and in the rough endoplasmic reticulum (diffuse black deposits) (Figure 7 ▶ ; B, C, and D). The latter pattern is typical of a secretary-type protein that is translated on the surface of rough endoplasmic reticulum and perinuclear space studded with polysomes and subsequently transported into the cisternae. Therefore, our findings clearly confirmed in situ production of MDC by DCs.
Figure 2.
Double staining for CD4 (blue) and CD1a (red) in the inflamed skin. One of representative figures among eight cases is presented. In the dermis, CD4+ T cells and CD1a+ DCs are gathered to form clusters (T cell-DC clusters; arrowheads). Arrows indicate CD1a+ epidermal LCs. No counterstaining. Original magnification, ×33.
Figure 3.
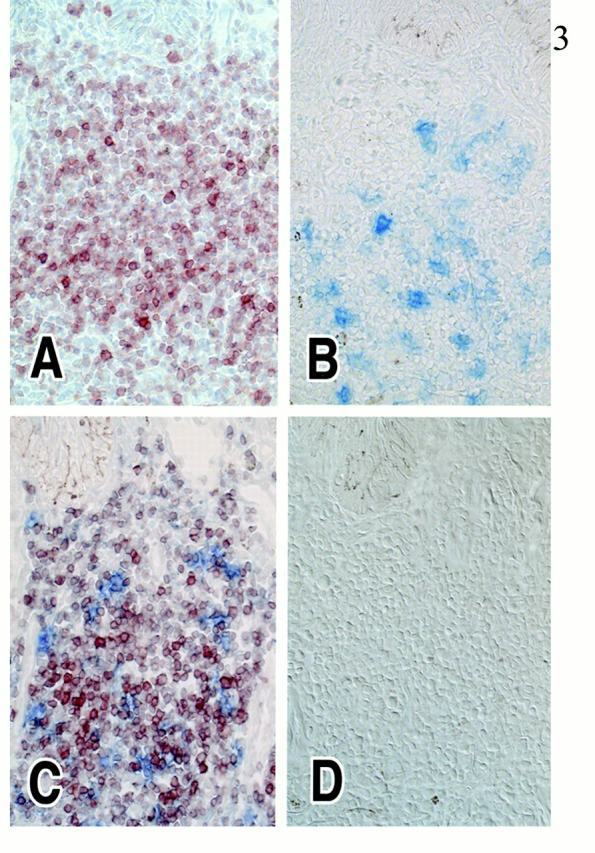
MDC and CCR4 expression in serial cryostat sections of inflamed skin. Eight cases were analyzed of this combination, and results of one representative case are presented. A: CCR4+ cells (red) are mostly small round-shaped cells and located within T cell-DC clusters of the dermis. B: MDC+ cells (blue) are relatively large cells located in the same area. C: Double immunohistochemistry revealed that MDC+ cells (blue) are surrounded by CCR4+ cells (red) in the inflamed dermis. MDC+ cells do not express CCR4. No counterstaining. D: The specificity of CCR4 and MDC immunostaining was demonstrated by using isotype-matched monoclonal antibody control and by the anti-MDC polyclonal antibody preabsorbed with the recombinant MDC protein, respectively. No staining was detected. No counterstaining. Original magnification, ×100 (A–D).
Figure 4.
Double immunohistochemistry for MDC (brown) and CD83 (black) in the T cell-DC clusters of inflamed dermis. Result is representative of eight experiments with inflamed skin. DCs are labeled with CD83 (black) along the cell surface and with MDC (brown) in the cytoplasm (arrows). Hematoxylin counterstaining. Original magnification, ×500.
Figure 5.
Double-immunofluorescence staining for CCR4 and cell markers in inflamed skin. Results shown in a, b, and c are representative figures of five experiments of this combination with inflamed skin. a: Numerous CCR4+ cells (green) and CD4+ cells (orange) are present in the T cell-DC cluster of dermis. CCR4+CD4+ cells (bright yellow) constitute approximately one-third of CD4+ cells. A part of CD4+ T cells in the epidermis also expressed CCR4. b: Double immunofluorescence for CCR4 (green) and CD1a (red) in the epidermis. Approximately half of epidermal LCs express CCR4 (yellow). c: Double immunofluorescence for CCR4 (green) and CD83 (red) in the inflamed dermis. CCR4+ cells and CD83+ cells are different. Focal yellow color may represent overlapping of cell membranes in thick frozen section. Original magnifications, ×100 (a), ×300 (b), ×320 (c).
Figure 6.
Double immunohistochemistry for MDC (red) and VEGFR-3 (blue) in the inflamed dermis. Result is a representative figure of eight experiments with inflamed skin. MDC+ DC (arrow) is located in the lumen of VEGFR-3+ lymphatic vessel (arrowheads) in the dermis. No counterstaining. Original magnification, ×200.
Figure 7.

Immunoelectron microscopy. Results shown in A to D are representative figures of five cases. A: CCR4+ lymphocytes (arrowheads), labeled by immunoperoxidase method as linear black deposits, are located around dendritic-shaped cells (DC) in the inflamed dermis. L, all lymphocytes in this figure. B: CD1a+ DC is labeled by small, black spherical particles on the plasma membrane by silver-enhanced gold particle method. C and D: Double-labeling immunoelectron microscopy for MDC and CD1a. MDC is expressed in the perinuclear space (arrowheads) and the rough endoplasmic reticulum (arrows) by diffuse black deposits by immunoperoxidase method, whereas CD1a is expressed along the plasma membrane by dot-like silver-enhanced gold particles (enlarged in D), identifying this cell as DC. Original magnifications, ×3,000 (A), × 2,000 (B), ×5,000 (C), ×12,000 (D). Scale bars, 1 μm.
Neck LNs
Both CCR4+ cells and MDC+ cells were located within the T cell area and generally absent in the follicle (Figure 8, A–D) ▶ . CCR4+ lymphocytes were clustered around the MDC+ DCs, showing a close similarity to the T cell-DC clusters in the inflamed dermis (see Figure 3C ▶ ). No cells were double-positive for MDC and CCR4. MDC+ cells in the T cell area were identified as DCs by their co-expression of CD1a or CD83 examined at both light and electron microscopic levels (not shown).
Figure 8.

MDC and CCR4 expression in serial sections of LN. Results shown in A to D are representative figures of experiments of this combination in eight cases. A: MDC+ DCs (blue) and CCR4+ lymphocytes (red) are located together in the T cell area of LN. B: CCR4+ cells (red) are small round-shaped cells. C: MDC+ cells (blue) are relatively large DC-like cells. D: CCR4+ cells (red) are located in the T cell area (T) of LN. CD20+ cells (blue) are present in the follicle (arrowheads; B) of LN. No counterstaining. Original magnification, ×100 (A–D).
Discussion
The present study morphologically revealed that CCR4+CD4+ T cells were clustered around MDC+ DCs within the T cell-DC clusters of both human inflamed skin and LNs. This suggests the importance of MDC-CCR4 system for the formation of T cell-DC clusters both in inflamed skin and LN.
MDC+ cells were identified as CD1a+ or CD83+ DCs both in inflamed skin and LN. This observation is consistent with earlier studies showing MDC expression by DCs in the T cell area of murine LN 4 and by dermal DCs in murine atopic dermatitis. 7 Our double-labeling immunoelectron microscopy confirmed that MDC protein is localized in the perinuclear space and the rough endoplasmic reticulum of CD1a+ or CD83+ DCs, demonstrating in situ production of MDC. We also found MDC+ DCs located within the lumen of lymphatic vessels. Our previous study showed that such DCs were of the mature phenotype expressing both B7-1 and B7-2. 17 Therefore, our morphological observation is consistent with the in vitro observation that MDC expression by DCs is up-regulated during their maturation process. 4,5 Galli and colleagues 25 reported MDC expression by T cells in human atopic dermatitis. However, we did not observe any staining of MDC in T cells. This discrepancy might be explained by the differences of diseases (a Th2-type disease versus microbial infection). One of the previous in vitro studies on MDC production proposed an autocrine role, suggesting that MDC expressed by DCs was chemotactic for DCs themselves to enhance DC accumulation at the site of inflammation. 3 According to this hypothesis, MDC+ cells might be expected to express CCR4. However, we did not observe any MDC+ DCs expressing CCR4 in both inflamed skin and LN.
CCR4 was mainly expressed by CD4+ T cells in both inflamed skin and LN. Our previous study revealed that CD4+ T cells in inflamed dermis were mostly CD45RO+, indicating memory/effector T cells. 17 Therefore, CCR4+ T cells within T cell-DC clusters are likely to be memory/effector T cells. This is consistent with the previous finding that CCR4 is selectively expressed on peripheral blood CD4+CD45RO+ T cells. 10 We observed that one-third of CD4+ T cells were positive for CCR4. Therefore, other chemokines are probably attracting the remaining CD4+ T cells.
CCR4 was also expressed by approximately half of the epidermal LCs in inflamed skin. This was contrasted with the lack of CCR4 expression in dermal DCs within T cell-DC clusters or in epidermal LCs in normal skin. Our morphological observation may suggest that CCR4 is involved in the trafficking of epidermal LCs at the inflamed site. Epidermal LCs were previously shown to express CCR6, whereas its ligand, liver, and activation-regulated chemokine (LARC)/macrophage inflammatory protein-3α (MIP-3α)/CCL20, was produced by inflamed tonsillar epithelial cells or normal epidermal keratinocytes. 26,27 It remains to be seen whether CCR4+ epidermal LCs are attracted to the epidermis by MDC or TARC via CCR4 in inflamed skin. CCR4+ epidermal LCs are also likely to transmigrate across the epidermal basement membrane toward the dermal T cell-DC clusters. During this transmigration, they may down-regulate CCR4 expression while up-regulating MDC expression.
One of the aims in this study was to compare inflamed skin tissues with LNs. A close similarity was observed in the staining pattern of MDC and CCR4 in inflamed skin and neck LNs. The microenvironments of inflamed skin tissues and LNs are probably quite different. Nevertheless, this similarity suggests that the basic mechanisms that lead to the formation of T cell-DC clusters are common in both inflamed skin and LN. Chemokines other than MDC may also be involved in the formation of T cell-DC clusters. For example, we also found up-regulation of TARC mRNA in both inflamed skin and LN by RT-PCR (unpublished data). However, we were unable to reveal the cellular localization of TARC protein because of a poor signal to noise ratio. It remains to be seen what other chemokines may be involved in the present dermal inflammatory process.
In summary, our results suggest that MDC and CCR4 play an important role in the formation of T cell-DC clusters in both inflamed skin and LN. This suggests that MDC and CCR4 are involved in both homeostatic and inflammatory conditions. In other words, our data support a view that inflammatory reactions leading to the formation of T cell-DC clusters in chronically inflamed tissues are similar to some extent to the physiological cell-to-cell interactions in the secondary lymphoid tissues. 2,28,29 The interaction between DCs and T cells in the peripheral inflamed regions may collaborate with those in the T cell area of LN in the protective immunity to infectious challenges.
Acknowledgments
We thank Dr. Nobukazu Shirai and Prof. Seishi Echigo, Departments of Oral and Maxillofacial Surgery I and II, Tohoku University, for supplying specimens.
Footnotes
Address reprint requests to Fuminori Katou, M.D., D.D.S., Dept. of Oral and Maxillofacial Surgery I, Tohoku University School of Dentistry, 4-1 Seiryo-machi, Aoba-ku, Sendai 980-8575, Japan. E-mail: fkatou@mail.cc.tohoku.ac.jp.
References
- 1.Zlotnik A, Yoshie O: Chemokines: a new classification system and their role in immunity. Immunity 2000, 12:121-127 [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Mackay CR, Lanzavecchia A: The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol 2000, 18:593-620 [DOI] [PubMed] [Google Scholar]
- 3.Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW: Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med 1997, 185:1595-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang HL, Cyster JG: Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science 1999, 284:819-822 [DOI] [PubMed] [Google Scholar]
- 5.Kanazawa N, Nakamura T, Tashiro K, Muramatsu M, Morita K, Yoneda K, Inaba K, Imamura S, Honjo T: Fractalkine and macrophage-derived chemokine: T cell-attracting chemokines expressed in T cell area dendritic cells. Eur J Immunol 1999, 29:1925-1932 [DOI] [PubMed] [Google Scholar]
- 6.Chantry D, Romagnani P, Raport CJ, Wood CL, Epp A, Romagnani S, Gray PW: Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood 1999, 94:1890-1898 [PubMed] [Google Scholar]
- 7.Vestergaard C, Yoneyama H, Murai M, Nakamura K, Tamaki K, Terashima Y, Imai T, Yoshie O, Irimura T, Mizutani H, Matsushima K: Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J Clin Invest 1999, 104:1097-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW: Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem 1998, 273:1764-1768 [DOI] [PubMed] [Google Scholar]
- 9.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O: The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem 1997, 272:15036-15042 [DOI] [PubMed] [Google Scholar]
- 10.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O: Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 1999, 11:81-88 [DOI] [PubMed] [Google Scholar]
- 11.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A: Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med 1998, 187:875-883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F: Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol 1998, 161:5111-5115 [PubMed] [Google Scholar]
- 13.Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, Fujisawa T, Pawankar R, Sano Y, Ohta K, Ishii A, Morita Y, Yamamoto K, Matsushima K, Yoshie O, Hirai K: Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol 2000, 165:2205-2213 [DOI] [PubMed] [Google Scholar]
- 14.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC: The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999, 400:776-780 [DOI] [PubMed] [Google Scholar]
- 15.Katou F, Echigo S, Ito M, Shirai N, Ohtani H, Motegi K: Reliability of internal jugular vein in oral microvascular reconstruction. Oral Surg Oral Med Oral Pathol 1998, 86:529-533 [DOI] [PubMed] [Google Scholar]
- 16.Katou F, Motegi K, Tagami H, Shirai N, Echigo S, Nagura H: Unique inflammatory features noted in intraorally transferred skin flaps: correlation with Candida albicans infection. Oral Surg Oral Med Oral Pathol 1999, 87:676-684 [DOI] [PubMed] [Google Scholar]
- 17.Katou F, Ohtani H, Saaristo A, Nagura H, Motegi K: Immunological activation of dermal Langerhans cells in contact with lymphocytes in a model of human inflamed skin. Am J Pathol 2000, 156:519-527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuhler G, Zobywalski A, Grunebach F, Brossart P, Reichardt VL, Barth H, Stevanovic S, Brugger W, Kanz L, Schlossman SF: Immune regulatory loops determine productive interactions within human T lymphocyte-dendritic cell clusters. Proc Natl Acad Sci USA 1999, 96:1532-1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 1998, 392:245-252 [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Palermo B, Lenig D, Miettinen M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M, Lanzavecchia A: Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol 1999, 29:1617-1625 [DOI] [PubMed] [Google Scholar]
- 21.Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, Bacon KB, Figdor CG: A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997, 387:713-717 [DOI] [PubMed] [Google Scholar]
- 22.Ngo VN, Tang HL, Cyster JG: Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J Exp Med 1998, 188:181-191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT: A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 1998, 95:258-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lymboussaki A, Partanen TA, Olofsson B, Thomas-Crusells J, Fletcher CD, de Waal RM, Kaipainen A, Alitalo K: Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol 1998, 153:395-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galli G, Chantry D, Annunziato F, Romagnani P, Cosmi L, Lazzeri E, Manetti R, Maggi E, Gray PW, Romagnani S: Macrophage-derived chemokine production by activated human T cells in vitro and in vivo: preferential association with the production of type 2 cytokines. Eur J Immunol 2000, 30:204-210 [DOI] [PubMed] [Google Scholar]
- 26.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C: Selective recruitment of immature and mature dendritic cells by distinct chemones expressed in different anatomic sites. J Exp Med 1998, 188:373-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charbonnier AS, Kohrgruber N, Kriehuber E, Stingl G, Rot A, Maurer D: Macrophage inflammatory protein 3α is involved in the constitutive trafficking of epidermal Langerhans cells. J Exp Med 1999, 190:1755-1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH: Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med 1996, 183:1461-1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM: Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med 1998, 188:1493-1501 [DOI] [PMC free article] [PubMed] [Google Scholar]