Abstract
Early chronic liver allograft rejection (CR) is characterized by distinctive cytological changes in biliary epithelial cells (BECs) that resemble cellular senescence, in vitro, and precede bile duct loss. If patients suffering from early CR are treated aggressively, the clinical and histopathological manifestations of CR can be completely reversed and bile duct loss can be prevented. We first tested whether the senescence-related p21WAF1/Cip1 protein is increased in BECs during early CR, and whether treatment reversed the expression. The percentage of p21+ BECs and the number of p21+ BECs per portal tract is significantly increased in early CR (26 ± 17% and 3.6 ± 3.1) compared to BECs in normal liver allograft biopsies or those with nonspecific changes (1 ± 1% and 0.1 ± 0.3; P < 0.0001 and P < 0.02), chronic hepatitis C (2 ± 3% and 0.7 ± 1; P < 0.0001 and P < 0.04) or obstructive cholangiopathy (7 ± 7% and 0.7 ± 0.6; P < 0.006 and P = 0.04). Successful treatment of early CR is associated with a decrease in the percentage of p21+ BECs and the number of p21+ BECs per portal tract. In vitro, nuclear p21WAF1/Cip1 expression is increased in large and multinucleated BECs, and is induced by transforming growth factor (TGF)-β. TGF-β1 also increases expression of TGF-β receptor II, causes phosphorylation of SMAD-2 and nuclear translocation of p21WAF1/Cip1, which inhibits BEC growth. Because conversion from cyclosporine to tacrolimus is an effective treatment for early CR, we next tested whether these two immunosuppressive drugs directly influenced BEC growth in vitro. The results show that cyclosporine, but not tacrolimus, stimulates BEC TGF-β1 production, which in turn, causes BEC mito-inhibition and up-regulation of nuclear p21WAF1/Cip1. In conclusion, expression of the senescence-related p21WAF1/Cip1 protein is increased in BECs during early CR and decreases with successful recovery. Replicative senescence accounts for the characteristic BEC cytological alterations used for the diagnosis of early CR and lack of a proliferative response to injury. The ability of cyclosporine to inhibit the growth of damaged BECs likely accounts for the relative duct sparing properties of tacrolimus.
Early chronic liver allograft rejection (CR) is characterized by cytological alterations in biliary epithelial cells (BECs), such as cellular and nuclear enlargement, multinucleation, aggregation of dense bundles of cytoplasmic filaments and cytoplasmic eosinophilia, which results in uneven nuclear spacing and thickening of the basement membrane. 1-8 These are well-described changes of cellular senescence in vitro and in vivo, 9-12 including primary cultures of human BECs, which enlarge, cease dividing, become multinucleated and squamoid-appearing before finally detaching from the plates. 13 Senescent cells also express p21WAF1/Cip1 9,14,15 and forced overexpression of p21WAF1/Cip1 causes cellular senescence and decreased replicative capacity. 14 Despite the BEC damage in early CR, there is no compensatory proliferation, which is unusual because BECs usually divide in response to injury. 16-18
If patients with early CR are treated aggressively, the clinical, biochemical, and histopathological manifestations of CR can be reversed 1,5,6,19-21 and bile duct loss, which is a late feature of CR, 7 can be prevented. Rare instances of spontaneous reversal of early CR have also been reported. 2,3 Interestingly, tacrolimus decreases the overall incidence of CR and liver allografts that do fail because of CR under tacrolimus show less bile duct loss than cyclosporine-treated recipients. 5-7 In vitro cyclosporine, but not tacrolimus, can induce epithelial production of transforming growth factor (TGF)-β, 22-24 which inhibits epithelial cell growth via induction of p21WAF1/Cip1.
In these studies, we tested the hypothesis that the characteristic phenotypic changes seen during early CR were because of BEC replicative senescence, and therefore, should be associated with increased expression of p21WAF1/Cip1. Because successful treatment of early CR reverses the BEC alterations, a decrease in p21WAF1/Cip1 should be observed in association with recovery. Furthermore, given the relative duct sparing qualities of tacrolimus in comparison to cyclosporine, 6 we hypothesized that the primary immunosuppressant drug might contribute to this process by influencing endogenous BEC TGF-β production and BEC growth.
Materials and Methods
Patient Selection and Immunohistochemistry for p21WAF1/Cip1
Patients were selected for study from two different groups. The first group consisted of patients who were converted from cyclosporine to tacrolimus during the original rescue trial conducted from 1989 until 1991. 21 Patients from this group were chosen to determine the effect of the primary immunosuppressant on BEC p21WAF1/Cip1 expression in various allograft syndromes: obstructive cholangiopathy (n = 3), chronic viral hepatitis (n = 3), early chronic rejection (n = 5), or nonspecific changes (n = 8). Individual patients were selected on the basis of the availability of a liver allograft biopsy obtained within 8 days before, and after conversion to tacrolimus, along with enough tissue remaining in each of the paraffin blocks to carry out the immunohistochemical stains. Biopsies were obtained at a median of 889 days after transplantation and all but one was obtained more than 3 months after transplantation. The second group consisted of randomly selected tacrolimus-treated patients who were experiencing obstructive cholangiopathy (n = 4); chronic hepatitis, viral type C (n = 5); or early chronic rejection (n = 3). These biopsies were obtained at a median of 938 days after transplantation.
An indirect immunolabeling procedure after microwave antigen retrieval in 10 mmol/L citrate buffer (pH 6.0), was used to localize p21WAF1/Cip1 protein expression in the human liver allograft biopsies (Waf-1, 1:50 dilution, Cat No. OP64; Oncogene Research, Cambridge, MA) and in primary cultures of mouse BECs (mBECs) (SX118; DAKO, Carpinteria, CA). Briefly, after antigen retrieval, samples were blocked with Blue Block (Shandon, Pittsburgh, PA) for 15 minutes, and then incubated with the primary antibody for 1 hour at room temperature. After washing with phosphate-buffered saline (PBS) plus 0.05% Tween 20, slides were incubated at room temperature for 30 minutes with biotinylated horse anti-mouse secondary antibody (1:200 dilution, BA 2000; Vector Laboratories, Burlingame, CA). The samples were then developed with Vectastain-Elite ABC (Vector Laboratories), stained with liquid Dab+ (DAKO) and counterstained with hematoxylin. An immunoglobulin class-matched nonimmune antibody was substituted for the primary antibody in the negative controls. For the mBECs, 10,000 BECs were seeded on collagen-coated glass cell chamber slides (Nunc, Rochester, NY) and cultured for 3 days in complete serum-free medium (described below), followed by formalin fixation. Staining for p21WAF1/Cip1 protein in the mBECs was enhanced by tyramide amplification (New England Nuclear–Life Sciences, Boston, MA).
For the human liver allograft biopsies, the total number of BECs showing strong nuclear p21WAF1/Cip1 positivity, the total number of BECs, and the total number of complete portal tracts were counted for each biopsy, without knowledge of whether the biopsy was obtained before or after conversion to tacrolimus, or the diagnosis. However, in most cases, the histopathological diagnosis was obvious, and true blinding of the samples was not possible. The expression of p21WAF1/Cip1 in BECs was expressed as both the percentage of BEC that were p21WAF1/Cip1-positive and the absolute number of p21WAF1/Cip1-positive BECs per portal tract.
Mice and Isolation of mBECs
Eight- to twelve-week-old male mice of a mixed strain consisting of 75% (CJ57BL/6) and 25% (SV129) were used in this study. The mice were housed in a pathogen-free environment at the University of Pittsburgh. Primary cultures of mBECs were prepared as previously reported. 25 For all experiments (except where noted), BECs were cultured in complete serum-free medium (C-SFM), consisting of Dulbecco’s modified Eagle medium/F12 medium (Sigma, St. Louis, MO) supplemented with 5.4 g/L d-glucose (Life Technologies, Inc., Rockville, MD), 50 μg/ml gentamicin (Life Technologies, Inc.), antibiotic-antimycotic (100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, 250 ng/ml amphotericin B; Life Technologies, Inc.), 10 mmol/L HEPES (Life Technologies, Inc.), 2.5 mg/ml bovine serum albumin (Sigma), insulin-transferrin-selenium X (10 mg/L insulin, 5.5 mg/L transferrin, 6.7 ng/L sodium selenite; Life Technologies, Inc.), 0.1 mmol/L minimal essential media nonessential amino acid solution (Life Technologies, Inc.), 2 mmol/L l-glutamine (Life Technologies, Inc.), 32 ng/ml thyroxin (Sigma), 10 ng/ml prostaglandin E1 (Sigma), 40 ng/ml hydrocortisone (Sigma), 10 μmol/L forskolin (Sigma), and 50 μg/ml trypsin inhibitor (Sigma).
Proliferation Assay for mBECs
For these experiments, mBECs were seeded at a density of 1 × 10 4 cells per well on collagen-coated 96-well flat-bottom plates (BD Falcon, Franklin Lakes, NJ) in C-SFM supplemented with 50 μg/ml of bovine pituitary extract (BPE) (Life Technologies, Inc.) and 10 ng/ml epidermal growth factor (EGF) (Life Technologies, Inc.). The cells were grown until 50% confluent when the media was removed. The cells were then washed two times at 37°C in Hanks’ balanced salt solution (HBSS) (Life Technologies, Inc.), and kept for 24 hours in simple serum-free medium (S-SFM) consisting of Dulbecco’s modified Eagle’s medium/F12 medium (Sigma) supplemented with 5.4g/L d-glucose (Life Technologies, Inc.), 50 μg/ml gentamicin (Life Technologies, Inc.), antibiotic-antimycotic (100 units/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, 250 ng/ml amphotericin B; Life Technologies, Inc.), 10 mmol/L HEPES (Life Technologies, Inc.), and 2.5 mg/ml bovine serum albumin (Sigma). The BECs were then switched to C-SFM alone or C-SFM supplemented with either cyclosporine A or tacrolimus for 48 hours (kindly donated from Dr. A. Zeevi and Dr. R. Venkataranam, University of Pittsburgh, Pittsburgh, PA). Twenty-four hours before harvesting, cells were treated with 1.0 μCi of [3H]-thymidine to each well.
Protein and mRNA Analysis
For both mRNA and protein studies, mBECs were seeded onto collagen-coated plates or flasks and grown to 70% confluence in C-SFM + BPE + EGF, washed twice at 37°C in HBSS, and then treated with cyclosporine, tacrolimus, or control media (C-SFM) for 48 hours. The media was removed and saved for analysis of TGF-β1 protein concentration by TGF-β1 Emax ImmunoAssay System (Promega, Madison, WI).
Total RNA was isolated for the cells using the TRIzol reagent (Life Technologies, Inc.) and TGF-β1 mRNA was assayed by RiboQuant ribonuclease protection assay (BD Pharmingen, San Diego, CA). Five μg of total RNA was assayed according to the manufacturer’s protocol. Gels were dried and analyzed by phosphoimaging (Molecular Dynamics, Sunnyvale, CA). The signals were quantified using the NIH Image analysis software.
Total cell lysates were prepared by treating the cells with RIPA buffer at 4°C for 1 hour. Lysates were cleared by centrifugation at 14,000 × g for 30 minutes and the supernatant was collected and stored at −80°C. Nuclear and cytosolic protein fractions were extracted by lysing the cells on ice with a buffer containing 10 mmol/L HEPES, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.1% Triton X-100, 10 μg/ml leupeptin, 4 μg/ml aprotinin, 1 mmol/L Na3VO4, 1 mmol/L phenylmethyl sulfonyl fluoride, and 1 mmol/L dithiothreitol for 10 minutes on ice. Lysates were centrifuged at 7,000 × g for 5 minutes at 4°C and the supernatant was saved as cytosolic protein at −80°C. The residual pellet was then incubated in a buffer containing 20 mmol/L HEPES, 1.5 mmol/L MgCl2, 0.1% Triton X-100, 0.2 mmol/L ethylenediaminetetraacetic acid, 420 mmol/L NaCl, 20% glycerol, 10 μg/ml leupeptin, 4 μg/ml aprotinin, 1 mmol/L Na3VO4, 1 mmol/L phenylmethyl sulfonyl fluoride, and 1 mmol/L dithiothreitol and then centrifuged for 15 minutes at 13,000 × g. The supernatant was saved as nuclear protein at −80°C. Forty μg of total cell lysate was separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, transferred to nitrocellulose membrane, and probed with anti-TGF-β receptor II (Santa Cruz Biotechnology, Santa Cruz, CA) or phospho-SMAD-2 (Upstate Biotechnology, Lake Placid, NY). The nuclear fraction (100 μg) was separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and used to probe for p21WAF1/Cip1 (Santa Cruz Biotechnology, Santa Cruz, CA).
Anti-TGF-β Blocking
This experiment was done to determine whether the BECs mito-inhibitory effect of TGF-β could be inhibited by neutralizing anti-TGF-β antibodies. BECs were prepared as above, and simultaneously treated with cyclosporine and anti-TGF-β neutralizing monoclonal antibody (R&D Systems) or control mouse IgG (Santa Cruz Biotechnology) for 48 hours and assayed for thymidine incorporation, as described above.
Isolation and Culture of Human BECs
Primary cultures of normal human BECs were prepared using a modification of a method reported by Strain and colleagues 26 ; instead of using liver biopsy pieces, small fragments of the isolated human biliary tree from normal livers were used as an explant source. The normal human livers were harvested for transplantation, but not used because of steatosis or other contra-indications that did not affect BEC viability. Liver tissue was supplied by Dr. S. Strom (University of Pittsburgh) through the NIH Liver Tissue Procurement and Distribution System. The biliary tree was isolated by digesting the entire liver via collagenase perfusion of the portal vein and removing hepatocytes, as previously reported. 27 Mechanical disruption with forceps and gentle shaking at 37°C in a 25% collagenase solution was used to remove any residual hepatocytes from the biliary tree, until the white portal connective tissue containing the bile ducts was isolated. Tiny fragments of the biliary tree were then washed three times in HBSS, resuspended in complete growth factor-supplemented serum-free medium (CGF-SFM), consisting of C-SFM + BPE + EGF + 20 ng/ml hepatocyte growth factor (Toyoba New York Inc., New York, NY), minced thoroughly, placed on collagen-coated plates with a minimal volume of C-SFM and allowed to attach for 1 hour at 37°C in a humidified incubator. The plate was then carefully covered with C-SFM + BPE + EGF + hepatocyte growth factor to avoid disrupting the attached fragments from the plates. The media was replaced after 24 hours and then every 4 to 5 days thereafter. BECs were seen growing from the explants within 2 to 3 weeks. After substantial growth of BECs, the cells were treated with cyclosporine or tacrolimus as per individual experiments.
Proliferation Assay for Human BECs
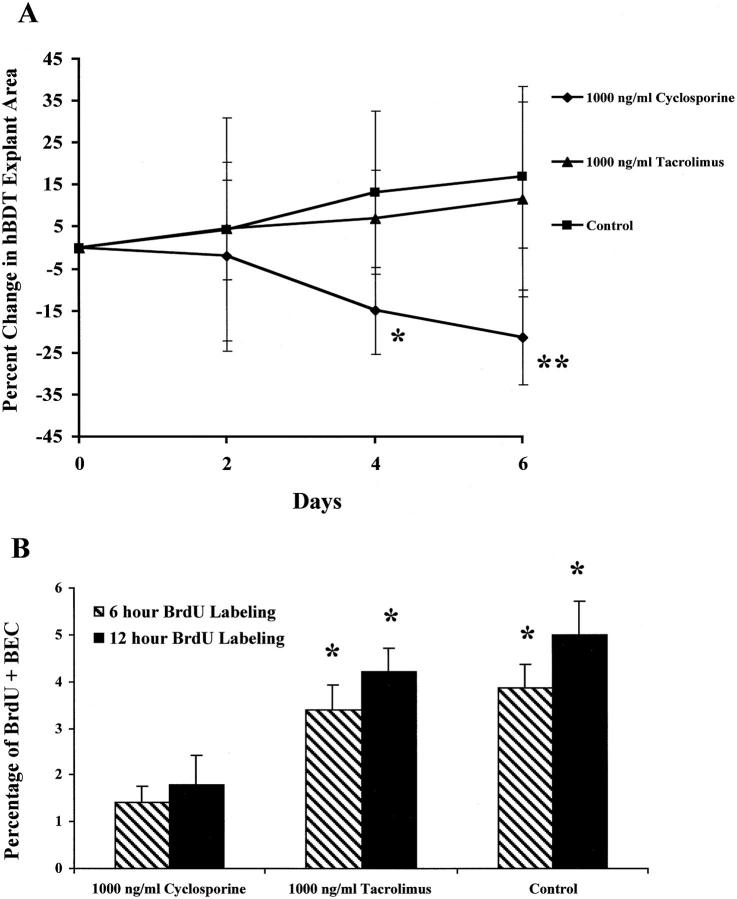
The biliary tree fragments were maintained in culture for several weeks until the normal human BECs began migrating from the explant. The total area of the explant and surrounding BEC colonies were then calculated using digital photography and area morphometry (Optimas 6.0 software; Media Cybernetics, Silver Springs, MD). An equal number of fragments showing an equivalent outgrowth of BECs were treated with either cyclosporine or tacrolimus, or left in CGF-SFM. Media was replaced every 2 days. Each explant was photographed every 2 days for a total of 6 days and the area determined was compared to that measured on day 0. These experiments were repeated twice.
Additionally, explants cultured as above for 6 days were also labeled with BrdU (10 μmol/L) for 6 or 12 hours before staining. To determine the percentage of labeled nuclei, 1,500 BECs were counted (500 cells, three times), without knowledge of treatment.
Statistical Analysis
All data are reported as the mean ± SD. Statistical analysis for the difference in BEC nuclear p21WAF1/Cip1 protein expression in human liver allograft biopsies between the various causes of liver allograft dysfunction used the Wilcoxon rank sum test. Statistical analysis for the difference in vitro assays used the Student’s t-test. A P value less than 0.05 was considered significant.
Results
Expression of p21WAF1/Cip1Protein in BECs in Liver Allograft Biopsies from Patients with Early Chronic Rejection and Other Causes of Late Liver Allograft Dysfunction
The percentage of p21+ BECs and the number of p21+ BECs per portal tract is significantly increased in early CR (26 ± 17% and 3.6 ± 3.1) compared to BECs in normal liver allograft biopsies or those with nonspecific changes (1 ± 1% and 0.1 ± 0.3; P < 0.0001 and P < 0.02), chronic hepatitis C (2 ± 3% and 0.7 ± 1; P < 0.0001 and P < 0.04), or obstructive cholangiopathy (7 ± 7% and 0.7 ± 0.6; P < 0.006 and P = 0.04). Immunohistochemical staining for p21 protein was also detected in periportal and perivenular hepatocytes in liver allografts with CR and is the subject of further study in our laboratories.
Cyclosporine-treated recipients suffering from the early stage of CR and rescued after conversion to tacrolimus, 1,21 all showed a decrease in the percentage of p21+ BECs and all but one showed a decrease in the number of p21+ BECs per portal tract. In the last biopsy with early CR before conversion to tacrolimus, 13 to 68% of the BECs were p21WAF1/Cip1+ and there was 1.5 to 12 p21+ BECs per portal tract (Figure 1, A and C) ▶ . After conversion to tacrolimus, the percentage of p21WAF1/Cip1+ BECs decreased to 2 to 27% and the number of p21+ BECs decreased to 0 to 3 per portal tract 11 to 233 days after conversion. Some of these patients were no longer considered to have early CR after conversion (Figure 1, B and C) ▶ . In two cyclosporine-treated recipients with early CR who failed to respond to tacrolimus conversion, follow-up biopsies and/or failed allograft showed loss of bile ducts in more than 90% of the small portal tracts, precluding evaluation of nuclear p21WAF1/Cip1. In contrast, neither the percentage nor the number of p21+ BECs per portal tract decreased after conversion to tacrolimus in patients without early CR (data not shown). In obstructive cholangiopathy, which can also cause ductopenia, the percentage of p21+ BECs was increased (7 ± 7%) compared to nonspecific changes (1 ± 1%; P < 0.01) or chronic hepatitis C (2 ± 3%; P < 0.06). However, when expressed as the number of p21+ BECs per portal tract the difference between obstructive cholangiopathy and nonspecific changes (P = 0.17) or chronic HCV (P = 0.14) was not (or only marginally) statistically significant.
Figure 1.
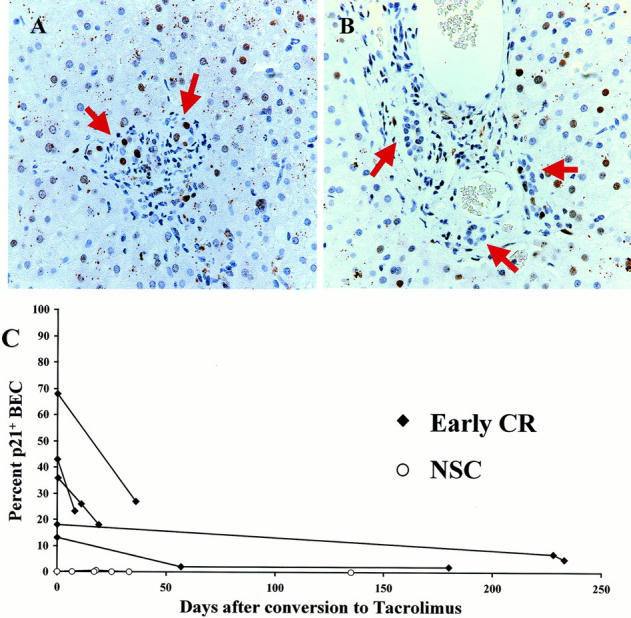
A: High-power photomicrograph of a representative portal tract from a liver allograft recipient with early CR stained for p21WAF1/Cip1 (brown-red nuclei). This biopsy was obtained 8 days before conversion to tacrolimus immunosuppression, when 36% of the BEC nuclei expressed p21WAF1/Cip1 protein (arrows). Note also the enlarged BEC cytoplasm and uneven nuclear spacing, features typical of early CR. B: Repeat biopsy of the same patient, 36 days after conversion to tacrolimus, showed that coincident with clinical improvement, the percentage of p21WAF1/Cip1+ BECs had decreased to 18% and the cytological changes had reversed, as shown in this representative portal tract. C: This graph shows the effect of conversion from cyclosporine to tacrolimus on BEC p21WAF1/Cip1 expression in liver allograft biopsies with either early CR or nonspecific changes. The values at time 0 are the percentages of BECs that stain positive for p21WAF1/Cip1 in the last biopsy obtained while the patient was on cyclosporine. The lines connect consecutive biopsies from the same patient. At time 0, note the increased percentage of p21WAF1/Cip1+ BECs in recipients with early CR compared to those with nonspecific changes. Note also, that the percentage of p21 WAF1/Cip1+ BEC decreases after conversion to tacrolimus in patients with early CR. In contrast, there was no change in the percentage of p21WAF1/Cip1+ BECs in biopsies with nonspecific changes, or in chronic HCV (data not shown).
Senescence of BECs, a Comparison of in Vivo to in Vitro
We had previously shown that as primary cultures of human BECs age, the cells enlarge, cease dividing, become multinucleated and squamoid-appearing, and detach finally from the plate; 13 the same is true of mBECs. These cytological alterations are quite similar to those seen in early CR (Figure 2A) ▶ and are well-described changes of cellular senescence, 9-12 as is p21WAF1/Cip1 expression. 14,15 We therefore determined whether senescent cells in primary cultures of mBECs showed expression of nuclear p21WAF1/Cip1 as did the BECs in early CR. The results are shown in Figure 2B ▶ . There was preferential p21WAF1/Cip1 nuclear labeling in the large, multinucleated, senescent BECs, whereas the smaller cells were unlabeled (Figure 2B) ▶ .
Figure 2.
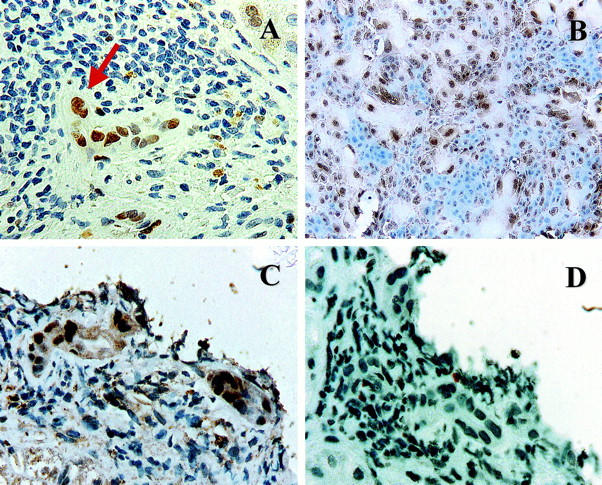
A: Immunohistochemical stain for p21WAF1/Cip1 (brown nuclei) in a liver allograft biopsy with early CR. Note the BEC cytological alterations, including multinucleation (arrow), uneven nuclear spacing, and cellular enlargement, along with the increased p21WAF1/Cip1 nuclear labeling. B: Immunohistochemical stain for p21WAF1/Cip1 in primary cultures of mBEC (brown nuclei). Note the similarity between the damaged BEC in the bile duct with early CR (A and C) and the enlarged senescent mBECs in the primary cultures. Both cell populations show multinucleation and cytoplasmic enlargement and occasional cells show nuclear pyknosis. Note also that both the enlarged cells in the primary BEC cultures and the BECs in damaged bile duct express nuclear p21WAF1/Cip1, but smaller cells do not. C and D: Staining of serial sections from a biopsy with early CR for p21WAF1/Cip1 (C) and Ki-67(Mib-1) (D) shows that the BEC up-regulation of p21WAF1/Cip1 is not associated with mitotic activity.
To exclude the possibility that BEC expression of p21WAF1/Cip1 was because of a postmitotic up-regulation, serial sections of liver allograft biopsies from two patients with increased p21WAF1/Cip1 expression were stained for p21WAF1/Cip1 and Ki-67 using the Mib-1 antibody (Figure 2, C and D ▶ , respectively). Despite the increased p21WAF1/Cip1, there was no Ki-67 labeling.
Effect of Cyclosporine and Tacrolimus on BEC Proliferation in Vitro
Conversion from cyclosporine to tacrolimus is the most effective treatment for early CR and successful recovery reverses the BEC cytological alterations, and decreases p21WAF1/Cip1 expression (Figure 1C) ▶ . In addition, less ductopenia is observed in liver allografts that fail because of CR in tacrolimus-treated patients. 6 We therefore hypothesized that the primary immunosuppressant drug might directly influence BEC viability and growth. Previous studies show that cyclosporine (but not tacrolimus) induces production of TGF-β in other epithelial cells, 22-24 which inhibits their growth, in vitro, via induction of p21WAF1/Cip1.
For these experiments, an equal number of normal human BEC colonies grown from bile duct explants in complete growth factor-supplemented SFM media (CGF-SFM) were changed to simple SFM for 24 hours before the addition of cyclosporine, tacrolimus, or CGF-SFM. Just before the addition of the drugs, the size of each colony was determined by digital photography and area morphometry, as described in the Materials and Methods. On average, five colonies were included in each treatment group. Measurements of colony area were made every 2 days for 6 days. Human BEC colonies kept in CGF-SFM supplemented with 1,000 ng/ml of cyclosporine showed a significant reduction in area compared to the same colonies on day 0 or the area of colonies kept in CGF-SFM or CGF-SFM supplemented with tacrolimus (1,000 ng/ml; Figure 3A ▶ ; 21.3% reduction, P = 0.006 versus control). In addition to colony area, treatment of the human BEC explants with 1,000 ng/ml of cyclosporine, but not tacrolimus, significantly inhibited hBEC BrdU labeling (Figure 3B) ▶ .
Figure 3.
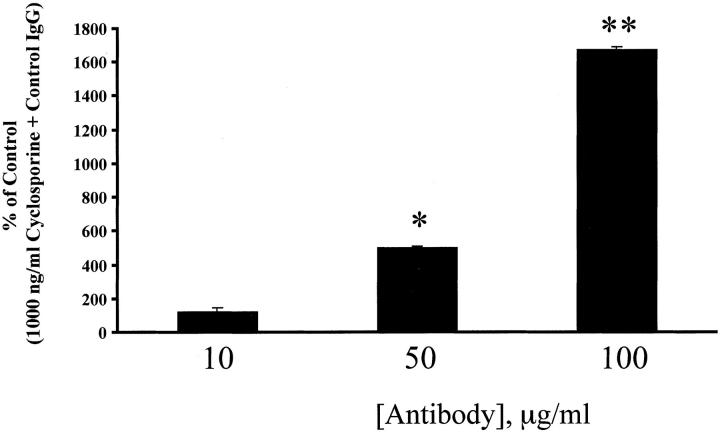
A: The effect of cyclosporine and tacrolimus on the growth of human BECs was tested on human bile-duct tree explants (hBDT), as described in the Materials and Methods. On average, five explant cultures were included in each treatment group. Measurements of culture size were made every 2 days for 6 days. Note the increase in culture size in both the control and tacrolimus-containing media, compared to the significant decrease in size of the cyclosporine-treated cultures (*, P < 0.02; **, P < 0.006 compared to control or tacrolimus-treated cultures). B: To show that the diminished size of the BEC cultures was related to mito-inhibition, the cultures were also labeled with BrdU for either 6 or 12 hours before fixation for anti-BrdU staining. Note the significantly lower percentage of BrdU+ BEC nuclei in the cyclosporine-treated cultures, compared to the others (*, P < 0.01 versus BEC cultures treated with 1,000 ng/ml cyclosporine).
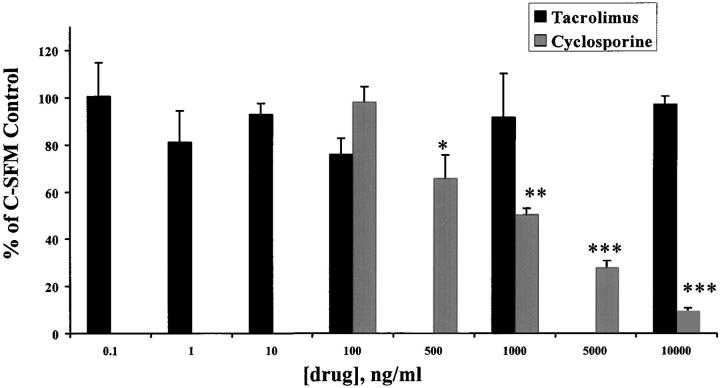
Because human BECs are not always conveniently available, we also determined whether either cyclosporine or tacrolimus also directly influenced mBEC proliferation, in vitro, in anticipation of further mechanistic studies. For these experiments, mBECs were cultured in C-SFM, without the addition of other growth factors or other growth supplements, other than the drugs. The addition of cyclosporine (100 ng/ml to 10,000 ng/ml) to mBECs caused a significant, concentration-dependent inhibition of [3H]-thymidine incorporation throughout a period of 48 hours (Figure 4) ▶ , compared to mBECs kept in control media or media supplemented with tacrolimus. In contrast, no significant growth inhibition was seen after the addition of 0.1 to 10,000 ng/ml tacrolimus to mBECs. Blood and bile cyclosporine concentrations generally range from 10- to 100-fold higher than tacrolimus. 28-32 However, even when tested in a similar concentration range, tacrolimus did not impact BEC growth compared to cyclosporine, which has been found in the bile of liver allograft recipients at concentrations ranging from 500 to >6,000 ng/ml. 33 Given that the human BECs and mBECs responded similarly to treatment with either tacrolimus or cyclosporine, the following mechanistic studies were conducted in mBECs because of their predictable availability.
Figure 4.
Treatment of mBECs kept in C-SFM with increasing concentrations of cyclosporine (100 to 10,000 ng/ml), caused a concentration-dependent decrease in mBECs [3H]-thymidine incorporation after 48 hours, compared to mBECs treated with tacrolimus (0.1 to 10,000 ng/ml). The results shown are representative of at least three separate experiments (*, P < 0.01; **, P < 0.001; ***, P < 0.0003).
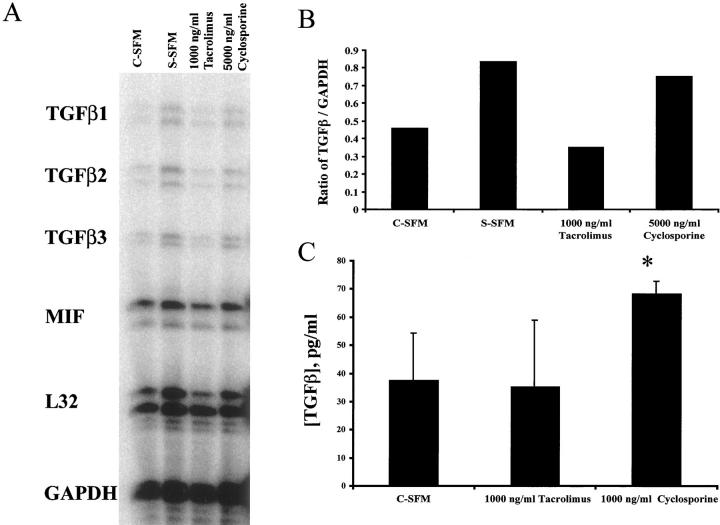
Effect of mBEC Treatment with Cyclosporine and Tacrolimus on TGF-β mRNA Expression and Protein Secretion
We next determined whether treatment of mBECs with either cyclosporine or tacrolimus caused an increase in mBEC TGF-β mRNA production and TGF-β protein secretion into the culture supernatant. Treatment of mBECs with a growth-inhibitory concentration of cyclosporine (5,000 ng/ml), but not a supraimmunosuppressive concentration of tacrolimus, increased mBEC TGF-β mRNA production, as determined by a standard ribonuclease protection assay (Figure 5, A and B) ▶ . Although the increase in TGF-β mRNA is small, the half-life of this mRNA is increased in cyclosporine-treated T cells. 34 Treatment of mBECs with cyclosporine (1,000 ng/ml) also resulted in a significant increase of TGF-β protein secretion in the mBEC supernatant, compared to mBECs kept either in the control media alone, or media supplemented with the same concentrations of tacrolimus (1,000 ng/ml; Figure 5C ▶ ).
Figure 5.
A: Treatment of mBECs kept in C-SFM with cyclosporine (5,000 ng/ml), but not tacrolimus (1,000 ng/ml) caused an increase of TGF-β mRNA production, as determined by a standard ribonuclease protection assay. If the mBECs were growth arrested by switching to simple serum-free medium (S-SFM) (see Materials and Methods) there was also an increase in TGF-β production. B: Densitometry tracing and comparison to GAPDH mRNA showed that TGF-β mRNA production is higher in the cyclosporine-treated than in the tacrolimus-treated cultures. The determinations were performed 24 hours after the addition of the drugs to the mBEC cultures kept in C-SFM. The results shown are representative of three separate experiments. C: Treatment of mBECs kept in C-SFM with cyclosporine (1,000 ng/ml), but not tacrolimus (1,000 ng/ml) for 48 hours caused an increase of TGF-β protein secretion into the culture supernatant, as determined by a standard enzyme-linked immunosorbent assay (*, P < 0.005).
Activation of TGF-β Intracellular Signaling
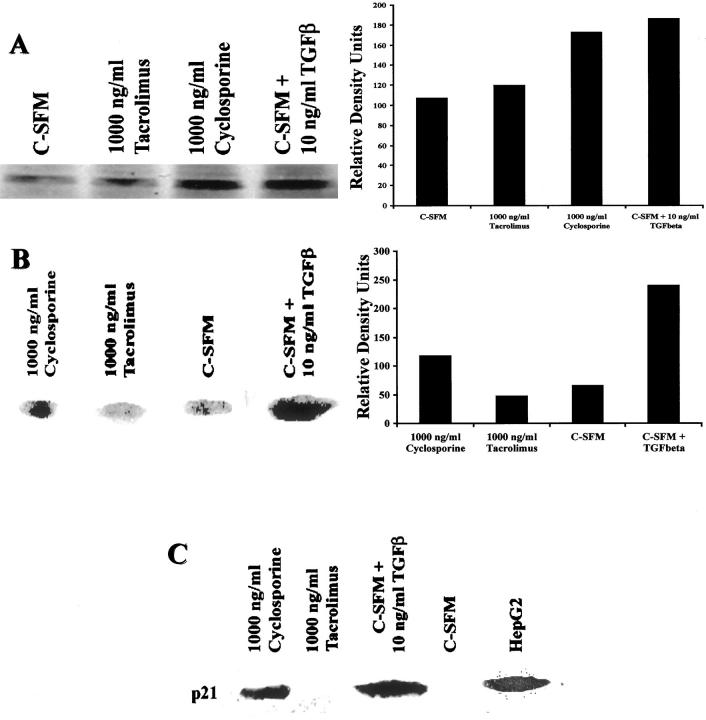
We have previously shown that TGF-β inhibits human BEC growth. 35 In most epithelial cells, TGF-β inhibits growth by interacting with the membrane bound TGF-β receptor II, which in turn, dimerizes with TGF-β receptor I and initiates intracellular signaling through serine/threonine kinase activity. TGF-β receptor I phosphorylates serine residues on SMAD-2, which associates with SMAD-4 and the complex is translocated into the nucleus. TGF-β signaling also increases expression of TGF-β receptor II. 36 Therefore, we next examined cyclosporine-treated mBECs for evidence of TGF-β-mediated signaling using immunoblotting for TGF-β receptor II expression and phosphorylation of SMAD-2. Figure 6A ▶ shows that cyclosporine treatment of mBEC increases TGF-β receptor II protein expression, compared to mBEC kept in tacrolimus-supplemented media; there is also increased SMAD-2 phosphorylation (Figure 6B) ▶ in the cyclosporine-treated, but not in the tacrolimus-treated mBECs.
Figure 6.
A: Treatment of mBECs kept in C-SFM with cyclosporine (1,000 ng/ml) or TGF-β (10 ng/ml) for 48 hours, but not tacrolimus (1,000 ng/ml) results in up-regulation of the TGF-β receptor type II protein compared to C-SFM controls (left) as determined by Western blotting. Densitometry tracing was used to illustrate the quantitative differences (right). B: TGF-β signaling pathway is mediated by phosphorylation of the transcription factor SMAD-2. In this Western blot (left), total cellular protein was probed for phospho-SMAD-2, which was detected in the mBECs treated with cyclosporine (1,000 ng/ml), but not with tacrolimus (1,000 ng/ml). The positive control consisted of mBECs treated with 10 ng/ml of exogenous TGF-β. Densitometry tracing was used to illustrate the quantitative differences (right). C: In this Western blot, BEC nuclear protein was probed for the cyclin-dependent kinase inhibitor protein, p21WAF1/Cip1, which is elevated 48 hours after treatment with either TGF-β (10 ng/ml; positive control) or cyclosporine (1,000 ng/ml), but not in the control C-SFM or after treatment with tacrolimus (1,000 ng/ml).
TGF-β inhibits epithelial cell proliferation via an increase in p21WAF1/Cip1, which is a universal inhibitor of cyclin-CDK activity. 37-40 Consistent with these studies, the cyclosporine-treated mBECs showed increased nuclear p21WAF1/Cip1 protein expression compared to tacrolimus-treated mBECs (Figure 6C) ▶ .
The Effect of Cyclosporine on mBEC Proliferation in Vitro Can Be Reversed by Neutralizing Anti-TGF-β Antibody
In an effort to determine whether the decreased proliferation seen in cyclosporine-treated mBEC cultures is attributable to TGF-β-mediated mito-inhibition, mBECs were simultaneously treated with a combination of 1,000 ng/ml cyclosporine and increasing concentrations of neutralizing anti-TGF-β antibody for 48 hours (Figure 7) ▶ . The addition of anti-TGF-β monoclonal antibody (10 to 100 μg/ml) resulted in a significant increase in [3H]-thymidine incorporation compared to mBECs treated with the combination of cyclosporine and control IgG at the same concentrations. These results confirm that autocrine/paracrine TGF-β signaling is responsible for the cyclosporine-mediated inhibition of BEC growth.
Figure 7.
The simultaneous addition of 1,000 ng/ml cyclosporine and neutralizing anti-TGF-β antibodies to mBECs in C-SFM caused a concentration-dependent increase in [3H]-thymidine incorporation after 48 hours, compared to mBECs treated with 1,000 ng/ml cyclosporine and control nonimmune immunoglobulin. This result further confirms that inhibition of BEC growth, in vitro, is mediated via TGF-β. The results shown are representative of two separate experiments (*, P < 0.002; **, P < 0.0003).
Discussion
This study shows that the disappearance of BECs in CR is preceded by a period of mito-inhibition and cellular senescence, marked by characteristic cytological alterations and up-regulation of nuclear p21WAF1/Cip1. Because recognition of the BEC cytological alterations in liver allograft biopsies is somewhat subjective but essential for establishing the diagnosis of early CR and distinguishing it from other causes of late liver allograft dysfunction, BEC p21WAF1/Cip1 expression can be used as an ancillary test to help establish the diagnosis. Caution is urged however, because BEC p21WAF1/Cip1 expression can also be increased in obstructive cholangiopathy, which can also cause ductopenia and be confused histologically with early CR. 7 Thus, it is not unreasonable to suggest that up-regulation of p21WAF1/Cip1 might be a common mechanism that precedes ductopenia in other liver diseases.
Both direct immunological damage and ischemia contribute to bile duct injury during early CR, 41,42 but molecular mechanism(s) responsible for disappearance of the BECs have not been clarified. The final common pathway seems to be BEC apoptosis, but the data are somewhat conflicting. Nawaz and colleagues 43 detected apoptotic BECs in acute rejection, but in CR, Afford and colleagues 44 were unable to detect significant BEC apoptotic activity using the terminal dUTP nick-end labeling assay. Given that BEC account for only a small fraction of the cells in the liver, and apoptosis of any individual cell is detectable for only a few hours, BEC apoptosis may be difficult to catalog in CR. In addition, p21WAF1/Cip1 can bind to procaspase 3 precursor 45 and block its processing and activation. Thus, increased p21WAF1/Cip1 expression might actually protect BECs against Fas-Fas-L-induced apoptosis, which is the mode of BEC injury in a graft-versus-host disease 46 that CR closely resembles.
It is likely that up-regulation of BEC p21WAF1/Cip1 occurs as a result of the prolonged bile duct injury in early CR, but given some of its functions, the increased expression of p21WAF1/Cip1 has the potential to importantly contribute to the pathophysiology. First, p21WAF1/Cip1 can potentially protect BECs from apoptosis. However, it also seems to exert its known mito-inhibitory function in early CR because the BECs do not proliferate in response to injury. 1,21 Secondly, forced overexpression of p21WAF1/Cip1 protein using an inducible promoter, causes cellular senescence, 14 including growth arrest and cytological changes such as those seen in the BECs in early CR. It also causes up-regulation of genes that encode for extracellular matrix components, such as fibronectin-1, plasminogen activator inhibitor-1, tissue-type plasminogen activator, Mac-2-binding protein, and extracellular matrix receptors (integrin β3), which might explain thickening of the basement membrane in CR. Lastly, clinical and histopathological recovery from CR is associated with decreased BEC nuclear p21WAF1/Cip1 expression.
The gene encoding p21WAF1/Cip1 is regulated by at least three classes of signals that result in growth arrest: 1) the tumor suppressor protein p53, which is activated by DNA damage; 2) extracellular growth factors, acting in a p53-independent manner, such as tumor necrosis factor-α (TNF-α), TGF-β, activin, phorbol esters, retinoic acid, and others; and 3) factors that induce cellular differentiation of many cell types. 47 In early CR, cytokines, growth factors, or other immunological effector mechanisms/molecules produced as a result of (or in response to) immunological injury (eg, TNF-α, TGF-β) are most likely to be the underlying cause for increased expression of BEC p21WAF1/Cip1. This contention is based on the fact that the number and severity of acute rejection episodes are the most significant risk factors for the development of CR. 5-7 In addition, Demirci and colleagues 48 showed increased TGF-β protein in the portal tracts and perivenular regions of liver allografts with CR. 48
When CR does occur, cyclosporine-treated recipients develop more bile duct loss and fibrosis than tacrolimus-treated recipients who develop CR. 5,6 In cyclosporine-treated patients, the median percentage of bile duct loss was 100% in allografts that failed because of CR; only one of 13 grafts had bile duct loss in <50% of the portal tracts. 5 In contrast, the median percentage of bile duct loss in allografts that failed from CR in tacrolimus-treated patients was only 43%. 6 Severe (bridging) perivenular fibrosis was also seen in most of the failed allografts from cyclosporine patients, 5 but was uncommon in the graft from the tacrolimus-treated cohort. 6 In addition, several clinical trials have shown that if cyclosporine-treated recipients with early CR are converted to tacrolimus, the clinical, biochemical, and histopathological manifestations of CR can be reversed 1,5,6,19-21 and bile duct loss, which is a late feature of CR, 7 can be prevented. Rare cases of spontaneous reversal of CR have also been reported. 2,3
The increased p21WAF1/Cip1 in early CR is immunologically mediated and clearly not drug-specific. However, the ability of cyclosporine, but not tacrolimus, to stimulate BEC production of TGF-β1, in vitro, along with the less potent immunosuppressive properties of cyclosporine, might explain the lower incidence of CR under tacrolimus therapy and it’s relative duct-sparing properties. Cyclosporine (but not tacrolimus), also inhibits the growth of mink lung epithelial cells, 24 human airway epithelial cells, 23 and murine proximal tubular cells 49 by activating the TGF-β promoter 50,51 and stimulating TGF-β production. Cyclosporine-induced BEC TGF-β production up-regulates TGF-β receptor II receptor expression, phosphorylation of the transcription factor SMAD-2 and nuclear translocation of p21WAF1/Cip1. Once immunological damage to the bile ducts has occurred, our in vitro studies provide mechanistic support for the clinical conclusion that tacrolimus is more effective in treating and preventing ductopenic rejection than cyclosporine. 19,21,52-57 Both cyclosporine and tacrolimus are metabolized by the liver and excreted in the bile, where both residual native drug (usually 1% or less) and metabolites (some of which have immunosuppressive properties) can be detected at concentrations higher than in the blood or serum. 28-32 Thus, any potential direct effect of these drugs on BEC physiology could be magnified by exposure both via the blood stream and the bile.
It is important to note that the differential effects of the immunosuppressive drugs on BEC growth were detected in primary BEC cultures, and the culture conditions are not equivalent to normal conditions in vivo. The culture conditions are stressful and the basal medium contains an adenylate cyclase stimulator, which increases BEC interleukin-6 production. 25 In contrast, the vast majority of normal BECs in vivo are in G0 phase of the cell cycle. 58 Thus, the in vitro conditions used in this study most accurately simulate a stressful BEC microenvironment, 25 such as that seen in early CR. This is consistent with the observation that neither cyclosporine nor tacrolimus alone induces either BEC cytological alterations or BEC up-regulation of p21WAF1/Cip1 in liver allograft recipients in the absence of early CR or other causes of ductal injury. Thus, in vivo, the difference between the two drugs becomes apparent only after BEC injury. This situation is similar to the ability of tacrolimus, but not cyclosporine, to promote the growth and repair of damaged peripheral nerves, 59,60 via a calcineurin-independent pathway, involving FK binding protein (FKBP)-52, steroid receptors, and hsp-90. Interestingly, both steroid receptors and hsp-90 are expressed in BECs, and up-regulated after BEC injury. 61 The profile of BEC FKBP expression has not been investigated.
Lastly, this study validates the notion that replicative senescence of epithelial cells can contribute to the pathobiology of chronic rejection. 62 Thus, even though CR of liver allografts is relatively uncommon, further study might provide useful information about the potential role of stimulation of epithelial growth on CR. 6
Footnotes
Address reprint requests to A. J. Demetris, M.D., E1548 BMST, University of Pittsburgh, Pittsburgh, PA 15261. E-mail: demetrisaj@msx.upmc.edu.
Supported by National Institutes of Health grants1 RO1DK49615-05, RO1AI40329-05, and RO1AI38899-01A2.
References
- 1.Demetris AJ, Fung JJ, Todo S, McCauley J, Jain A, Takaya S, Alessiani M, Abu-Elmagd K, Van Thiel DH, Starzl TE: FK 506 used as rescue therapy for human liver allograft recipients. Transplant Proc 1991, 23:3005-3006 [PMC free article] [PubMed] [Google Scholar]
- 2.Freese DK, Snover DC, Sharp HL, Gross CR, Savick SK, Payne WD: Chronic rejection after liver transplantation: a study of clinical, histopathological and immunological features. Hepatology 1991, 13:882-891 [PubMed] [Google Scholar]
- 3.Hubscher SG, Buckels JA, Elias E, McMaster P, Neuberger J: Vanishing bile-duct syndrome following liver transplantation—is it reversible? Transplantation 1991, 51:1004-1010 [DOI] [PubMed] [Google Scholar]
- 4.Lautenschlager I, Hockerstedt K, Jalanko H, Loginov R, Salmela K, Taskinen E, Ahonen J: Persistent cytomegalovirus in liver allografts with chronic rejection. Hepatology 1997, 25:190-194 [DOI] [PubMed] [Google Scholar]
- 5.Blakolmer K, Seaberg EC, Batts K, Ferrell L, Markin R, Wiesner R, Detre K, Demetris A: Analysis of the reversibility of chronic liver allograft rejection implications for a staging schema. Am J Surg Pathol 1999, 23:1328-1339 [DOI] [PubMed] [Google Scholar]
- 6.Blakolmer K, Jain A, Ruppert K, Gray E, Duquesnoy R, Murase N, Starzl TE, Fung JJ, Demetris AJ: Chronic liver allograft rejection in a population treated primarily with tacrolimus as baseline immunosuppression: long-term follow-up and evaluation of features for histopathological staging. Transplantation 2000, 69:2330-2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, Gouw A, Gustafsson B, Haga H, Harrison D, Hart J, Hubscher S, Jaffe R, Khettry U, Lassman C, Lewin K, Martinez O, Nakazawa Y, Neil D, Pappo O, Parizhskaya M, Randhawa P, Rosoul-Rockenschaub S, Reinholt F, Reynes M, Robert M, Tsamandas A, Wanless I, Weisner R, Wernerson A, Wrba F, Wyatt J, Yamabe H: Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology 2000, 31:792-799 [DOI] [PubMed] [Google Scholar]
- 8.Fennell RH, Jr, Vierling JM: Electron microscopy of rejected human liver allografts. Hepatology 1985, 5:1083-1087 [DOI] [PubMed] [Google Scholar]
- 9.Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR, Jr, Reid LM, Gupta S: Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol 1999, 276:G1260-G1272 [DOI] [PubMed] [Google Scholar]
- 10.Brodsky WY, Uryvaeva IV: Cell polyploidy: its relation to tissue growth and function. Int Rev Cytol 1977, 50:275-332 [DOI] [PubMed] [Google Scholar]
- 11.Printseva OY, Tjurmin AV: Proliferative response of smooth muscle cells in hypertension. Am J Hypertens 1992, 5:S118-S123 [DOI] [PubMed] [Google Scholar]
- 12.Gotloib L, Shostak A, Wajsbrot V, Kushnier R: High glucose induces a hypertrophic, senescent mesothelial cell phenotype after long in vivo exposure. Nephron 1999, 82:164-173 [DOI] [PubMed] [Google Scholar]
- 13.Demetris AJ, Markus BH, Saidman S, Fung JJ, Makowka L, Graner S, Duquesnoy R, Starzl TE: Isolation and primary cultures of human intrahepatic bile ductular epithelium. In Vitro Cell Dev Biol 1988, 24:464-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB: Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci USA 2000, 97:4291-4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB: Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 1999, 18:4808-4818 [DOI] [PubMed] [Google Scholar]
- 16.Popper H, Kent G, Stein R: Ductular cell reaction in the liver in hepatic injury. J Mt Sinai Hosp 1957, 24:551-556 [PubMed] [Google Scholar]
- 17.Popper H: The relation of mesenchymal cell products to hepatic epithelial systems [Review]. Prog Liver Dis 1990, 9:27-38 [PubMed] [Google Scholar]
- 18.Demetris AJ: Immunopathology of the human biliary tree. Biliary and Pancreatic Ductal Epithelia. Pathobiology and Pathophysiology. 1997:pp 127-180 DS Longnecker. New York, Marcel Dekker, Inc., Edited by AE Sirica
- 19.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A: FK 506 for liver, kidney, and pancreas transplantation. Lancet 1989, 2:1000-1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung JJ, Todo S, Tzakis A, Demetris A, Jain A, Abu-Elmaged K, Allessiani M, Starzl TE: Conversion of liver allograft recipients from cyclosporine to FK 506-based immunosuppression: benefits and pitfalls. Transplant Proc 1991, 23:14-21 [PMC free article] [PubMed] [Google Scholar]
- 21.Demetris AJ, Fung JJ, Todo S, McCauley J, Jain A, Takaya S, Allessiani M, Abu-Elmagd K, Van Thiel DH, Starzl TE: Conversion of liver allograft recipients from cyclosporine to FK506 immunosuppressive therapy—a clinicopathologic study of 96 patients. Transplantation 1992, 53:1056-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JG, Walmsley MW, Moy JV, Cunningham AC, Talbot D, Dark JH, Kirby JA: Differential effects of cyclosporin A and tacrolimus on the production of TGF-beta: implications for the development of obliterative bronchiolitis after lung transplantation. Transpl Int 1998, 11:S325-S327 [DOI] [PubMed] [Google Scholar]
- 23.Mohamed MA, Walmsley M, Robertson H, Kirby JA, Talbot D: The effect of cyclosporin A and tacrolimus on cultured human epithelial cells: the role of TGF-beta. Transplant Proc 1999, 31:1173. [DOI] [PubMed] [Google Scholar]
- 24.Khanna A, Li B, Stenzel KH, Suthanthiran M: Regulation of new DNA synthesis in mammalian cells by cyclosporine. Demonstration of a transforming growth factor beta-dependent mechanism of inhibition of cell growth. Transplantation 1994, 57:577-582 [PubMed] [Google Scholar]
- 25.Yokomuro S, Lunz JG, III, Sakamoto T, Ezure T, Murase N, Demetris AJ: The effect of interleukin-6 (IL-6)/gp130 signaling on biliary epithelial cell growth, in vitro. Cytokine 2000, 12:727-730 [DOI] [PubMed] [Google Scholar]
- 26.Strain AJ, Wallace L, Joplin R, Daikuhara Y, Ishii T, Kelly DA, Neuberger JM: Characterization of biliary epithelial cells isolated from needle biopsies of human liver in the presence of hepatocyte growth factor. Am J Pathol 1995, 146:537-545 [PMC free article] [PubMed] [Google Scholar]
- 27.Strom SC, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, Schuetz EG: Use of human hepatocytes to study P450 gene induction. Methods Enzymol 1996, 272:388-401 [DOI] [PubMed] [Google Scholar]
- 28.Burckart GJ, Starzl TE, Venkataramanan R, Hashim H, Wong L, Wang P, Makowka L, Zeevi A, Ptachcinski RJ, Knapp JE, Iwatsuki S, Esquivel C, Sanghrvi A, Van Thiel DH: Excretion of cyclosporine and its metabolites in human bile. Transplant Proc 1986, 18:46-49 [PMC free article] [PubMed] [Google Scholar]
- 29.Venkataramanan R, Jain A, Warty VW, Abu-Elmagd K, Furakawa H, Imventarza O, Fung J, Todo S, Starzl TE: Pharmacokinetics of FK 506 following oral administration: a comparison of FK 506 and cyclosporine. Transplant Proc 1991, 23:931-933 [PMC free article] [PubMed] [Google Scholar]
- 30.Zeevi A, Eiras G, Burckart G, Makowka L, Venkataramanan R, Wang CP, Van Thiel DH, Murase N, Starzl TE, Duquesnoy R: Immunosuppressive effect of cyclosporine metabolites from human bile on alloreactive T cells. Transplant Proc 1988, 20:115-121 [PMC free article] [PubMed] [Google Scholar]
- 31.Venkataramanan R, Warty VS, Zemaitis MA, Sanghvi AT, Burckart GJ, Seltman H, Todo S, Makowka L, Starzl TE: Biopharmaceutical aspects of FK-506. Transplant Proc 1987, 19:30-35 [PMC free article] [PubMed] [Google Scholar]
- 32.Venkataramanan R, Jain A, Cadoff E, Warty V, Iwasaki K, Nagase K, Krajack A, Imventarza O, Todo S, Fung J, Starzl TE: Pharmacokinetics of FK 506: preclinical and clinical studies. Transplant Proc 1990, 22:52-56 [PMC free article] [PubMed] [Google Scholar]
- 33.Scanlon L, Baloh R, Gridelli B, Rao KM, Shaw B, Starzl T, Sanghvi A: Determination of cyclosporine concentration in bile. Transplantation 1986, 41:657-659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahuja SS, Shrivastav S, Danielpour D, Balow JE, Boumpas DT: Regulation of transforming growth factor-beta 1 and its receptor by cyclosporine in human T lymphocytes. Transplantation 1995, 60:718-723 [DOI] [PubMed] [Google Scholar]
- 35.Yokomuro S, Tsuji H, Lunz JG, III, Sakamoto T, Ezure T, Murase N, Demetris AJ: Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology 2000, 32:26-35 [DOI] [PubMed] [Google Scholar]
- 36.Kleeff J, Wildi S, Friess H, Korc M: Ligand induced upregulation of the type II transforming growth factor (TGF-beta) receptor enhances TGF-beta responsiveness in COLO-357 cells. Pancreas 1999, 18:364-370 [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama A, Nagaki M, Shidoji Y, Moriwaki H, Muto Y: Regulation of cell cycle-related genes in rat hepatocytes by transforming growth factor beta1. Biochem Biophys Res Commun 1997, 238:539-543 [DOI] [PubMed] [Google Scholar]
- 38.Saha P, Eichbaum Q, Silberman ED, Mayer BJ, Dutta A: p21cip1 and Cdc25a: competition between an inhibitor and an activator of cyclin-dependent kinases. Mol Cell Biol 1997, 17:4338-4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Florenes VA, Bhattacharya N, Bani MR, Ben-David Y, Kerbel RS, Slingerland JM: TGF-beta mediated G1 arrest in a human melanoma cell line lacking p15INK4B: evidence for cooperation between p21Cip1/WAF1 and p27Kip1. Oncogene 1996, 13:2447-2457 [PubMed] [Google Scholar]
- 40.Miyazaki M, Ohashi R, Tsuji T, Mihara K, Gohda E, Namba M: Transforming growth factor-beta 1 stimulates or inhibits cell growth via down- or up-regulation of p21/Waf1. Biochem Biophys Res Commun 1998, 246:873-880 [DOI] [PubMed] [Google Scholar]
- 41.Oguma S, Belle S, Starzl TE, Demetris AJ: A histometric analysis of chronically rejected human liver allografts: insights into the mechanisms of bile duct loss: direct immunologic and ischemic factors. Hepatology 1989, 9:204-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oguma S, Zerbe T, Banner B, Belle S, Starzl TE, Demetris AJ: Chronic liver allograft rejection and obliterative arteriopathy: possible pathogenic mechanisms. Transplant Proc 1989, 21:2203-2207 [PMC free article] [PubMed] [Google Scholar]
- 43.Nawaz S, Fennell RH: Apoptosis of bile duct epithelial cells in hepatic allograft rejection. Histopathology 1994, 25:137-142 [DOI] [PubMed] [Google Scholar]
- 44.Afford SC, Hubscher S, Strain AJ, Adams DH, Neuberger JM: Apoptosis in the human liver during allograft rejection and end-stage liver disease. J Pathol 1995, 176:373-380 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M: Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene 1998, 17:931-939 [DOI] [PubMed] [Google Scholar]
- 46.Ueno Y, Ishii M, Yahagi K, Mano Y, Kisara N, Nakamura N, Shimosegawa T, Toyota T, Nagata S: Fas-mediated cholangiopathy in the murine model of graft versus host disease. Hepatology 2000, 31:966-974 [DOI] [PubMed] [Google Scholar]
- 47.Moustakas A, Kardassis D: Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci USA 1998, 95:6733-6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demirci G, Nashan B, Pichlmayr R: Fibrosis in chronic rejection of human liver allografts: expression patterns of transforming growth factor-TGFbeta1 and TGF-beta3. Transplantation 1996, 62:1776-1783 [DOI] [PubMed] [Google Scholar]
- 49.Wolf G, Thaiss F, Stahl RA: Cyclosporine stimulates expression of transforming growth factor-beta in renal cells. Possible mechanism of cyclosporines antiproliferative effects. Transplantation 1995, 60:237-241 [DOI] [PubMed] [Google Scholar]
- 50.Wolf G, Zahner G, Ziyadeh FN, Stahl RA: Cyclosporin A induces transcription of transforming growth factor beta in a cultured murine proximal tubular cell line. Exp Nephrol 1996, 4:304-308 [PubMed] [Google Scholar]
- 51.Prashar Y, Khanna A, Sehajpal P, Sharma VK, Suthanthiran M: Stimulation of transforming growth factor-beta 1 transcription by cyclosporine. FEBS Lett 1995, 358:109-112 [DOI] [PubMed] [Google Scholar]
- 52.Neuhaus P, Bechstein WO, Blumhardt G, McMaster P, Mayer D, Buckels J, Calne R, Friend P, Joughin C, Pichlmayr R, Winkler M, Ringe B, Otto G, Bleyl J, William R, Devlin J, O’Grady J, Groth C, Erizon B, Duraj F, Bismuth H, Samuel D, Rucay P: Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group. Lancet 1994, 344:423-4287520105 [Google Scholar]
- 53.Group TUSMFLS: A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med 1994, 331:1110-1115 [DOI] [PubMed] [Google Scholar]
- 54.McDiarmid SV, Klintmalm GB, Busuttil RW: FK506 conversion for intractable rejection of the liver allograft. Transpl Int 1993, 6:305-312 [DOI] [PubMed] [Google Scholar]
- 55.Fung JJ, Eliasziw M, Todo S, Jain A, Demetris AJ, McMichael JP, Starzl TE, Meier P, Donner A: The Pittsburgh randomized trial of tacrolimus compared to cyclosporine for hepatic transplantation. J Am Coll Surg 1996, 183:117-125 [PMC free article] [PubMed] [Google Scholar]
- 56.Sher LS, Cosenza CA, Michel J, Makowka L, Miller CM, Schwartz ME, Busuttil R, McDiarmid S, Burdick JF, Klein AS, Esquivel C, Levy M, Roberts JP, Lake JR, Kalayoglu M, D’Alessandro AM, Gordon RD, Stieber AC, Shaw BW, Jr, Thistlethwaite JR, Whittington P, Wiesner RH, Porayko M, Cosimi AB, Bynon JS, Eckhoff DE, Freeman RB, Rohrer RJ, Lewis WD, Marsh JW, Peters M, Powelson J, Cosimi AB: Efficacy of tacrolimus as rescue therapy for chronic rejection in orthotopic liver transplantation: a report of the U. S. Multicenter Liver Study Group. Transplantation 1997, 64:258-263 [DOI] [PubMed] [Google Scholar]
- 57.Taibi A, Adham M, Ducerf C, Chevallier M, Bizollon T, Delaroche E, Aljuaid N, Berthoux N, Baulieux J: Rescue FK506 therapy for acute rejection and early chronic rejection after liver transplantation: report of 14 cases. Transplant Proc 1998, 30:1411-1412 [DOI] [PubMed] [Google Scholar]
- 58.Liu Z, Sakamoto T, Ezure T, Yokomuro S, Murase N, Michalopoulos G, Demetris AJ: Interleukin-6, hepatocyte growth factor, and their receptors in biliary epithelial cells during a type I ductular reaction in mice: interactions between the periductal inflammatory and stromal cells and the biliary epithelium. Hepatology 1998, 28:1260-1268 [DOI] [PubMed] [Google Scholar]
- 59.Gold BG, Katoh K, Storm-Dickerson T: The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci 1995, 15:7509-7516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gold BG: FK506 and the role of the immunophilin FKBP-52 in nerve regeneration. Drug Metab Rev 1999, 31:649-663 [DOI] [PubMed] [Google Scholar]
- 61.Jorge AD, Stati AO, Roig LV, Ponce G, Jorge OA, Ciocca DR: Steroid receptors and heat-shock proteins in patients with primary biliary cirrhosis. Hepatology 1993, 18:1108-1114 [PubMed] [Google Scholar]
- 62.Halloran PF, Melk A, Barth C: Rethinking chronic allograft nephropathy: the concept of accelerated senescence. J Am Soc Nephrol 1999, 10:167-181 [DOI] [PubMed] [Google Scholar]