Abstract
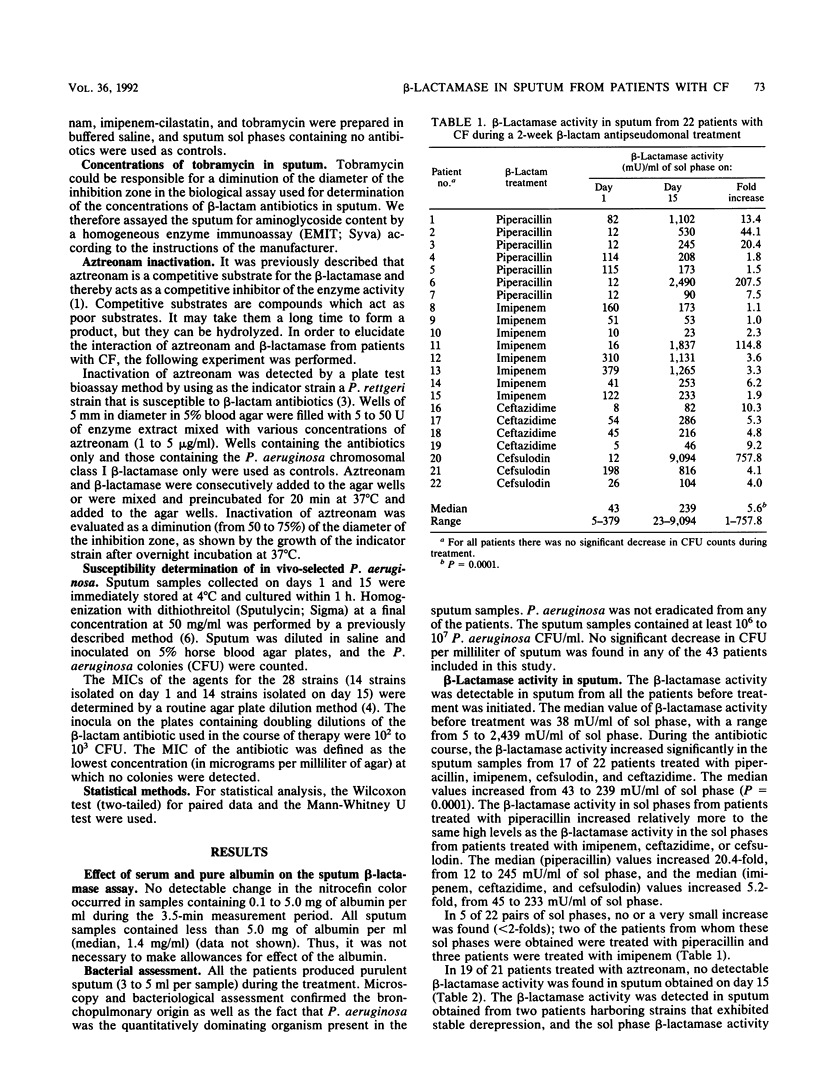
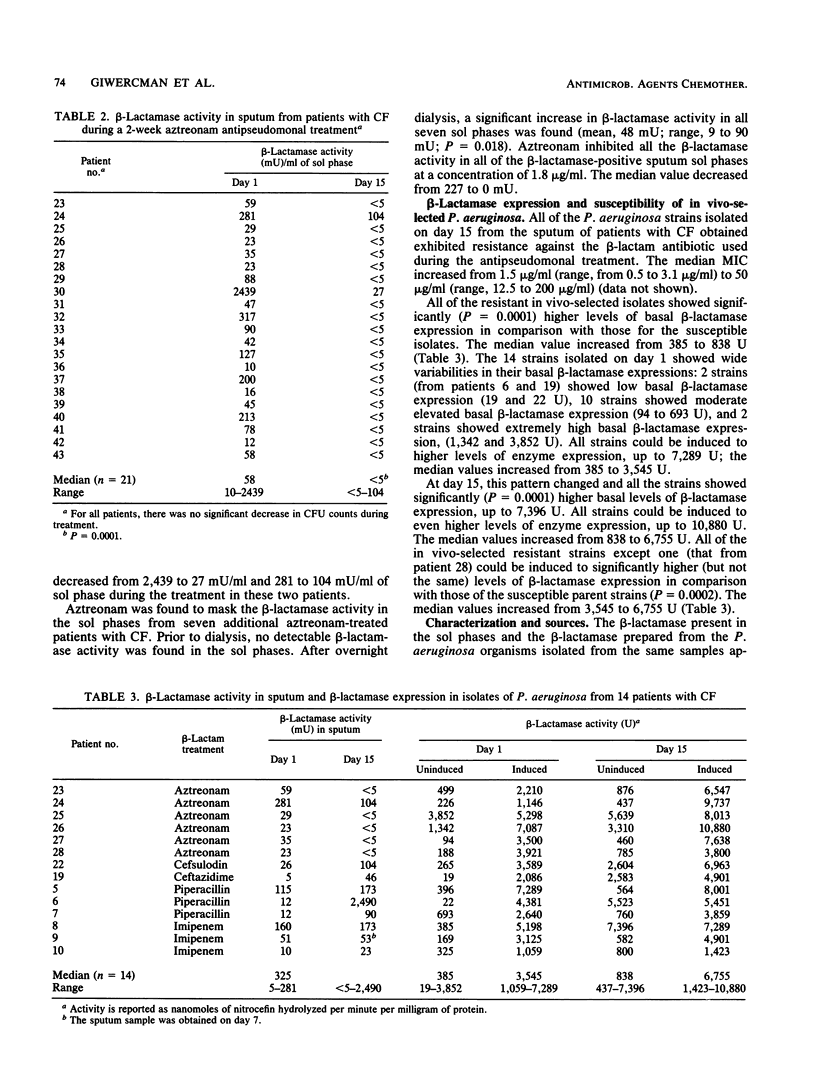
The in vivo activity and source of beta-lactamase in sputum samples from 43 patients with cystic fibrosis (CF) during a 2-week antipseudomonal treatment were studied. A colorimetric method, based on the conversion of nitrocefin, was used for quantitation of the sputum beta-lactamase activity. beta-Lactamases in sputum were characterized by isoelectric focusing and inhibition profile and were compared with the beta-lactamases extracted from Pseudomonas aeruginosa isolated from the paired sputum samples. We found that the beta-lactamase activity increased to high levels in sputum from patients with CF during the course of piperacillin, ceftazidime, cefsulodin, or imipenem therapy. Aztreonam therapy lead to opposite results because the beta-lactamase activity decreased and aztreonam was able to mask beta-lactamase activity by acting as an inhibitor. All sputum beta-lactamases displayed characteristics indicative of a class I enzyme, identical to the beta-lactamases extracted from P. aeruginosa. The presence of beta-lactamase at such levels could lead to in vivo inactivation of beta-lactam antibiotics. This study supports the hypothesis that beta-lactamase production is an important in vivo resistance mechanism in P. aeruginosa-infected patients with CF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush K., Sykes R. B. beta-Lactamase inhibitors in perspective. J Antimicrob Chemother. 1983 Feb;11(2):97–107. doi: 10.1093/jac/11.2.97. [DOI] [PubMed] [Google Scholar]
- Dragicevic P., Hill S. L., Burnett D., Merrikin D., Stockley R. A. Activities and sources of beta-lactamase in sputum from patients with bronchiectasis. J Clin Microbiol. 1989 May;27(5):1055–1061. doi: 10.1128/jcm.27.5.1055-1061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne J., Vézina G., Levesque R. C. Molecular cloning and expression of the imipenem-hydrolyzing beta-lactamase gene from Pseudomonas maltophilia in Escherichia coli. Rev Infect Dis. 1988 Jul-Aug;10(4):806–817. doi: 10.1093/clinids/10.4.806. [DOI] [PubMed] [Google Scholar]
- Giwercman B., Lambert P. A., Rosdahl V. T., Shand G. H., Høiby N. Rapid emergence of resistance in Pseudomonas aeruginosa in cystic fibrosis patients due to in-vivo selection of stable partially derepressed beta-lactamase producing strains. J Antimicrob Chemother. 1990 Aug;26(2):247–259. doi: 10.1093/jac/26.2.247. [DOI] [PubMed] [Google Scholar]
- Hammerschlag M. R., Harding L., Macone A., Smith A. L., Goldmann D. A. Bacteriology of sputum in cystic fibrosis: evaluation of dithiothreitol as a mucolytic agent. J Clin Microbiol. 1980 Jun;11(6):552–557. doi: 10.1128/jcm.11.6.552-557.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livermore D. M. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur J Clin Microbiol. 1987 Aug;6(4):439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rosdahl V. T., Vejlsgaard V., Rosdahl N., Vejlsgaard R. A micromethod for determination of antibiotics in serum. Dan Med Bull. 1969 May;16(5):133–139. [PubMed] [Google Scholar]
- Sanders C. C., Gates M. L., Sanders W. E., Jr Heterogeneity of class I beta-lactamase expression in clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1988 Dec;32(12):1893–1895. doi: 10.1128/aac.32.12.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]


