Abstract
Nitric oxide generated by the inducible form of nitric oxide synthase (iNOS) may contribute to the pathogenesis of multiple sclerosis (MS). In this report, we studied postmortem tissues of MS patients for the expression of iNOS by in situ hybridization and immunocytochemistry. Immunocytochemistry for nitrotyrosine, a putative footprint for peroxynitrite formation was also performed. In acute MS lesions, intense reactivity for iNOS mRNA and protein was detected in reactive astrocytes throughout the lesion and in adjacent normal appearing white matter. Staining of macrophages, inflammatory cell infiltrates, and endothelial cells was variable from case to case, but generally detected only in acute lesions. In chronic MS lesions reactive astrocytes at the lesion edge were positive for iNOS whereas the lesion center was nonreactive. Normal appearing white matter demonstrated little reactivity, as did tissues from noninflamed control brains. Staining for nitrotyrosine was also detected in acute but not chronic MS lesions, and displayed a diffuse parenchymal, membranous, and perivascular pattern of immunoreactivity. These results support the conclusion that iNOS is induced in multiple cell types in MS lesions and that astrocyte-derived nitric oxide could be important in orchestrating inflammatory responses in MS, particularly at the blood-brain barrier.
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) that is thought to be mediated by an autoimmune attack directed against components of the myelin sheath. MS lesions are characterized by loss of myelin, oligodendrocytes, and axons associated with a mononuclear inflammatory infiltrate and a reactive gliosis. Although the mechanisms that lead to loss of function associated with these events remain poorly understood, the activation of T cells and macrophages that secrete freely diffusable factors has been widely implicated. Included in these factors are the pro-inflammatory cytokines interleukin (IL)-1, tumor necrosis factor-α, IL-12, and interferon (IFN)-γ, and reactive oxygen and reactive nitrogen species. All of these factors have been shown to be elevated in active MS lesions, and animal models support a role for them in disease pathogenesis. 1,2
The anti-proliferative and/or cytotoxic effects of nitric oxide (NO) have been associated with the persistent production of high levels of NO that occurs after the activation of the inducible form of nitric oxide synthase (iNOS). 3 The expression of this enzyme in various cell types is known to be transcriptionally regulated and to be activated by a combination of pro-inflammatory signals such as ligands that activate toll-like receptors and/or cytokines such as IL-1, tumor necrosis factor-α, and interferon-γ (IFN-γ). 3 NO by itself demonstrates only weak toxic activity, but congeners formed by auto-oxidation such as NO2·, N2O3, and S-nitrosothiols enhance its cytotoxic potential. The toxicity of NO is also greatly enhanced when it combines with O2− to generate peroxynitrite (ONOO−), an oxidant capable of damaging lipids, proteins, and DNA. 4-6 Detection of nitrotyrosine at inflammatory sites serves as a biochemical marker for peroxynitrite formation. 7,8 Activated macrophages/microglia are a major source of reactive oxygen intermediates, whereas iNOS has been detected in a wide range of different cell types.
The factors that lead to the activation of iNOS have been shown to be both cell-type- and species-specific. So, for example, it is known that in cells of human origin enhancer elements are located upstream of −4.7 kb within a 10-kb promoter region that contains four functional nuclear factor-kappa B (NF-κB) elements 8 and two activatory protein-1 (AP-1) sites. 9 This pattern of cis-element expression contrasts markedly with the murine iNOS promoter, where only 1.0 kb of 5′-flanking sequence containing two NF-κB sites is required for lipopolysaccharide and cytokine responsiveness and no AP1 sites are detected. 10 These data strongly suggest that the factors that regulate iNOS expression differ between mice and humans and this has been supported by studies in which it has been shown that activation of iNOS in human monocytes/macrophages is relatively refractory to lipopolysaccharide and cytokine activation, and comparatively little is known about the regulation of this enzyme in these cells. 11,12 However, iNOS can be readily induced in human cell types of varying lineages by a combination of cytokines, and in human fetal astrocytes activation by IL-1 in combination with IFN-γ forms a potent inducing stimulus. 13
Because IL-1 and IFN-γ are known to be up-regulated in active MS lesions, these data would suggest that astrocytes should be activated to express iNOS at these sites. Two early reports that examined the expression of NADPH diaphorase staining as an indicator of NO production supported a role for astrocytes as a source of NO in MS lesions, 14,15 however, two subsequent studies using a reverse transcriptase in situ polymerase chain reaction (PCR) hybridization and/or immunocytochemical approach failed to detect iNOS in astrocytes, and instead implicated cells of the monocyte/macrophage lineage. 16,17 More recently, a study of brain biopsies from two acute cases of MS in young adults detected signal for iNOS in both reactive astrocytes and perivascular monocytes/macrophages, whereas no signal was found in more chronic MS cases. 18 These data suggest that the extent of lesion activity may critically affect which cell types express iNOS in the lesion. To address this possibility in greater detail we have examined MS lesions of varying activity and age for iNOS expression using a combination of in situ hybridization for iNOS mRNA and immunocytochemistry for iNOS protein and nitrotyrosine production. The results support the conclusion that in active MS lesions multiple cell types, including astrocytes both within and outside of the lesions, express both iNOS mRNA and protein whereas the distribution of peroxynitrite is more restricted.
Materials and Methods
Tissue
Tissues were derived from archival autopsy material from the Clinical Neuropathology Service of the Albert Einstein College of Medicine or from a brain bank established by Dr C. S. Raine at the same institution. All tissue collection and use was approved by the Committee on Clinical Investigation of the Albert Einstein College of Medicine. Early postmortem tissues (4 to 18 hours) were studied from 12 patients (Tables 1 and 2) ▶ ▶ with a clinical diagnosis of primary progressive (case 2) or secondary progressive MS (cases 3 to 7 and 10 to 13). Case 1 came to autopsy with a diagnosis of progressive multifocal leukoencephalopathy, but was reclassified as Balo’s concentric sclerosis after neuropathological examination. Tissues were either snap-frozen and embedded in OCT medium and stored at −80°C until use, or processed for conventional paraffin embedding. The classification of the lesions followed the recommendations of the recent workshop and were determined after extensive analysis of the distribution of inflammatory cells, T cells, macrophages, and evidence of myelin breakdown in these tissues. 19
Table 1.
iNOS Immunoreactivity in Multiple Sclerosis Tissue
| Case no. | Age/sex | Number slides examined and lesion type | iNOS reactivity* | Cell localization |
|---|---|---|---|---|
| 1 | 54 /F | 4, Balo’s concentric sclerosis (acute MS) | 4+ | Astrocytes, inflammatory cells and endothelial cells |
| 2, NAWM | negative | |||
| 2 | 31 /F | 6, Acute MS | 4+ | Astrocytes, rare vessels & inflammatory cells |
| 3 | 50 /F | 3, Chronic active MS | 2+ | Astrocytes |
| 4 | 55 /F | 2, Chronic silent MS | 1+ | Astrocytes |
| 2, NAWM | None | |||
| 5 | 43 /F | 1, Chronic silent MS | 1+ | Rare astrocytic reactivity |
| 6 | 43 /F | 2, Chronic active MS | 2+ | Astrocytes |
| 7 | 51 /F | 1, Chronic active MS | 2+ | Astrocytes |
| 8 | 60 /F | 1, Tropical spastic paraparesis | 2+ | Astrocytes |
| 9 | 41 /M | 2, Normal | None | |
| 10 | 35 /F | 2, Normal | None |
*iNOS immunoreactivity was assessed using a semi-quantitative scale of 0 to 4, reflecting the number of positive cells per area.
NAWM: normal-appearing white matter.
Table 2.
Nitrotyrosine Immunoreactivity in Multiple Sclerosis Tissue
| Case no. | Age/sex | Sections examined and diagnosis | Nitrotyrosine reactivity* | Regional localization |
|---|---|---|---|---|
| 1 | 54 /F | 5, Balo’s concentric sclerosis | 4+ | Lesion center and perilesional white matter |
| 2, NAWM | None | |||
| 2 | 31 /F | 2, Acute MS | 3+ | Lesion center |
| 12 | 34 /F | 5, Acute MS | 2+ | Lesion center & perilesional white matter |
| 11 | 33 /F | 4, Chronic active MS | + | Perilesional white matter |
| 4 | 55 /F | 4, Chronic silent MS | ± | Lesion edge |
| 1, NAWM | None | |||
| 13 | 71 /F | 2, Chronic silent MS | None | |
| 14 | 46 /M | 4, Chronic silent MS | ± | Perilesional white matter |
| 1, NAWM | None | |||
| 9 | 41 /M | 2, Normal | None | |
| 10 | 35 /F | 2, Normal | None |
*Nitrotyrosine immunoreactivity was assessed using an arbitrary scale of 0 to 4.
NAWM: normal-appearing white matter.
In Situ Hybridization
The presence and distribution of iNOS mRNA in MS lesions was determined by in situ hybridization using both radioactive and nonradioactive hybridization procedures. Radioactive in situ hybridization was performed on two acute MS lesions (cases 1 and 2; Table 1 ▶ ) using riboprobes that were constructed by PCR using primers for a 325 bp of iNOS (3′CCG TGA CCC AGA ACC CCG AA and 5′ CCA GCA TCT CCT CCT GGT AG) as described. 9 The PCR product was cloned into the TA vector (Invitrogen) and orientation and nucleotide sequence of inserts confirmed by automated sequencing. Antisense and sense probes were transcribed in the presence of 35S-UTP from appropriately cleaved template using Sp6 or T7 RNA polymerases.
Cryostat sections were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) for 20 minutes at room temperature, rinsed in phosphate-buffered saline (PBS), and treated with proteinase-K (1 μg/ml) in 1× PBS for 15 minutes at 37°C. Slides were then rinsed in 1× PBS and treated with 0.1 mol/L triethanolamine, pH 8.0, and 0.25% acetic anhydride for 5 minutes at room temperature. Additional acetic anhydride was added to make a 0.5% solution, and slides were incubated for another 5 minutes. They were then rinsed in 2× standard saline citrate (SSC) for 5 minutes and dehydrated through graded alcohols. Hybridization solution (50% formamide, 0.3 mol/L NaCl, 20 mmol/L NaAc, pH 5.0, 5 mmol/L ethylenediaminetetraacetic acid, 10% dextran sulfate, 2% Denhardt’s solution, 50 mmol/L dithiothreitol, 10 mg/ml yeast t-RNA, and probe at 10 4 cpm/μl) was heated to 80°C for 2 minutes and then applied to the slides, which were incubated overnight at 42°C. Slides were washed in 4× SSC and 10 mmol/L dithiothreitol three times throughout 1 hour. Slides were then dehydrated in graded alcohols, incubated for 15 minutes at 50°C in 50% formamide, 300 mmol/L NaCl, 200 mmol/L NaAc, 10 mmol/L ethylenediaminetetraacetic acid, and 1 mmol/L dithiothreitol, and then washed with 2× SSC. Slides were then treated with RNase A in 20 μg/ml of RNase buffer (0.5 mol/L NaCl, 10 mmol/L Tris-HCl, and 1 mmol/L ethylenediaminetetraacetic acid) for 30 minutes at 37°C and then washed with a large volume (1 L) of the RNase buffer for 30 minutes at 37°C. Slides were then washed with 4 to 5 changes of 2× SSC for 1 hour. Finally, the slides were washed with 0.1× SSC for 15 minutes at 50°C before being dehydrated in graded alcohol washes. Slides were dipped in emulsion and incubated for 14 to 21 days at −80°C before development and counterstaining with hematoxylin. Primary cultures of human fetal astrocytes activated with cytokines as described 13 were used as positive and negative controls for the antisense and sense probes respectively.
Nonradioactive in Situ Hybridization
Three MS cases showing acute, chronic active, and chronic silent MS lesions were studied. Anti-sense probes consisted of a 32-base oligonucleotide complimentary to mRNA in the carboxy terminus of iNOS (5′AGA GCG CTG ACA TCT CCA GGC TGC TGG GCT GC) and the 3′ PCR primer described above. As a control, sense probes were generated using an oligonucleotide complimentary to that described above (GCA GCC CAG CAG CCT GGA GAT GTC AGC GCT CT) and the 5′ PCR primer. These probes were labeled by 3′ tailing with digoxigenin-UTP according to the manufacturer’s instructions (Boehringer Mannheim, Indianapolis, IN).
Paraffin sections were dewaxed and rehydrated through xylene and a series of graded alcohol washes, then treated with 0.2 N HCl for 20 minutes at room temperature, 2× SSC for 15 minutes at 70°C, and proteinase-K 1 μg/ml, 1× PBS) for 15 minutes at 37°C, washed in PBS, and then postfixed with 4% paraformaldehyde. After another wash in PBS, sections were incubated in 0.1 mol/L triethanolamine, pH 8.0, and 0.25% acetic anhydride for 5 minutes at room temperature. Additional acetic anhydride was added for a total of 0.5% in the solution and slides were incubated for another 5 minutes. Slides were rinsed in 2× SSC, rehydrated through graded alcohol, then air-dried. Slides were incubated in hybridization solution (50% deionized formamide, 4× SSC, 1× Denhardt’s solution, 0.5 μg/salmon sperm DNA, 0.25 μg/yeast tRNA solution, and 20% Dextran sulfate for 1 hour before the addition of probe, 200 ng in 30 μl of each oligonucleotide). Sections were incubated with 10 to 15 μl of hybridization solution for 18 to 24 hours at 45°C in a humidified chamber. Slides were rinsed with 2× SSC and then washed for 15 minutes in 2× SSC and 50% formamide at 45°C a total of four times. A final wash of 1× SSC at room temperature was performed for 1 hour. Labeling of sections by probe was detected by immunostaining for digoxigenin with nitroblue tetrazolium as the chromogen according to the manufacturer’s instructions (Boehringer-Mannheim). Sections were counterstained with nuclear Fast Red.
Immunocytochemistry
Paraffin sections were microwaved for 7 minutes in distilled water for antigen retrieval and then incubated with 3% H2O2 for 30 minutes, followed by incubation with 10% goat serum for 1 hour. Rabbit serum raised against the human iNOS carboxy terminal peptide corresponding to amino acids 1135 to 1153 (Santa Cruz Biochemical, Santa Cruz, CA) was then applied at a dilution of 1:100 in 10% normal goat serum for 16 hours at 4°C. The sections were then washed and incubated with biotin-conjugated anti-rabbit IgG (DAKO, Carpinteria, CA) for 30 minutes at room temperature and then processed using the ABC technique and diaminobenzidine as substrate (Pierce, Rockford, IL). The specificity of the reaction was determined by pre-absorption with the specific iNOS peptide (20-fold excess for 2 hours, room temperature; Santa Cruz). Parallel sections were stained for immunoreactivity to glial fibrillary acidic protein (BioGenex, San Ramon, CA), S100β (DAKO), or CD68 (KP-1; DAKO). For double labeling for iNOS and cell-type-specific markers, sections were first immunostained for iNOS using diaminobenzidine (brown) as the chromogen, followed by incubation with mouse monoclonal antibodies directed against GFAP or CD68 and alkaline-phosphatase-conjugated secondary antibodies for 4 hours room temperature. Nitroblue tetrazolium was used as the chromogen.
Nitrotyrosine Immunocytochemistry
Sections were microwaved for 7 minutes in distilled water for antigen retrieval and then incubated with a rabbit antibody against nitrotyrosine (Upstate Biotechnology, Lake Placid, NY) at 1:200 in 10% normal goat serum in PBS for 16 hours at room temperature. Further incubations with secondary antibody and avidin-biotin complex were performed as above. Diaminobenzidine was used as the chromogen. Positive controls consisted of sections treated with 0.5 mmol/L of tetranitromethane (Sigma Chemical Co., St. Louis, MO) in 50 mmol/L KH2PO2 for 15 minutes, and negative controls using tissues from cases 1 and 2 consisted of sections treated with antibody in the presence of 10 mmol/L nitrotyrosine, as previously described. 20
Results and Discussion
iNOS mRNA Expression in MS Lesions as Determined by in Situ Hybridization
In the first set of experiments, we used highly sensitive radioactive riboprobes on two acute MS lesions to determine whether iNOS mRNA was present in sufficient abundance to be detected by regular in situ hybridization procedures, or whether a more sensitive reverse transcriptase in situ PCR protocol would be required. As shown in Figure 1 ▶ , strong reactivity for iNOS mRNA was detected in the active lesions. Figure 1A ▶ depicts the edge of an acute lesion with the lesion center to the left and normal appearing white matter to the right. In the lesion, prominent accumulations of silver grains were noted decorating large cells, the size, shape, and distribution of which was consistent with hypertrophic reactive astrocytes. In addition, an elevated distribution of silver grains was observed throughout the lesioned area, with the edge of the lesion clearly demarcated by a drop-off in the intensity of silver grain deposition. Elevated accumulations of silver grains were also detected in association with blood vessels both within the lesion as well as in the adjacent normal appearing white matter (Figure 1, A and C) ▶ . A higher power view of the lesion shown in Figure 1A ▶ depicts intense labeling of large cells consistent with hypertrophic astrocytes (arrow) and a lower level reactivity decorating smaller cells in the lesion (Figure 1B) ▶ . Using a combination of in situ reactivity for iNOS mRNA and immunoreactivity for CD68, some of these smaller cells were positive for both (Figure 1B ▶ inset), indicating that these cells belonged to the macrophage lineage. In Figure 1C, a ▶ higher power view of an inflamed vessel shows both a linear array of reactivity, as well as focal accumulations of silver grains, consistent with reactivity associated with perivascular infiltrates. In contrast, no silver grains were detected in the same tissues hybridized with a control sense probe (Figure 1D) ▶ .
Figure 1.
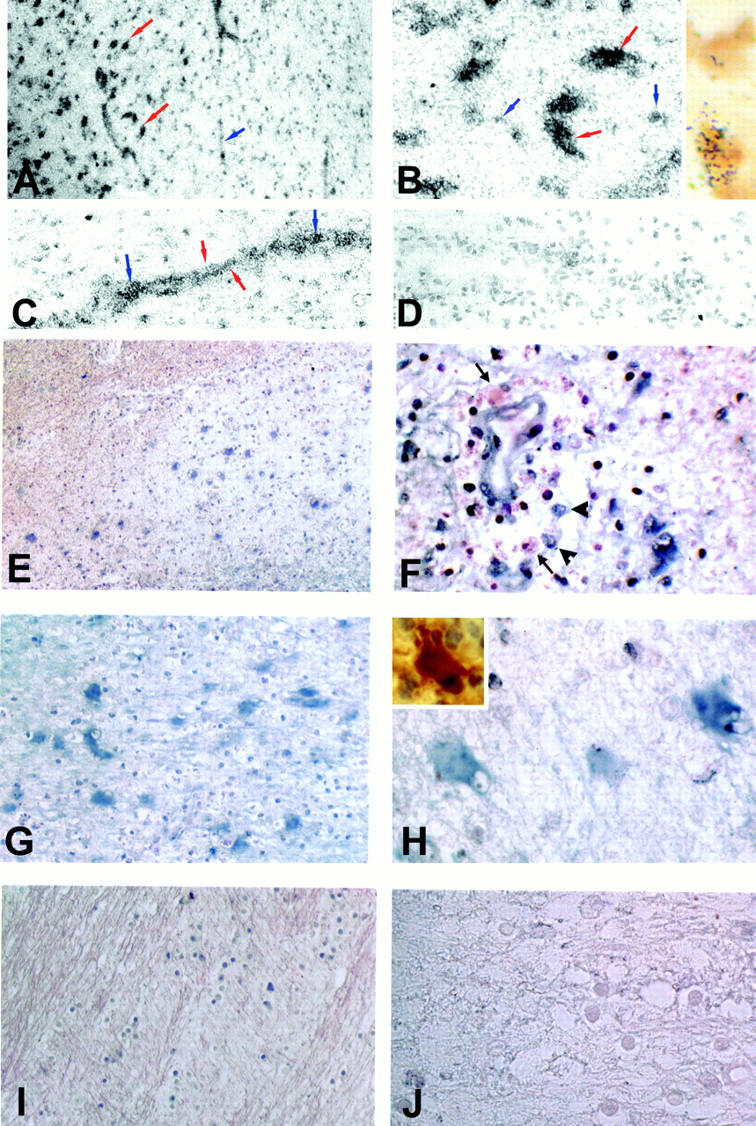
In situ hybridization for iNOS mRNA in MS lesions. To assess the distribution of iNOS mRNA in MS lesions tissues were reacted either with probes labeled with 35S (A–D) or with digoxygenin (E–J). A: A low-power view of the edge of an acute MS lesion shows prominent deposition of silver grains over large cells (red arrows) within the lesion (left), as well as in association with vascular elements (blue arrow). B: High-power view of the lesion center demonstrating intense labeling of large cells consistent with reactive astrocytes (red arrows), and a lower level reactivity decorating smaller cells (blue arrows). Inset shows the same lesion double-labeled for CD68 antigen (brown) and iNOS mRNA (silver grains). C: High-power view of an inflamed vessel. Note the linear array of silver grains consistent with reactivity of the vessel wall (red arrows) and more focal accumulations suggestive of reactivity in inflammatory cells (blue arrows). D: A control section reacted with sense RNA probe. E: Nonradioactive in situ hybridization for iNOS mRNA (blue) in an acute MS lesion. F: High-power view of a perivascular inflammatory cell cuff demonstrating inflammatory cells that are positive (arrowhead) as well as negative (arrows) for iNOS mRNA. Note that the endothelial cells also demonstrate iNOS mRNA reactivity. G: Higher power view of the lesion demonstrating intense reactivity (blue) throughout the lesion center. H: Large cells within the lesion center expressing intense iNOS mRNA reactivity (blue) are compatible with hypertrophic astrocytes stained for S100β in serial sections (inset). I: Normal appearing adjacent white matter showing lack of iNOS mRNA expression. J: Same lesion as in E hybridized with a control sense probe. Original magnifications: ×200 (A and E), ×400 (C, D, I, and J), ×800 (B, F, and H), and ×1000 (I, insets in B and I).
We then examined these same lesions, as well as additional MS tissues, using a nonradioactive probe directed against a different region of the iNOS gene. In acute MS lesions reactivity for iNOS mRNA (blue) was detected in large cells distributed throughout the lesion center (Figure 1E) ▶ . When this same lesion was examined at higher magnification, inflamed vessels within the lesion displayed reactivity for iNOS mRNA within endothelial cells as well as in some of the inflammatory cells present in the inflammatory cuffs (Figure 1F) ▶ . Within the lesion center the large reactive iNOS mRNA reactive cells displayed the morphology and characteristic distribution of hypertrophic astrocytes (Figure 1, G and H) ▶ , as illustrated by staining of parallel sections with S100β (Figure 1H ▶ , inset). From these data we conclude that multiple cell types can be activated to express iNOS mRNA in MS lesions. In contrast to inflamed areas of the CNS, no reactivity was detected in white matter areas distant from the lesions (Figure 1I) ▶ . When the acute lesions of the brain were reacted with a sense probe no reactivity was detected (Figure 1J) ▶ . Chronic-silent MS lesions showed minimal reactivity (data not shown).
iNOS Immunoreactivity in MS Lesions
To examine the distribution of iNOS protein in these same lesions, the tissues were reacted for immunohistochemistry using a human iNOS-specific antibody. In previous studies we have shown that this antibody reacts strongly with primary cultures of human fetal astrocytes that have been activated by cytokines and that can be shown to release high levels of NO as determined by the Griess reaction, and which by Western blot express a single band of ∼130 kd. 13 In all lesions from seven different cases of MS, iNOS immunoreactivity was detected within lesioned areas of the brain, with levels that correlated with lesion activity (Table 1 ▶ and Figure 2 ▶ ). A case of tropical spastic paraparesis was also studied and showed a moderate degree of iNOS expression (Table 1) ▶ . Two normal control cases, as well as normal appearing white matter in MS tissues, showed little or no iNOS reactivity.
Figure 2.
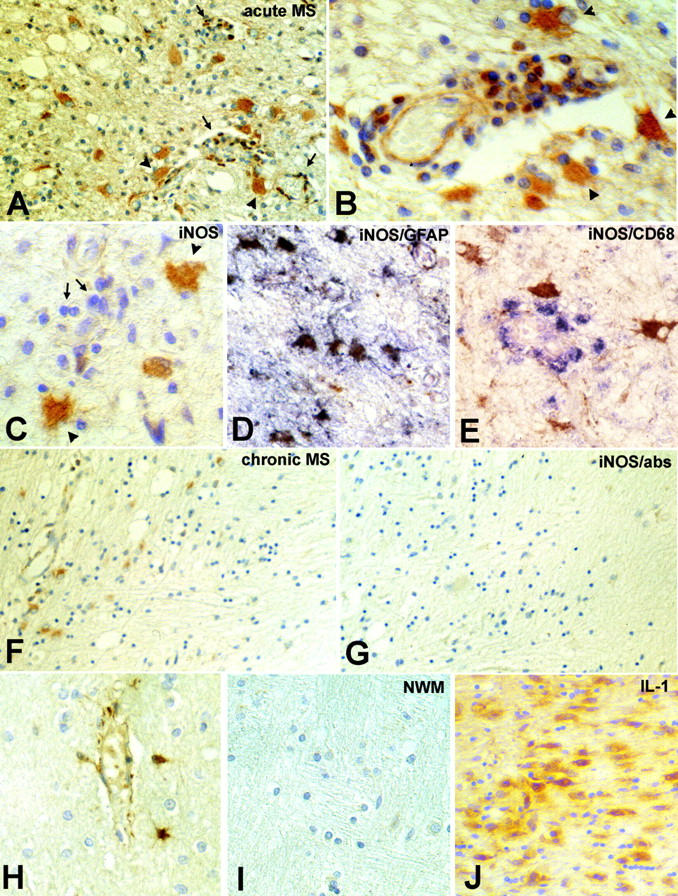
iNOS immunoreactivity in MS lesion. Shown are an acute MS lesion from a case of Balo’s concentric sclerosis (A and B), and an acute MS lesion (C, D, and E). A: A low-power view of the lesion demonstrating immunoreactivity for iNOS protein in hypertrophic astrocytes (arrowheads) as well as in inflammatory cells present in inflammatory cuffs (arrows). B: Higher power view of the lesion demonstrating reaction product in hypertrophic astrocytes (arrowheads), inflammatory cells, and endothelial cells. C: An acute lesion demonstrating immunoreactivity for iNOS protein in hypertrophic astrocytes (arrowheads). Note that most inflammatory cells were nonreactive (arrows). D: Double labeling of serial sections for GFAP (purple) and iNOS (brown) and in E for CD68 (purple) and iNOS (brown). F: A chronic active lesion demonstrating lack of staining in the lesion center (right) and iNOS immunoreactivity in reactive astrocytes at the lesion edge (left). G: Same lesion as in F after staining with anti-iNOS antibody absorbed with the specific peptide. H: Occasional staining of astrocytes was detected in noninflamed areas. I: Normal appearing white matter from normal brain was nonreactive. J: Acute MS lesion immunoreacted for IL-1. Original magnifications: ×250 (A, F, G, and H), ×500 (B, C, D, and E), and ×200 (I and J).
In MS lesions the distribution of iNOS immunoreactivity was consistent with that found for analysis of iNOS mRNA. Within the center of acute lesions, strong iNOS immunoreactivity was detected in hypertrophic astrocytes, as well as in some perivascular infiltrating cells and occasional endothelial cells (Figure 2, A and B) ▶ . Reactivity associated with astrocytes was confirmed by double labeling with glial fibrillary acidic protein (Figure 2, C and D) ▶ . The extent of iNOS reactivity in macrophages was variable from lesion to lesion, and in some active lesions perivascular macrophages (as identified by labeling with CD68) were not stained with the iNOS antibody (Figure 2E) ▶ . In more chronic lesions, the lesion center was generally nonreactive for iNOS protein, and iNOS reactivity was localized to scattered reactive astrocytes along the border of the lesion (Figure 2F) ▶ . The specificity of the immunoreactivity for iNOS protein was then determined by pre-absorption of the antibody with the specific peptide supplied by the manufacturer (Figure 2G) ▶ . iNOS immunoreactivity was also noted in reactive astrocytes at some distance from the lesion, as well as in association with noninflamed blood vessels (Figure 2H) ▶ . The expression of iNOS immunoreactivity in astrocytes at some distance from the lesion has been noted in the animal model of MS, experimental autoimmune encephalomyelitis (EAE), and the authors speculate that astrocyte-derived NO at these sites may be protective and serve to limit the spread of the inflammatory process. 21 This interesting hypothesis is discussed further in light of the data for peroxynitrite (see below). Normal appearing white matter remote from the lesion, as well as white matter from a normal control brain was nonreactive (Figure 2I) ▶ . IL-1, which has been implicated in the induction of iNOS in astrocytes, 13 was also prominently expressed in the acute MS lesions (Figure 2J) ▶ .
Nitrotyrosine Immunoreactivity in MS Lesions
Seven MS cases were examined immunohistochemically for evidence of nitrotyrosine, a relatively specific and stable biochemical marker for peroxynitrite formation, particularly when large amounts are present. 7,8,22 Because the deposition of peroxynitrite represents a reaction between reactive nitrogen and reactive oxygen species, its production cannot be localized to one cell but its presence within the tissues is indicative of the presence of both NO and O2−. The results are summarized in Table 2 ▶ and illustrated in Figure 3 ▶ . In acute lesions intense peroxynitrite staining was detected within the parenchyma with a distribution pattern suggestive of immunoreactivity on cell membranes and/or myelin membrane staining, as well as in perivascular and interstitial locations (Figure 3 ▶ ; A, B, and C). This distribution of nitrotyrosine reactivity is remarkably similar to that recently documented in patients with AIDS dementia complex, 23 and supports previous studies that have detected the presence of nitrotyrosine in association with disease activity in the CNS of patients with MS, as well as in EAE. 8,22,24 This close apposition of nitrotyrosine as well as iNOS immunoreactivity to vessels (illustrated in Figure 3, E and F ▶ ) likely contributes to the damage to the blood brain barrier that is a cardinal feature of active MS lesions. 1 In addition, a small number of discrete nitrotyrosine-positive cells were detected in three cases, whereas in two other cases, weak staining was detected in many cells (Table 2 ▶ and Figure 3 ▶ ). Pre-absorption of the antibody with nitrotyrosine led to loss of immunoreactivity, including the diffuse immunoreactivity associated with the lesion center (Figure 3D) ▶ and inflamed vessels (Figure 3G) ▶ , demonstrating the specificity of the reaction. Chronic MS lesions (Figure 3H) ▶ as well as normal white matter in three MS cases and in two normal brains showed no immunoreactivity (Table 2) ▶ .
Figure 3.
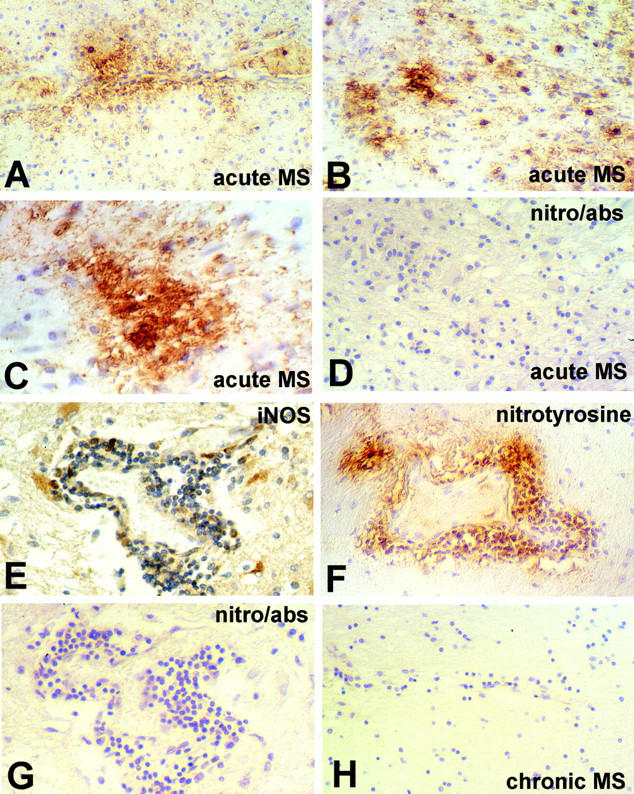
Nitrotyrosine immunoreactivity in MS lesions. A through D are from an acute MS lesion. A: At the lesion edge nitrotyrosine immunoreactivity can be seen surrounding an inflamed vessel. B: A higher power view of the lesion demonstrates intense nitrotyrosine immunoreactivity in association with small round cells, as well as staining of membranes in the adjacent parenchyma. C: The distribution of nitrotyrosine immunoreactivity throughout the lesion center that is lost when stained with antibody in the presence of excess nitrotyrosine (D). E through G: An inflamed vessel reacted for iNOS (E), nitrotyrosine (F), and nitrotyrosine antibody in the presence of excess nitrotyrosine (G). H: The edge of a chronic active lesion demonstrating lack of immunoreactivity for nitrotyrosine. Original magnifications: ×250 (A, B, D–H), ×500 (C).
Taken together, the results of this study provide strong support for the conclusion that iNOS mRNA and protein are abundantly expressed in active MS lesions, with many different cell types, including reactive astrocytes, macrophages, and endothelial cells, showing reactivity for both iNOS mRNA and protein. The abundant expression of iNOS mRNA and protein in reactive astrocytes both within the lesion and in the adjacent normal appearing white matter is, therefore, consistent with the initial results obtained with NADPH-diaphorase staining in MS lesions, 14,15 as well as with a more recent report that studied two active cases of MS. 18 The expression of iNOS by reactive astrocytes is also consistent with the in vitro data, and with evidence from animals with EAE, where reactivity has been noted in these cells both within the lesion center as well as in hypertrophic astrocytes located at some distance from the lesion itself. 22 It is likely that the induction of iNOS in these cells represents a response to the presence of pro-inflammatory cytokines such as IL-1 and IFN-γ in the lesion, 15 and our studies further demonstrated prominent reactivity for IL-1 in these tissues.
This distribution of iNOS expression is in general agreement with the notion that NO generated via iNOS may contribute to lesion pathogenesis in MS, but also raises the question as to the function of NO within these tissues. 6,25 Although most early reports focused on the potential role of NO as a mediator of tissue injury, after the observation that NO is toxic for oligodendrocytes and neurons, 26,27 enhances conduction failure in demyelinated axons, 28 and that inhibitors of NO production can protect animals against EAE, 29-31 more recent studies have questioned a purely destructive role for NO in these lesions. In particular, data from mice with targeted deletions of the iNOS gene have shown enhanced susceptibility to EAE 32,33 and specific inhibitors of iNOS have been found to reactivate EAE in recovered animals, 34 suggesting a more significant role for the anti-inflammatory/immunosuppressive properties of NO. Studies of other inflammatory disorders in these mice have also supported an anti-inflammatory role for NO produced via the activation of iNOS. 35,36 Nevertheless, it should be kept in mind that the immunosuppressive activities of NO are thought to be mediated principally through the induction of apoptosis in lymphocytes, again attesting to the cytotoxic potential of this free radical.
As noted by Willenborg and colleagues in their recent review, 6 an alternative interpretation is that NO is generally an immunosuppressive anti-inflammatory molecule whereas in the target tissues some downstream molecule, such as peroxynitrite, is responsible for tissue destruction. In the lesions studied here intense immunoreactivity for nitrotyrosine was detected in most but not all active MS lesions. This concept is also consistent with the data from animals with EAE, where widespread evidence of peroxynitrite was detected in animals with active disease, particularly hyperacute EAE, but not during disease regression even though the animals still displayed evidence of inflammation and clinical activity, 22 and the observations that inhibitors/scavengers of peroxynitrite protect against disease expression. 31,37 Of greater relevance to the current studies are the data from Cross and colleagues, 8 who demonstrated nitrotyrosine immunoreactivity in active MS lesions and significantly elevated levels of nitrate (the product of NO and peroxynitrite) in the cerebrospinal fluid of patients in clinical relapse. Autopsy CNS tissues from patients with AIDS dementia complex also showed intense and widespread deposition of nitrotyrosine, which was not detected in patients who had died with HIV encephalitis not associated with dementia. 24 The authors suggested that the formation of peroxynitrite in these lesions represented an interaction between activated macrophages/microglia that functioned as a source of cytokines such as IL-1 and reactive oxygen intermediates, and astrocytes that were activated by these cytokines to produce NO. We would suggest yet a further involvement of activated T cells as a source of IFN-γ, a cytokine that is known to act synergistically with other activators in the sustained production of high levels of iNOS in many different cell types, including astrocytes. 3,9,10,13,38 From this scenario we predict that the production of the highly toxic downstream products of NO would require interactions between appropriately activated lymphocytes, macrophages/microglia, and astrocytes. Such a complex multiple signaling pathway requiring cooperative activity between multiple cell types is encountered frequently in immune-based diseases where the potential for causing irreversible damage to adjacent uninvolved tissues constitutes a significant risk. In summary, our results support a role for multiple cell types, including hypertrophic astrocytes, macrophages/microglia, and endothelial cells, as sources of NO production in highly acute MS lesions, whereas as the lesion ages astrocytes become the more predominant cell type expressing iNOS mRNA and protein. This would be consistent with the known immunoregulatory role for astrocytes in CNS inflammation. In addition, the close proximity of astrocytes to the blood brain barrier and the expression of iNOS in activated astrocytes as well as peroxynitrite at sites adjacent to the blood brain barrier also support the possibility that astrocyte iNOS may contribute to vasodilation and damage to the blood brain barrier in MS, in addition to immunoregulatory and cytotoxic roles.
Acknowledgments
We thank Dr. Cedric S. Raine for information regarding the designation of MS lesion age and activity; Dr. Linda Goodman for contributing an MS case; and Michael Cammer of the Analytical Imaging Facility for assistance with the figures.
Footnotes
Address reprint requests to Celia F. Brosnan, Ph.D., Albert Einstein College of Medicine, Department of Pathology, 1300 Morris Park Ave., Bronx, NY 10461. E-mail: brosnan@aecom.yu.edu.
Supported in part by National Multiple Sclerosis Society grant RG 2771 and United States Public Health Service grants NS 11920, NS 31919, and T32GM07288.
References
- 1.Martin R, McFarland HF: Immunology of multiple sclerosis and experimental allergic encephalomyelitis. Multiple Sclerosis: Clinical and Pathogenetic Aspects. Edited by CS Raine, HF McFarland, WW Tourtellotte. London, Chapman and Hall, pp 221–239
- 2.Brosnan CF, Raine CS: Mechanisms of immune injury in multiple sclerosis. Brain Pathol 1996, 6:243-257 [DOI] [PubMed] [Google Scholar]
- 3.Nathan C, Xie QW: Regulation of biosynthesis of nitric oxide. J Biol Chem 1994, 269:13725-13728 [PubMed] [Google Scholar]
- 4.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS: A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 1993, 364:626-632 [DOI] [PubMed] [Google Scholar]
- 5.Crow JP, Beckman JS: The role of peroxynitrite in nitric oxide-mediated toxicity. Curr Top Microbiol Immunol 1995, 196:57-73 [DOI] [PubMed] [Google Scholar]
- 6.Willenborg DO, Staykova MA, Cowden WB: Our shifting understanding of the role of nitric oxide in autoimmune encephalomyelitis: a review. J Neuroimmunol 1999, 100:21-35 [DOI] [PubMed] [Google Scholar]
- 7.Beckman JS, Koppenol WH: Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 1996, 271:C1424-C1437 [DOI] [PubMed] [Google Scholar]
- 8.Cross AH, Manning PT, Keeling RM, Schmidt RE, Misko TP: Peroxynitrite formation within the central nervous system in active multiple sclerosis. J Neuroimmunol 1998, 88:45-56 [DOI] [PubMed] [Google Scholar]
- 9.Geller DA, Lowenstein CJ, Shapiro RA, Nussler AK, Di Silvio M, Wang SC, Nakayama DK, Simmons RL, Snyder SH, Billiar TR: Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci USA 1993, 90:3491-3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marks-Konczalik J, Chu SC, Moss J: Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kB-binding sites. J Biol Chem 1998, 273:22201-22208 [DOI] [PubMed] [Google Scholar]
- 11.Nathan C, Xie Q-W: Nitric oxide synthases: roles, tolls, and controls. Cell 1994, 78:915-918 [DOI] [PubMed] [Google Scholar]
- 12.Bertholet S, Tzeng E, Felley-Bosco E, Mauel J: Expression of the inducible NO synthase in human monocytic U937 cells allows high output nitric oxide production. J Leukoc Biol 1999, 65:50-58 [DOI] [PubMed] [Google Scholar]
- 13.Lee SC, Dickson DC, Liu W, Brosnan CF: Induction of nitric oxide synthase activity in human astrocytes by IL-1 and IFN-γ. J Neuroimmunol 1993, 46:19-24 [DOI] [PubMed] [Google Scholar]
- 14.Bo L, Dawson TM, Wesselingh S, Mork S, Choi S, Kong PA, Hanley D, Trapp BD: Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol 1994, 36:778-786 [DOI] [PubMed] [Google Scholar]
- 15.Brosnan CF, Battistini L, Raine CS, Dickson DW, Casadevall A, Lee SC: Reactive nitrogen intermediates in human neuropathology: an overview. Dev Neurosci 1994, 16:152-161 [DOI] [PubMed] [Google Scholar]
- 16.Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, Tawadros R, Koprowski H: Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci USA 1995, 92:12041-12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Groot CJA, Ruuls SR, Theeuwes JWM, Dijkstra CD, Van der Valk P: Immunocytochemical characterization of the expression of inducible and constitutive isoforms of nitric oxide synthase in demyelinating multiple sclerosis lesions. J Neuropathol Exp Neurol 1997, 56:10-20 [DOI] [PubMed] [Google Scholar]
- 18.Oleszak EL, Zaczynska E, Bhattacharjee M, Butunoi C, Legido A, Katsetos CD: Inducible nitric oxide synthase and nitrotyrosine are found in monocytes/macrophages and/or astrocytes in acute, but not in chronic, multiple sclerosis. Clin Diagn Lab Immunol 1998, 5:438-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassmann H, Raine CS, Antel J, Prineas JW: Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol 1998, 86:213-217 [DOI] [PubMed] [Google Scholar]
- 20.Downen M, Amaral TD, Hua LL, Zhao M-L, Lee SC: Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia 1999, 28:114-127 [PubMed] [Google Scholar]
- 21.Tran EH, Hardin-Pouzet H, Verge G, Owens T: Astrocytes and microglia express inducible nitric oxide synthase in mice with experimental allergic encephalomyelitis. J Neuroimmunol 1997, 74:121-129 [DOI] [PubMed] [Google Scholar]
- 22.van der Veen RC, Hinton DR, Incardonna F, Hofman FM: Extensive peroxynitrite activity during progressive stages of central nervous system inflammation. J Neuroimmunol 1997, 77:1-7 [DOI] [PubMed] [Google Scholar]
- 23.Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HS: Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol 1999, 162:4319-4327 [PubMed] [Google Scholar]
- 24.Cross AH, Manning PT, Stern MK, Misko TP: Evidence for the production of peroxynitrite in inflammatory CNS demyelination. J Neuroimmunol 1997, 80:121-130 [DOI] [PubMed] [Google Scholar]
- 25.Giovannoni G, Heales SJ, Land JM, Thompson EJ: The potential role of nitric oxide in multiple sclerosis. Mult Scler 1998, 4:212-216 [DOI] [PubMed] [Google Scholar]
- 26.Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE: Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience 1994, 61:575-585 [DOI] [PubMed] [Google Scholar]
- 27.Dawson VL, Dawson TM: Nitric oxide in neurodegeneration. Prog Brain Res 1998, 118:215-229 [DOI] [PubMed] [Google Scholar]
- 28.Smith KJ, Kapoor R, Felts PA: Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 1999, 9:69-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao W, Tilton RG, Corbett JA, McDaniel ML, Misko TP, Williamson JR, Cross AH, Hickey WF: Experimental allergic encephalomyelitis in the rat is inhibited by aminoguanidine, an inhibitor of nitric oxide synthase. J Neuroimmunol 1996, 64:123-133 [DOI] [PubMed] [Google Scholar]
- 30.Hooper DC, Bagasra O, Marini JC, Zborek A, Ohnishi ST, Kean R, Champion JM, Sarker AB, Bobroski L, Farber JL, Akaike T, Maeda H, Koprowski H: Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc Natl Acad Sci USA 1997, 94:2528-2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okuda Y, Sakoda S, Fujimura H, Yanagihara T: Aminoguanidine, a selective inhibitor of the inducible nitric oxide synthase, has different effects on experimental allergic encephalomyelitis in the induction and progression phase. J Neuroimmunol 1998, 81:201-210 [DOI] [PubMed] [Google Scholar]
- 32.Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS: Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol 1998, 160:2940-2946 [PubMed] [Google Scholar]
- 33.Sahrbacher UC, Lechner F, Eugster HP, Frei K, Lassmann H, Fontana A: Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur J Immunol 1998, 28:1332-1338 [DOI] [PubMed] [Google Scholar]
- 34.O’Brien NC, Charlton B, Cowden WB, Willenborg DO: Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol 1999, 163:6841-6847 [PubMed] [Google Scholar]
- 35.Perner A, Rask-Madsen J: Review article: the potential role of nitric oxide in chronic inflammatory bowel disorders. Aliment Pharmacol Ther 1999, 13:135-144 [DOI] [PubMed] [Google Scholar]
- 36.Hesse M, Cheever AW, Jankovic D, Wynn TA: NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol 2000, 157:945-955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross AH, San M, Stern MK, Keeling RM, Salvemini D, Misko TP: A catalyst of peroxynitrite decomposition inhibits murine experimental autoimmune encephalomyelitis. J Neuroimmunol 2000, 107:21-28 [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Zhao M-L, Brosnan CF, Lee SC: Expression of type II nitric oxide synthase in primary human astrocytes and microglia: role of IL-1 and IL-1 receptor antagonist. J Immunol 1996, 157:3569-3576 [PubMed] [Google Scholar]


