Abstract
Progression of breast cancer involves cross-talk between epithelial and stromal cells. This cross-talk is mediated by growth factors and cytokines secreted by both cancer and stromal cells. We previously reported expression of interleukin (IL)-1α in a subset of breast cancers and demonstrated that IL-1α is an autocrine and paracrine inducer of prometastatic genes in in vitro systems. To understand the role of IL-1α in breast cancer progression in vivo, we studied the growth of MCF-7 breast cancer cells overexpressing a secreted form of IL-1α (MCF-7IL-1α) in nude mice. MCF-7IL-1α cells formed rapidly growing estrogen-dependent tumors compared to parental cells. Interestingly, IL-1α expression alone was not sufficient for metastasis in vivo although in vitro studies showed induction of several prometastatic genes and matrix metalloproteinase activity in response to cross-talk between IL-1α-expressing cancer cells and fibroblasts. Animals implanted with MCF-7IL-1α cells were cachetic, which correlated with increased leptin serum levels but not other known cachexia-inducing cytokines such as IL-6, tumor necrosis factor, or interferon gamma. Serum triglycerides, but not blood glucose were lower in animals with MCF-7IL-1α cell-derived tumors compared to animals with control cell-derived tumors. Cachexia was associated with atrophy of epidermal and adnexal structures of skin; a similar phenotype is reported in triglyceride-deficient mice and in ob/ob mice injected with leptin. Mouse leptin-specific transcripts could be detected only in MCF-7IL-1α cell-derived tumors, which suggests that IL-1α increases leptin expression in stromal cells recruited into the tumor microenvironment. Despite increased serum leptin levels, animals with MCF-7IL-1α cell-derived tumors were not anorexic suggesting only peripheral action of tumor-derived leptin, which principally targets lipid metabolism. Taken together, these results suggest that cancer cell-derived cytokines, such as IL-1α, induce cachexia by affecting leptin-dependent metabolic pathways.
Progression of breast cancer from a benign to a malignant stage is accompanied by overexpression of several growth factors, cytokines, and chemokines by cancer cells. 1-4 These growth factor/cytokine expression patterns can predict clinical outcome because they can influence disease progression by enhancing metastasis or inducing cachexia without any distant metastasis. In fact, 30% of cancer mortality is because of cachexia rather than tumor burden or metastasis. 5
The major circulating cytokines implicated in breast cancer progression include tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8. 4,6 In various experimental models, all three of these cytokines can promote cancer progression by enhancing both metastasis and cachexia. 7-9 Others and we reported the expression of IL-1α in primary breast cancer and breast cancer cell lines with highly metastatic phenotype. 10,11 Invasive breast cancers and ductal carcinoma in situ express higher levels of IL-1α compared to benign tumors. 11 In breast cancer cell lines, increased IL-1α expression correlated with constitutive DNA binding of extracellular signal-activated transcription factor nuclear factor (NF)-κB, and expression of prometastatic (IL-6 and IL-8) and anti-apoptotic genes (TRAF-1 and cIAP-2). 10,12,13 Furthermore, IL-1α from breast cancer cells induced NF-κB in stromal cells, which was accompanied by increased expression of urokinase plasminogen activator (uPA), IL-6, and IL-8 in stromal fibroblasts. 10,14 Results of these in vitro studies suggest that IL-1α is involved in invasion and metastatic growth of breast cancer.
IL-1α is usually expressed as a preprotein, which is secreted only after cleavage by the calpain family of proteases. 15 Calpains cleave pre-IL-1α and release the N-terminal propiece-IL-1α and the C-terminal secreted IL-1α. 16 Unlike IL-1β, which is biologically active only as a secreted mature molecule, membrane-associated IL-1α, propiece-IL-1α, and mature secreted IL-1α show distinct biological activities. 15 The propiece-IL-1α has transforming activity whereas secreted IL-1α has paracrine and autocrine activities similar to IL-1β. 15,17 Membrane-associated IL-1α potentiates anti-tumor immunity. 18 Transformed but not normal epithelial cells secrete IL-1α, possibly because of overexpression of calpain family proteases by cancer cells. 15,19 Because IL-1α is overexpressed in a variety of cancers including breast, squamous cell carcinoma, and melanoma, 11,20,21 we initiated this study to specifically address the role of secreted IL-1α in breast cancer progression. The aims of this study were to investigate the effects of a cancer cell-derived secreted form of IL-1α on general metabolic status and to test whether IL-1α expression alone is sufficient to convert MCF-7 breast cancer cells from nonmetastatic to metastatic phenotype. MCF-7 cells do not express IL-1α and form estrogen-dependent, nonmetastatic tumors in nude mice. 10,22 We show that IL-1α expression alone is not sufficient to induce metastasis of these cells despite increasing prometastatic gene expression in stromal cells in in vitro studies. However, IL-1α alone was able to induce profound cachexia. Cachexia was accompanied with atrophy of epidermal and adnexal structures of skin. Interestingly, cachexia in animals with IL-1α-overexpressing cell-derived tumors correlated with elevated serum leptin and reduced triglyceride levels but not with any other known cachexia-inducing cytokines.
Materials and Methods
Breast Cancer Cell Lines and Generation of Breast Cancer Cells Overexpressing IL-1α
MCF-7 and human lung fibroblasts (HLF-1) were purchased from American Type Culture Collection, Rockville, MD, and maintained in minimal essential medium plus 10% fetal calf serum and antibiotics. To generate MCF-7 cells overexpressing the human mature secreted form of IL-1α, we amplified sequences corresponding to amino acids 122 to 271 of full-length IL-1α 23 by polymerase chain reaction (PCR) and cloned it into BamHI-XbaI sites of the pcDNA3 or the modified pCMV4 vector (pCMV4 vector has an alfalfa mosaic virus translation enhancer, which increases translation efficiency). MCF-7 cells were transfected with these vectors and grown in the presence of G418 (600 μg/ml) to select transfected cells. G418-resistant colonies were isolated and grown individually.
Implantation of Cells into Mammary Fat Pads of Nude Mice, Tumor, and Animal Weight Measurements
All animal studies were performed with the approval from Institutional Animal Care and Use Committee and as per the National Institutes of Health guidelines. MCF-7 breast cancer cells with or without HLF-1 were injected into the mammary fat pads of 6- to 8-week-old nu/nu mice (Harlan Sprague Dawley, Indianapolis, IN) as described previously. 24 Eight to ten animals per group were used in every experiment described in the text and experiments were repeated three times. Estrogen pellets (17β estradiol, 0.72 mg/pellet, 60 day release; Innovative Research of America, Sarasota, FL) were implanted a day before tumor cell injection. Tumor growth was measured once a week using a caliper (in mm) and tumor weight (mg) was calculated using the formula tumor weight (mg) = (a 2 × b)/2 where a is the width in mm and b is the length in mm. 25,26 Actual tumor weight was also measured at the time of sacrifice to further confirm the results obtained with the above formula. Animal weight was measured once weekly and final body weight was calculated after subtracting tumor weight.
Measurement of Blood Glucose, Serum Cytokines, Triglycerides, Leptin, and Calcium
Blood glucose was measured using the Accu-Check advantage blood glucose monitor (Roche, Indianapolis, IN). Serum was collected at the time of sacrifice. Serum cytokines were measured using LINCOplex multiplex immunoassay system (Linco Research, Inc., Missouri, MO). This assay system is highly sensitive and measures cytokines as low as 3.2 pg/ml of serum (www.lincoresearch.com). Leptin was also measured similarly. Serum calcium was measured as described previously. 27 IL-1α was measured using an enzyme-linked immunosorbent assay from R&D Systems as per the manufacturer’s recommendation (R&D Systems, Minneapolis, MN). For enzyme-linked immunosorbent assay, 1 × 106 cells were plated for 2 days in 60-mm plates. After washing in PBS, cells were incubated with 5 ml of serum-free media for 24 hours and media was analyzed for IL-1α. The sensitivity of the assay was 3.9 pg/ml.
Electrophoretic Mobility Shift Assays, Western Blotting, Northern Blotting, and Gelatin Zymography
Electrophoretic mobility shift assay with whole cell extracts was performed as described previously. 28 For Western blotting, finely minced thigh muscle was resuspended in radioimmunoassay buffer (RIPA, 50 mmol/L Tris, pH 7.5, 0.25% sodium deoxycholate, 1% Nonidet P-40, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 100 μmol/L sodium orthovanadate, 1 mmol/L sodium fluoride, 1 mmol/L β-glycerophosphate, 0.5 mmol/L phenylmethyl sulfonyl fluoride, 2 μg/ml aprotinin, leupeptin, and pepstatin) and homogenized. Soluble protein was used for Western blotting as described previously. 29 Northern blotting for IL-8 expression was performed as described previously. 28 For gelatin zymography, conditioned media (CM) from 1 × 105 cells plated overnight were used. For co-culturing, an equal number of cancer cells and HLF-1 were used. Zymography was performed as described previously. 30
Measurement of Proteasomal Activity
Thigh muscle collected at the time of sacrifice was homogenized in buffer Y (50 mmol/L Tris, pH 7.4, 250 mmol/L NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, and 1 mmol/L ethylenediaminetetraacetic acid) 31 and 20S proteasome activity was measured using an assay kit from Chemicon International (Temecula, CA).
Analysis of Micrometastasis by PCR of Lung DNA
Lung DNA was isolated and subjected to PCR analysis as described by Endo and colleagues. 32 One μg of DNA was subjected to 24 cycles of PCR using primers 5′AGAGCCATCTATTGCTTACA3′ and 5′TATGACATGAACTTAACCAT3′. PCR products were identified by Southern blotting using the internal primer 5′ACACAACTGTGTTCACTAGC3′ as a probe.
Analysis of Tumors for the Expression of Leptin and Lipid-Mobilizing Factor (LMF)
RNA from flash-frozen tumors was isolated by RNAzol (Tel-Test Inc., Friendswood, TX). Five μg of RNA was reverse-transcribed using random primers and reverse transcription (RT)-PCR kit (Stratagene, La Jolla, CA). Reverse-transcribed RNA (1/25 volume) was subjected to PCR using specific primers. Primers used for amplification of leptin were 5′GGAGACCCCTGTGTCGGTTC3′ and 5′TCCAGGCTCTCTGGCTTCTG3′; the internal primer used was 5′GATGACACCAAAACCCTCATC3′. The primers used for LMF were 5′CTGTCCTGCTGTCTCTGCTG3′ and 5′TGGGCTGAGACTTCCTGTCT3′; the internal primer used was 5′CTCCACTGGGCTGTCCAAGC3′. GAPDH primers that can amplify both human and mouse GAPDH were 5′GAGGACCAGGTTGTCTCC3′ and 5′CCTTGGAGGCCATGTAGG3′. Southern blotting was performed as described previously. 10
Quantitative Measurements and Statistical Analysis
Expression levels of MyoD and ubiquitinated proteins were quantitated by densitometric scanning of Western blots. Data were analyzed with GD-STAT or Graphpad softwares. Analysis of variance was used to determine P values between mean measurements. A P value of <0.05 was deemed significant. Error bars on all graphs represent standard errors between measurements.
Results
Generation and Analysis of MCF-7 Breast Cancer Cells Overexpressing IL-1α
To study the effect of the secreted form of IL-1α on growth of breast cancer cells in nude mice, we generated a mammalian expression vector that codes for only the mature secreted form (amino acids 122 to 271) of human IL-1α (pcDNA3-IL-1α). 23 The expression of the secreted form of IL-1α in MCF-7 cell colonies obtained after transfection with either expression vector alone (pcDNA3-1, 2, and 3) or IL-1α expression vector (MCF-7IL-1α-4, 5, and 6) was measured by enzyme-linked immunosorbent assay. No measurable IL-1α could be detected in the CM of pcDNA3 clones. In contrast, IL-1α5 and IL-1α6 CM contained 100 and 24 pg/ml of IL-1α, respectively. We also measured NF-κB DNA-binding activity in these cells because IL-1α expression should lead to autocrine activation of NF-κB. 33 Indeed, active NF-κB protein levels, as measured by DNA-binding activity, were elevated in MCF-7IL-1α cells compared to pcDNA3 cells (Figure 1A) ▶ . NF-κB activation in cancer cells correlated with increased expression of proinvasive and prometastatic genes IL-8 (data not shown) and CXCR4. 34 However, MCF-7IL-1α cells failed to express the NF-κB regulated prometastatic gene uPA, which could be because of methylation of the uPA promoter in these cells. 35
Figure 1.
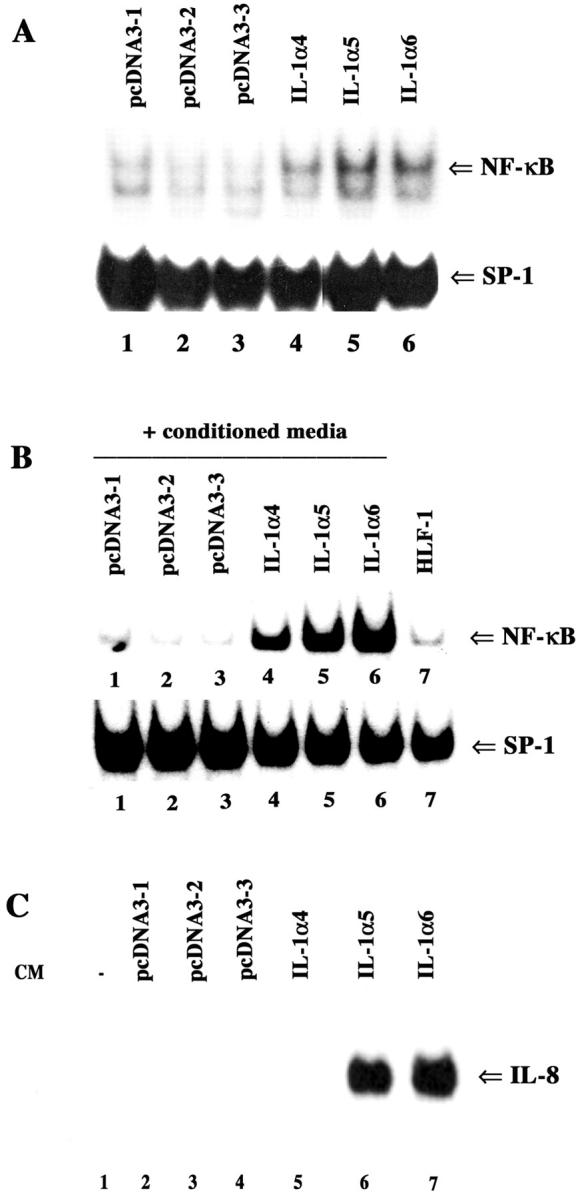
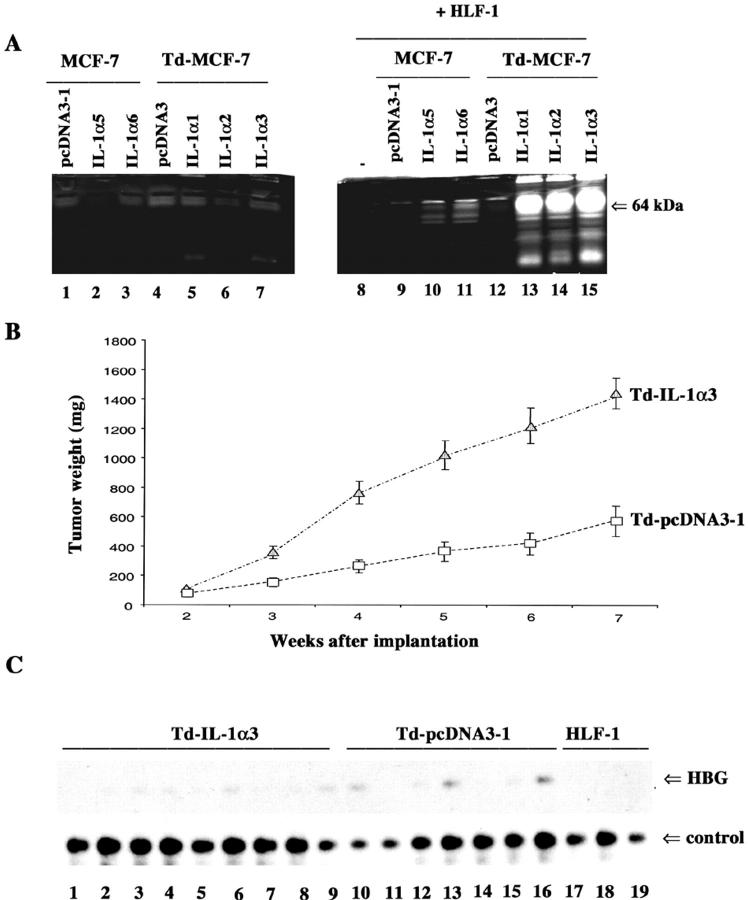
Generation of IL-1α-overexpressing MCF-7 cells. A: NF-κB DNA-binding activity in MCF-7 cells transfected with pcDNA3 or IL-1α expression vector. Individual G418-resistant colonies were examined for NF-κB DNA-binding activity by electrophoretic mobility shift assay. DNA binding of the general transcription factor SP-1 is also shown. B: Induction of NF-κB in HLF-1 by the CM from pcDNA3 and IL-1α-overexpressing clones. HLF-1 cells were incubated with CM from indicated cell lines for 1 hour. NF-κB and SP-1 DNA-binding activities were measured as described above. CM from ∼90% confluent cells was collected after overnight incubation in serum-free media. C: Induction of IL-8 in HLF-1 cells by CM from various cell types. HLF-1 was incubated with CM for 4 hours and IL-8 expression was measured by Northern blotting of total RNA.
To further confirm that the secreted form of IL-1α is biologically active, we treated HLF-1 with CM from pcDNA3 or MCF-7IL-1α cells. CM from MCF-7IL-1α but not pcDNA3 cells induced NF-κB in HLF-1 (Figure 1B) ▶ . The NF-κB:DNA complex obtained with HLF-1 cell extracts was a heterodimer of p65:p50 subunits of NF-κB as determined by antibody supershift assay (data not shown). Induction of NF-κB in HLF-1 by CM of MCF-7IL-1α cells correlated with increased expression of the NF-κB responsive gene IL-8 (Figure 1C) ▶ .
To determine the influence of IL-1α on growth of MCF-7 cells in nude mice, we implanted pcDNA3-1, IL-1α5, and IL-1α6 cells (5 × 106) into mammary fat pads with or without estrogen pellets. Tumors were obtained only in animals with estrogen pellet implants, which were in general larger in animals implanted with MCF-7IL-1α cells. Because tumor intake was not uniform, we isolated tumors from animals and grew them in culture in the presence of G418. All animal experiments described below were performed with cells that have been passed through nude mice once. This approach has been used by a number of investigators to improve tumor intake in xenograft models. 3,36,37
Properties of Tumor-Derived pcDNA3 and IL-1α Cells
We first determined IL-1α expression in CM of tumor-derived cell lines (named Td-pcDNA3 and Td-IL-1α) by enzyme-linked immunosorbent assay. IL-1α levels in CM of Td-pcDNA3-1, Td-IL-1α1, Td-IL-1α2, and Td-IL-1α3 were 0, 218 ± 4, 293 ± 15, and 288 ± 4 pg/ml, respectively. Reasons for the marked increase in IL-1α expression in tumor-derived IL-1α clones compared to the original IL-1α clones in culture are not known. Although parental IL-1α-overexpressing cells were of clonal origin, some cells may have lost IL-1α expression during prolonged culture because of lack of selection pressure. In contrast, clonal selection of high IL-1α-expressing cells in animals may have contributed to elevated IL-1α expression in Td-IL-1α cells.
To determine the autocrine activity of IL-1α, we measured NF-κB DNA-binding activity in tumor-derived cell lines and found that only Td-IL-1α cells contained constitutive NF-κB DNA-binding activity (Figure 2A ▶ , lanes 1 to 4). Furthermore, CM from only Td-IL-1α cells induced NF-κB DNA-binding activity in HLF-1 (Figure 2A ▶ , lanes 5 to 9). Neutralizing antibody against IL-1α but not leukemia inhibitory factor blocked Td-IL-1α1 cell CM-mediated NF-κB activation in HLF-1 (Figure 2B) ▶ . Induction of NF-κB by CM in HLF-1 correlated with increased expression of IL-6, IL-8, and uPA in these cells (data not shown).
Figure 2.
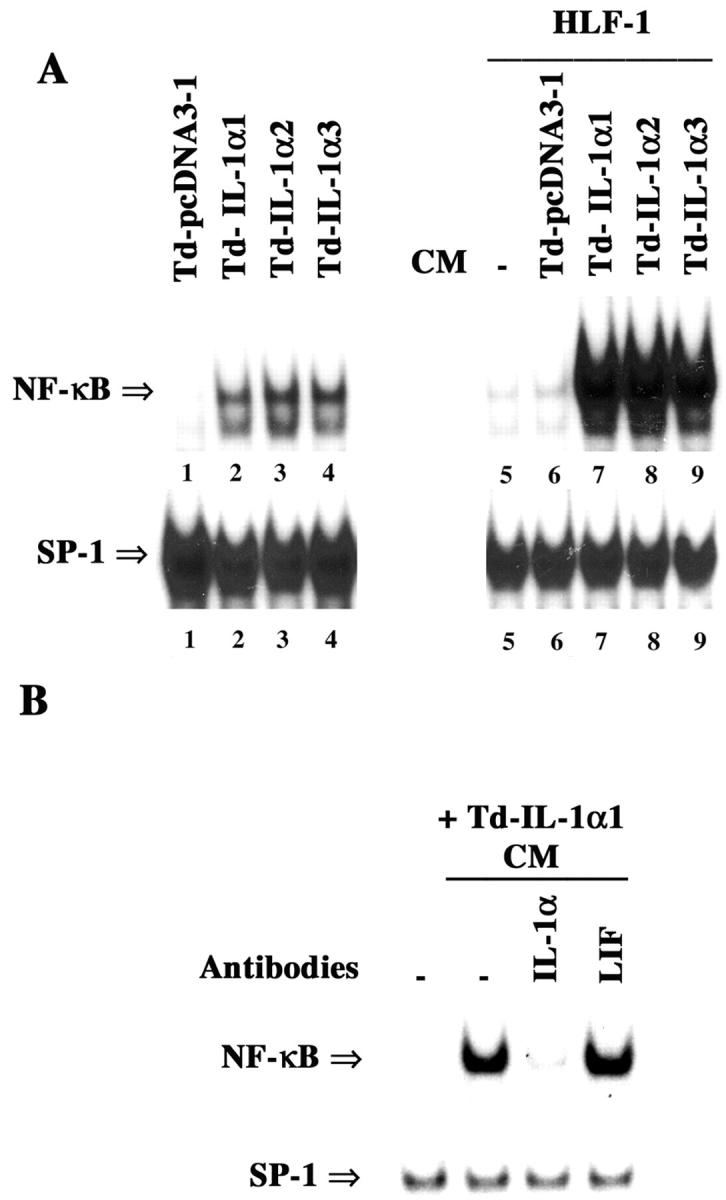
Properties of tumor-derived pcDNA3- and IL-1α-overexpressing clones. A: NF-κB DNA-binding activity in pcDNA3- and IL-1α-overexpressing cells isolated from tumors and grown in culture (lanes 1 to 4). CM from the same cells was tested for their ability to induce NF-κB in HLF-1 cells (lanes 5 to 9). NF-κB DNA binding was measured as in Figure 1A ▶ . B: Neutralizing antibody against IL-1α blocks NF-κB activation by Td-IL-1α1 CM. CM was treated with the indicated neutralizing antibodies (3 μg/ml) for 1 hour at room temperature. Cells were treated with CM for 1 hour.
Td-pcDNA3-1 and Td-IL-1α3 cells (5 × 106) were implanted into the mammary fat pads with or without estrogen pellets. No tumors were obtained with either cell type in the absence of estrogen. Thus, IL-1α cannot confer hormone-independent growth properties to MCF-7 cells. Similarly, MCF-7 cells grown in the presence of exogenous IL-1α failed to become hormone-independent (data not shown). Both Td-pcDNA3-1 and Td-IL-1α3 cells formed tumors in all animals with estrogen pellet implants. After 7 weeks of implantation, tumor weight for the Td-IL-1α3 and Td-pcDNA3-1 groups was 2042 ± 383 mg and 1044 ± 297 mg, respectively. Thus, it seems that IL-1α enhances the rate of tumor growth. Enhanced growth of Td-IL-1α3-derived tumors is less likely because of the increased sensitivity of these cells to estrogen, at least at the genomic level because estrogen receptor activity was similar in both Td-pcDNA3-1 and Td-IL-1α3 cells in in vitro assays (data not shown). Although Td-IL-1α3-derived tumors grew faster, hematoxylin and eosin (H&E) staining of lungs collected at the time of sacrifice did not reveal any metastasis. Also, a similar level of micrometastasis to lungs, as determined by PCR analysis of lung DNA for the presence of human β-globin sequences, 32 was observed with both groups (data not shown). Lack of metastasis of Td-IL-1α3-derived tumor is not because of silencing of the transfected IL-1α gene in the tumor because the serum of animals with Td-IL-1α3-derived tumors but not Td-pcDNA3-1-derived tumors had measurable IL-1α (15 to 69 pg/ml). Despite the lack of metastasis, weight loss was observed in animals with Td-IL-α3-derived tumors (19.3 ± 1.1 g) compared to animals with Td-pcDNA3-1-derived tumors (22.3 ± 0.9 g). Note that animals in both groups were of similar weight at the time of tumor cell implantation.
The Effect of Fibroblasts on Growth of Td-IL-1α3 and Td-pcDNA3-1 Cells in Nude Mice
The failure of Td-IL-1α3-derived tumors to metastasize could be because of the inability of these tumors cells to recruit stromal cells, which provide matrix metalloproteinases (MMPs) required for invasion and metastasis. 38 To test this possibility, we first determined MMP activity in CM of parental pcDNA3 and IL-1α clones, Td-pcDNA3-1 and Td-IL-1α clones, and all cell types co-cultured with HLF-1 using gelatin zymography. 30 CM of pcDNA3, HLF-1, or IL-1α clones showed very little MMP activity (Figure 3A ▶ , lanes 1 to 8). MMP activity was modestly higher in the CM of Td-pcDNA3-1 and HLF-1 co-culture compared to pcDNA3 and HLF-1 co-culture (Figure 3A ▶ , compare lanes 9 and 12). This increase in MMP activity in CM of Td-pcDNA3-1 is independent of IL-1α because no measurable IL-1α could be detected in CM of both pcDNA3 and Td-pcDNA3-1 clones. CM of Td-IL-1α cells co-cultured with HLF-1 displayed very strong MMP activity (Figure 3A ▶ , lanes 9 to 15). Based on the molecular weight, it appears that secreted MMPs correspond to MMP-9 and MMP-2, which are secreted by mammary epithelial cells. 39 Neutralizing antibody against IL-1α reduced MMP activity suggesting that IL-1α is required for MMP activity (data not shown). At present it is not clear whether MMPs are produced by Td-IL-1α cells or HLF-1 cells. However, a similar study published recently indicates that fibroblasts but not MCF-7 cells produce MMP-2 and MMP-9 under co-culture conditions. 40 Co-culturing is required for MMP production as incubating HLF-1 cells with CM of Td-IL-1α cells or vice versa did not result in significant increase in MMP activity (data not shown). No uPA activity was detected under any culture conditions when zymography was performed using human plasminogen as a substrate (data not shown).
Figure 3.
MMP activation and growth of Td-pcDNA3-1 and Td-IL-1α3 cells in the presence of HLF-1 cells. A: Gelatin zymography using CM from either cancer cells alone (lanes 1 to 7), HLF-1 (lane 8), or cancer cells in combination with HLF-1 cells. B: Rate of tumor growth. Cancer cells (2 × 106) with or without HLF-1 cells (2 × 105) were injected into the mammary pads of nude mice with estrogen pellet implants. Tumor growth was measured once a week. C: Analysis of lung DNA for metastasis of cancer cells. DNA from lungs was isolated and subjected to PCR with human β-globin-specific primers. As a control, PCR was also performed with primers that amplify mouse requiem genomic DNA. 77 Southern blotting using an internal primer identified PCR products.
We implanted both Td-pcDNA3-1 and Td-IL-1α3 cells with or without HLF-1 cells because there was specific increase in MMP activity in co-cultured cells. Unlike in experiments described above, the number of tumor cells was reduced to 2 × 106 per animals with or without 2 × 105 HLF-1. Tumors derived from Td-IL-1α3 cells with or without HLF-1 grew much faster than Td-pcDNA3-1 cells and HLF-1 did not significantly alter the growth rate. Therefore, Figure 3B ▶ shows combined tumor growth rate of three independent experiments with or without HLF-1 (n = ∼30). With lower numbers of implanted cells compared to the previous experiment, differences in growth rates between Td-pcDNA3-1 and Td-IL-1α3-derived tumors are more apparent. The failure of HLF-1 to provide additional growth advantage to Td-IL-1α3 cells in vivo could be because of IL-1α-dependent recruitment and/or growth of mouse-derived stromal cells. Growth-stimulating ability of IL-1α was manifested within the tumor microenvironment but not in vitro because pcDNA3, IL-1α, Td-pcDNA3, and Td-IL-1α clones grew at a similar rate in culture (data not shown). H&E staining of tumors revealed extensive central necrosis in Td-IL-1α3 cell-derived tumors (data not shown). Despite enhanced growth of Td-IL-1α3 cell-derived tumors, H&E staining of lungs did not reveal metastasis of either cell type. Furthermore, PCR analysis of lung DNA for human β-globin gene revealed similar levels of micrometastasis of Td-IL-1α3 and Td-pcDNA3-1 tumor cells (Figure 3C) ▶ . Thus, IL-1α expression alone is not sufficient to promote metastasis of MCF-7 cells in an in vivo setting. However, we cannot rule out IL-1α-dependent metastasis of other breast cancer cell types.
IL-1α Expression Leads to Severe Cachexia
Mice with Td-IL-1α3-derived tumors showed lordokyposis (hunchback spine), which is an early aging-associated phenotype in mice. 41 A significant progressive weight loss was observed in animals injected with Td-IL-1α3 cells compared to animals injected with Td-pcDNA3-1 cells (P = 0.0003) (Figure 4A) ▶ . Animals in all three groups (nontumor and Td-pcDNA3-1- and Td-IL-1α3-derived tumor containing group) grew for first 3 weeks after implantation. Although the weight of animals remained steady during rest of the study in nontumor and Td-pcDNA3-1 implanted animals, Td-IL-1α-implanted animals displayed progressive loss of weight. Thus, loss of body weight is an active cachetic process but not simply because of IL-1α-induced arrest of growth. Note that all animals were of the same age group and weight at the time of tumor cell implantation. We performed H&E staining of dorsal skin to further analyze cachexia. Skin of Td-IL-1α tumor-bearing mice showed diffused atrophy of both epidermis and adnexal structures (hair follicle buds and sweat glands) compared to animals with Td-pcDNA3 tumor-bearing animals (Figure 4B) ▶ . Similar skin abnormalities have been observed in ob/ob mice injected with leptin and in mice lacking acyl coA:diacylglycerol acyltransferase, which is essential for triglyceride synthesis. 42 Skin abnormalities are frequently observed during premature aging and cachexia. 41 Skin abnormalities detected with Td-IL-1α tumor-bearing mice closely resembled the prematurely aged skin of mice overexpressing dominantly acting p53 but not that of mice with defects in DNA repair. 41,43
Figure 4.
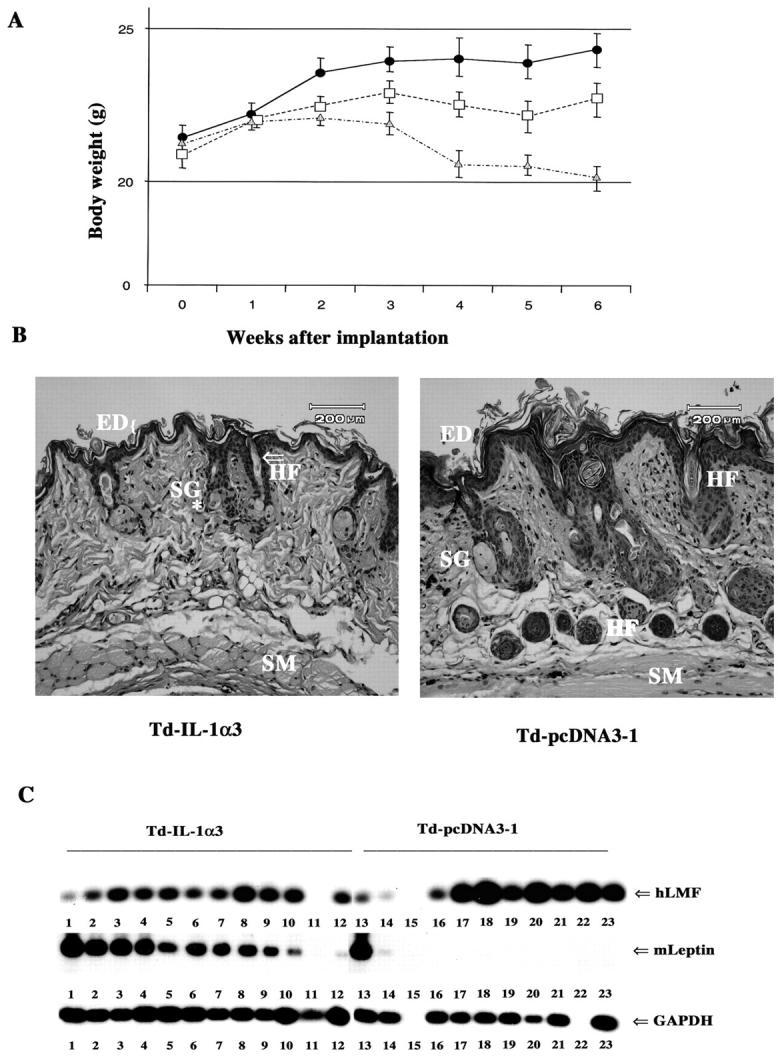
The effect of IL-1α expression in cancer cells on body weight and mouse leptin transcripts. A: Weight of animals implanted with Td-pcDNA3-1 (square) or Td-IL-1α3 (triangle) (n = ∼30). Weight of animals without any tumor is also shown (circle). P = <0.0001 nontumor versus Td-IL-1α3; P = 0.0003 Td-pcDNA3-1 versus Td-IL-1α3; P = 0.0839 nontumor versus Td-pcDNA3-1. B: Skin phenotype of Td-pcDNA3 and Td-IL-1α tumor-bearing animals. Cross sections of dorsal skin show atrophy in Td-IL-1α tumor-bearing animals compared to Td-pcDNA3 tumor-bearing animals. SM, skeletal muscle; HF, hair follicle; SG sebaceous glands; ED, epidermis. C: Human LMF and mouse leptin-specific transcript levels in tumor samples. Total RNA from tumors was subjected to RT-PCR (35 cycles) using primers that specifically amplify mouse leptin RNA. Primers corresponding to human LMF were used to amplify LMF (35 cycles). Quality of RNA as well as cDNA synthesis was verified by PCR amplification of the housekeeping gene GAPDH (18 cycles). Southern blotting of PCR products with an internal primer as a probe and autoradiography identified PCR products. Because of limited amplification, PCR products were not visible by ethidium bromide staining.
Animals with Td-IL-1α3-Derived Tumors Contain Elevated Serum Leptin
To investigate whether IL-1α-induced cachexia correlates with any changes in metabolic pathways, we measured glucose, triglycerides, calcium, and leptin levels in blood/serum. Blood glucose was measured twice (after 6 weeks of implantation and at the time of sacrifice) whereas calcium, triglycerides and leptin were measured once at the time of sacrifice. There was no significant difference in blood glucose between Td-IL-1α3 and Td-pcDNA3-1 groups (Table 1) ▶ . In contrast, the Td-IL-1α3 group showed lower levels of triglycerides compared to the Td-pcDNA3-1 group. The differences were highly significant (P = 0.0007). Altered triglyceride levels have been reported in cachexia patients. 44,45 Higher levels of serum calcium were detected in the Td-IL-1α3 group compared to animals in the Td-pcDNA3-1 group (Table 1) ▶ . Most importantly, leptin levels were higher in serum of animals in the Td-IL-1α3 group compared to animals in the Td-pcDNA3-1 group (Table 1) ▶ . Higher leptin levels have been detected in serum of breast cancer patients and in breast cancer specimens and elevated leptin levels correlated with cachexia parameters. 46,47
Table 1.
Blood Glucose, Triglycerides, Serum Calcium, Leptin Level, Food Intake, and 20S Proteasome Activity
| Td-IL-1α group | Td-pcDNA3 group | p Values | |
|---|---|---|---|
| Blood glucose (mg/dl) | 75.4 ± 4.4 | 78.63 ± 4.3 | 0.6233 |
| Triglycerides (mg/dl) | 55.5 ± 5.3 | 85.9 ± 5.2 | 0.0006 |
| Serum calcium (mg/dl) | 10.4 ± 1.5 | 9.2 ± 1.4 | 0.0423 |
| Leptin (ng/ml) | 1.95 ± 0.18 | 1.03 ± 0.32 | 0.0133 |
| 20S Proteasome activity | 19.5 ± 0.8 | 23.1 ± 1.2 | 0.0512 |
| Food intake (g/mouse/day) | 6.3 ± 0.3 | 4.7 ± 0.3 | 0.0032 |
Blood glucose was measured twice during the course of the experiment and average with standard error from both experiments is presented. Triglycerides, calcium, and leptin were measured in serum collected at the time of sacrifice. Proteasome activity is expressed as arbitrary units.
Leptin has been shown to control food intake by acting through the central nervous system. 48 It is possible that cachexia in Td-IL-1α tumor-bearing animals is an indirect consequence of leptin-mediated decrease in food intake. To test this possibility, we measured food intake twice weekly between weeks 4 and 7 after implantation. To our surprise, food consumption was modestly higher in Td-IL-1α3 group compared to Td-pcDNA3-1 group (Table 1) ▶ . Therefore, loss of body weight in Td-IL-1α3 tumor-bearing animals is not because of anorexia.
Tumors from the Td-IL-1α3 Group Contain Higher Levels of Leptin but Not LMF Transcripts
Leptin is generally secreted by adipocytes. However, examination of the skin (Figure 4B) ▶ or abdomen did not reveal any increase in adipocytes in animals with Td-IL-1α3 compared to animals with Td-pcDNA3-1-derived tumors. Therefore, the tumor itself is the likely source of leptin. To test this possibility, we performed RT-PCR analysis of RNA from tumor tissue for human and mouse leptin. PCR-amplified products were not detected with primers that specifically amplify human leptin. In contrast, mouse leptin transcripts could be detected in RNA from Td-IL-1α3 groups but not Td-pcDNA3-1 groups (Figure 4C) ▶ . Similar results were obtained when PCR was performed with a different set of primers (data not shown). H&E staining of tumor samples failed to detect any adipocytes in tumors of both Td-IL-1α3 or Td-pcDNA3-1 groups (data not shown). These results suggest that IL-1α produced by tumor cells recruits nonadipocyte cells that can produce leptin.
Previous studies have shown that altered lipid metabolism in cancer patients is mediated by LMF. 49 LMF, which is identical to Zn-α2-glycoprotein, is expressed in breast cancer cells. 50 To determine whether IL-1α directly modulates LMF expression, we measured human LMF expression by RT-PCR. LMF does not appear to be the direct target of IL-1α (Figure 4C) ▶ . In fact, LMF transcripts appear to be higher in the Td-pcDNA3-1 group compared to the Td-IL-1α3 group.
Loss of Body Weight in Animals with Td-IL-1α3-Derived Tumors Is Not Associated with Increased Levels of Other Known Inducers of Cachexia
NF-κB has been proposed to promote cachexia by down-regulating MyoD mRNA. 51 Because IL-1α can potentially reduce MyoD through activation of NF-κB, we measured the level of MyoD proteins in muscle by Western blotting (Figure 5A) ▶ . Densitometric scanning analysis showed that MyoD protein levels tended to be lower in muscles of animals with Td-IL-1α3-derived tumors compared to Td-pcDNA3-1-derived tumors (P = 0.0542). Recent reports indicate that ubiquitination of proteins in muscle is increased during cachexia, particularly when cachexia is mediated by proteolysis-inducing factor (PIF). 52,53 However, the level of ubiquitinated proteins was similar in both Td-IL-1α3 and Td-pcDNA3-1 groups (Figure 5A) ▶ . Also, the muscle from both groups lacked myostatin/GDF8, which has recently been proposed to be a major cachexia-inducing protein expressed in muscle (data not shown). 54 We measured 20S proteasome activity in the muscle of Td-pcDNA3-1 and Td-IL-1α3 tumor-bearing animals to further clarify the role of PIF in cachexia. Proteasomal activity was similar in both groups, which rules out the involvement of PIF in IL-1α-induced cachexia (Table 1) ▶ .
Figure 5.
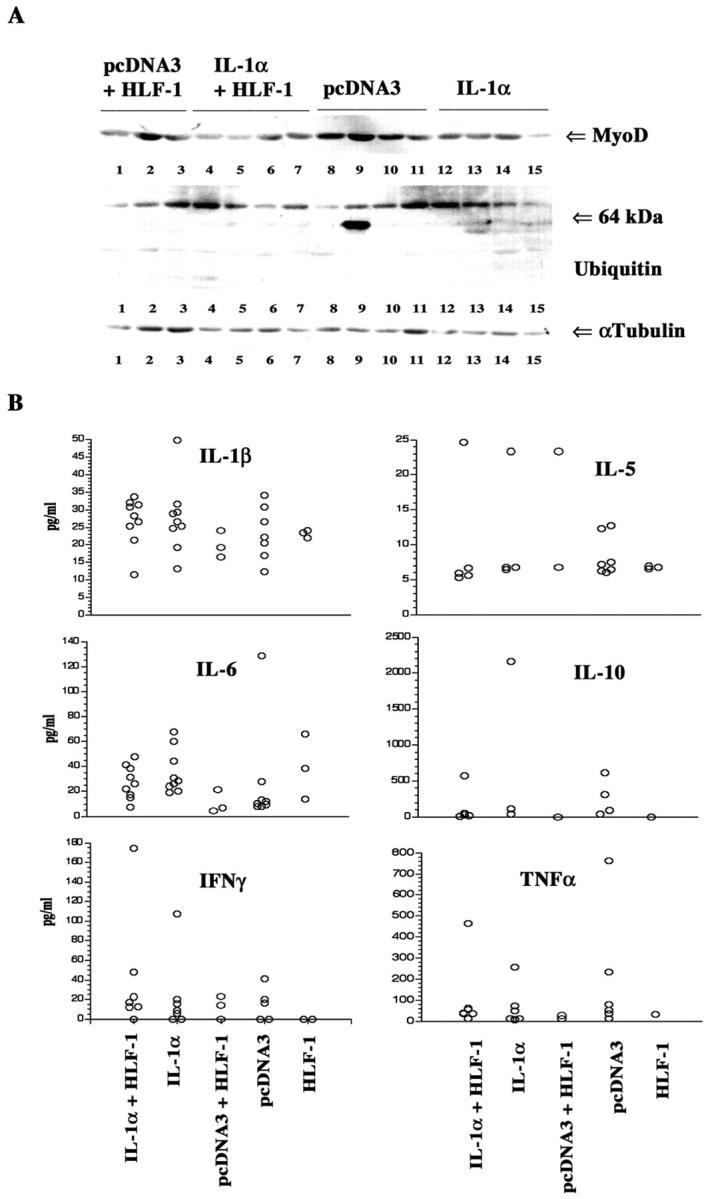
The effect of IL-1α expression in cancer cells on muscle protein status and serum cytokine profile. A: Differences in the level of MyoD, ubiquitinated protein, and α-tubulin in leg muscle. Muscle extracts were prepared using RIPA buffer and 50 μg of protein was subjected to Western blotting with indicated antibodies. B: Profile of various cytokines in serum of animals implanted with different cell types.
Several cytokines including TNF-α and IL-6 induce cachexia and both TNF-α and IL-6 can be induced by IL-1α through activation of NF-κB. 55,56 To test whether any of these circulating cytokines are elevated in animals with Td-IL-1α3-derived tumors compared to animals with Td-pcDNA3-1-derived tumors, serum was subjected to LINCOplex cytokine multiplex immunoassay. The assay simultaneously measures the level of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, interferon (IFN)-γ, GM-CSF, and TNF-α with a sensitivity of 3.2 pg/ml of serum. Only those cytokines that are present in most of the animals are shown in Figure 5B ▶ . There were no significant differences in any of the cytokines tested although the IL-6 level appears to be slightly elevated in the Td-IL-1α3 group. Note that the difference in IL-6 is statistically significant if one animal in the Td-pcDNA3-1 group, which showed unusually high level of IL-6, is excluded from the calculation. Taken together, it appears that IL-1α-induced weight loss is less likely because of up-regulation of TNF-α and IL-6 by IL-1α.
Discussion
IL-1s are present in abundance in the tumor microenvironment and are believed to play a role in growth, invasiveness, and anti-tumor immunity. 57 Anti-tumor activity of IL-1α has been demonstrated for lymphoid tumors and fibrosarcoma using an animal model. 18,58 Membrane-associated form of IL-1α but not the secreted form is believed to initiate anti-tumor immunity. 18 Because IL-1α is overexpressed in ductal carcinoma in situ and in invasive but not in benign mammary tumors, it is likely that breast cancer cells have somehow overcome the anti-tumor activity of IL-1α, as with many cancers. 11,59 Furthermore, IL-1α expression is observed mostly in estrogen receptor α-negative breast cancer, which are usually more invasive and metastatic and is associated with poor prognosis. 60 It is suggested that IL-1α is important in regulating protumorigenic activities within the tumor microenvironment. 60 To understand the effect of IL-1α overexpression on cancer progression in vivo, we used the nude mice model. Despite limitations of this model, we observed two major effects of IL-1α, one on the growth of tumor and the other on the metabolic status. When this manuscript was under revision, Voronov and colleagues 61 reported a similar finding using IL-1α knockout animals. IL-1α is required for tumor invasiveness and angiogenesis. Growth stimulation by IL-1α appears to depend on tumor cell-stromal cell interaction because parental and IL-1α-overexpressing cells grew at a similar rate in vitro (data not shown). The growth-promoting factors induced as a consequence of tumor cell-stromal cell interaction remain to be identified. One possible candidate is leptin, whose expression is increased in animals with IL-1α-producing tumors. Leptin has been shown to increase the proliferation of MCF-7 cells in vitro. 62 Leptin also increases endothelial cell proliferation, which can lead to increased angiogenesis and tumor cell proliferation. 48,63
A major observation of our study is cachexia in animals injected with IL-1α-overexpressing cancer cells. Cachexia was accompanied with changes in the skin architecture, which resembled that of premature aging. Cachexia is generally a consequence of loss of lipids, enhanced proteolysis or both, although lipid depletion occurs out of proportion to the protein loss. 64,65 IL-6, IL-8, TNF-α, IFN-γ, leukemia inhibitory factor, myostatin/GDF8, PIF, LMF, and toxohormone are some of the factors involved in cachexia. 54,55,66,67 IFN-γ in combination with TNF-α has been shown to induce cachexia through NF-κB-dependent destabilization of MyoD mRNA in muscle. 51 We were unable to measure all of these factors in serum because of limited sample availability or lack of commercially available antibodies. However, among the factors measured, we did not see any significant differences between Td-IL-1α3 and Td-pcDNA3-1 groups. Moreover, differences in MyoD were marginal with no difference in ubiquitinated proteins and proteasome activity in muscle between groups, which rules out the involvement of IFN-γ, TNF-α, and PIF in cachexia in our model. 53,68
Cachexia in the Td-IL-1α3 group correlated with elevated leptin level. Elevated leptin is observed in breast cancer patients with cachexia and increased leptin-like signaling by cytokines is the hallmark of cachexia. 46,69 Similarly, elevated leptin is linked to cachexia in patients with chronic heart failure. 70 Leptin is secreted mainly by adipocytes. However leptin expression in nonadipocytes, including breast cancer cell lines, has been observed and its expression is IL-1α-inducible. 47,71-73 Histological analysis of tumors did not indicate any effect of IL-1α on adipocyte content in the tumor (data not shown). RT-PCR analysis of tumor RNA with human leptin-specific primers failed to detect human leptin (data not shown). In contrast, mouse-specific leptin transcripts could be detected in total RNA of tumors derived from Td-IL-1α3 cells (Figure 4) ▶ . Thus, IL-1α increases leptin expression in mouse-derived stromal cells or in infiltrating immune cells. Interestingly, Td-IL-1α3 tumor-bearing animals were not anorexic despite elevated leptin levels. Thus, cachexia is not a consequence of reduced food intake, which is consistent with some of the clinical observations. 65 It is recognized recently that leptin acts both at the central nervous system and at the peripheral level. 48 While action at the central nervous system controls food intake, action at the periphery controls insulin action, glucose transport, lipogenesis, and lipid partitioning. For example, leptin directly inhibits de novo synthesis of fatty acids and increases the release and oxidation of fatty acids in adipocytes. 48,74 Moreover, leptin reduces incorporation of oleate into triglycerides, 48 which can explain for reduced triglycerides and body weight in Td-IL-1α3 tumor-bearing animals. It is interesting that LIF, which induces cachexia by mobilizing lipids, causes a modest decrease in triglyceride levels in leptin-sensitive wild-type mice but not in leptin-deficient ob/ob mice. 49 Based on the recent realization that leptin biology is much more complex than originally envisioned, 48 we propose that leptin is a central player in cachexia involving impaired lipid metabolism. At present, we cannot conclude that leptin alone is responsible for enhanced tumor growth and cachexia in mice implanted with Td-IL-1α3 cells. Additional studies with neutralizing antibodies against leptin are essential, which we believe is beyond the scope of this investigation. This study at least provides a basis for future investigation in this direction.
One of the surprising observations is the failure of IL-1α-overexpressing tumor cells to metastasize, although in vitro studies supported such a possibility. Failure of IL-1α to initiate metastasis could be because of expression of a dominant metastasis suppressor gene in MCF-7 cells or alternatively, genes that initiate metastasis are not expressed in these cells or are not induced by IL-1α. In this regard, it was shown recently that loss of metastasis suppressor gene expression is essential for metastatic progression of prostate cancer. 75 Also, it was reported that the promoter of uPA is methylated in MCF-7 cells, thus making it inaccessible to IL-1α-induced NF-κB. 35 uPA is one of the major proteases involved in initiation of metastasis. 76 It will be interesting to determine whether enforced expression of uPA in Td-IL-1α3 cells can initiate metastasis in vivo.
Acknowledgments
We thank P. Bhat-Nakshatri, C. Stauss, and J. Dunn for technical assistance; Y. C. Yang for IL-1α cDNA; Suzan C. Hufferd, Gregory Reid Gibson, Ronald McClintock, and Mark Deeg for serum cytokine, calcium, and leptin measurements; and Andrea Carperell-Grant and Robert Harris for advice.
Footnotes
Address reprint requests to Harikrishna Nakshatri, R4-202 Indiana Cancer Research Institute, 1044 West Walnut St., Indianapolis, IN 46202. E-mail: hnakshat@iupui.edu.
Supported by the National Cancer Institute (Public Services award CA-82208 and CA-89153) and the American Institute for Cancer Research (grant 00A047 to H. N.).
S. K. and H. K. contributed equally to this study.
References
- 1.Liotta LA, Stetler-Stevenson WG, Steeg PS: Cancer invasion and metastasis: positive and negative regulatory elements. Cancer Invest 1991, 9:543-551 [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA: An attractive force in metastasis. Nature 2001, 410:24-25 [DOI] [PubMed] [Google Scholar]
- 3.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A: Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410:50-56 [DOI] [PubMed] [Google Scholar]
- 4.Lewis CE, Leek R, Harris A, McGee JO: Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol 1995, 57:747-751 [DOI] [PubMed] [Google Scholar]
- 5.van Eys J: Nutrition and cancer: physiological interrelationships. Annu Rev Nutr 1985, 5:435-461 [DOI] [PubMed] [Google Scholar]
- 6.Leek RD, Harris AL, Lewis CE: Cytokine networks in solid human tumors: regulation of angiogenesis. J Leukoc Biol 1994, 56:423-435 [DOI] [PubMed] [Google Scholar]
- 7.Tisdale MJ: Wasting in cancer. J Nutr 1999, 129:243S-246S [DOI] [PubMed] [Google Scholar]
- 8.Baracos VE: Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition 2000, 16:1015-1018 [DOI] [PubMed] [Google Scholar]
- 9.Wolf JS, Chen Z, Dong G, Sunwoo JB, Bancroft CC, Capo DE, Yeh NT, Mukaida N, Van Waes C: IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res 2001, 7:1812-1820 [PubMed] [Google Scholar]
- 10.Bhat-Nakshatri P, Newton TR, Goulet R, Jr, Nakshatri H: NF-kappaB activation and interleukin 6 production in fibroblasts by estrogen receptor-negative breast cancer cell-derived interleukin 1alpha. Proc Natl Acad Sci USA 1998, 95:6971-6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurtzman SH, Anderson KH, Wang Y, Miller LJ, Renna M, Stankus M, Lindquist RR, Barrows G, Kreutzer DL: Cytokines in human breast cancer: IL-1alpha and IL-1beta expression. Oncol Rep 1999, 6:65-70 [DOI] [PubMed] [Google Scholar]
- 12.Newton TR, Patel NM, Bhat-Nakshatri P, Stauss CR, Goulet RJ, Jr, Nakshatri H: Negative regulation of transactivation function but not DNA binding of NF-kappaB and AP-1 by IkappaBbeta1 in breast cancer cells. J Biol Chem 1999, 274:18827-18835 [DOI] [PubMed] [Google Scholar]
- 13.Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, Gelfanov V, Boswell SH, Goulet RJ, Jr, Sledge GW, Jr, Nakshatri H: Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene 2000, 19:4159-4169 [DOI] [PubMed] [Google Scholar]
- 14.Nozaki S, Sledge GW, Jr, Nakshatri H: Cancer cell-derived interleukin 1alpha contributes to autocrine and paracrine induction of pro-metastatic genes in breast cancer. Biochem Biophys Res Commun 2000, 275:60-62 [DOI] [PubMed] [Google Scholar]
- 15.Dinarello CA: Biologic basis for interleukin-1 in disease. Blood 1996, 87:2095-2147 [PubMed] [Google Scholar]
- 16.Carruth LM, Demczuk S, Mizel SB: Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. J Biol Chem 1991, 266:12162-12167 [PubMed] [Google Scholar]
- 17.Stevenson FT, Turck J, Locksley RM, Lovett DH: The N-terminal propiece of interleukin 1 alpha is a transforming nuclear oncoprotein. Proc Natl Acad Sci USA 1997, 94:508-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voronov E, Weinstein Y, Benharroch D, Cagnano E, Ofir R, Dobkin M, White RM, Zoller M, Barak V, Segal S, Apte RN: Antitumor and immunotherapeutic effects of activated invasive T lymphoma cells that display short-term interleukin 1alpha expression. Cancer Res 1999, 59:1029-1035 [PubMed] [Google Scholar]
- 19.Watanabe N, Kobayashi Y: Selective release of a processed form of interleukin 1 alpha. Cytokine 1994, 6:597-601 [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Colon I, Ortiz N, Callister M, Dong G, Pegram MY, Arosarena O, Strome S, Nicholson JC, Van Waes C: Effects of interleukin-1alpha, interleukin-1 receptor antagonist, and neutralizing antibody on proinflammatory cytokine expression by human squamous cell carcinoma lines. Cancer Res 1998, 58:3668-3676 [PubMed] [Google Scholar]
- 21.Kock A, Schwarz T, Urbanski A, Peng Z, Vetterlein M, Micksche M, Ansel JC, Kung HF, Luger TA: Expression and release of interleukin-1 by different human melanoma cell lines. J Natl Cancer Inst 1989, 81:36-42 [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Kharbanda S, McLeskey SW, Kern FG: Overexpression of fibroblast growth factor 1 in MCF-7 breast cancer cells facilitates tumor cell dissemination but does not support the development of macrometastases in the lungs or lymph nodes. Cancer Res 1999, 59:5023-5029 [PubMed] [Google Scholar]
- 23.March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, Gillis S, Henney CS, Kronheim SR, Grabstein K, Conlon PJ, Hopp TP, Cosman D: Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature 1985, 315:641-647 [DOI] [PubMed] [Google Scholar]
- 24.Sledge GW, Jr, Qulali M, Goulet R, Bone EA, Fife R: Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice. J Natl Cancer Inst 1995, 87:1546-1550 [DOI] [PubMed] [Google Scholar]
- 25.Ovejera AA, Houchens DP, Barker AD: Chemotherapy of human tumor xenografts in genetically athymic mice. Ann Clin Lab Sci 1978, 8:50-56 [PubMed] [Google Scholar]
- 26.Kishimoto H, Urade M, Sakurai K, Noguchi K: Isolation and characterisation of adenoid squamous carcinoma cells highly producing SCC antigen and CEA from carcinoma of the maxillary sinus. Oral Oncol 2000, 36:70-75 [DOI] [PubMed] [Google Scholar]
- 27.Demiralp B, Chen HL, Koh AJ, Keller ET, McCauley LK: Anabolic actions of parathyroid hormone during bone growth are dependent on c-fos. Endocrinology 2002, 143:4038-4047 [DOI] [PubMed] [Google Scholar]
- 28.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW, Jr: Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 1997, 17:3629-3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhat-Nakshatri P, Sweeney CJ, Nakshatri H: Identification of signal transduction pathways involved in constitutive NF-kappaB activation in breast cancer cells. Oncogene 2002, 21:2066-2078 [DOI] [PubMed] [Google Scholar]
- 30.Fife RS, Sledge GW, Jr: Effects of doxycycline on in vitro growth, migration, and gelatinase activity of breast carcinoma cells. J Lab Clin Med 1995, 125:407-411 [PubMed] [Google Scholar]
- 31.Li B, Dou QP: Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci USA 2000, 97:3850-3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endo Y, Sasaki T, Harada F, Noguchi M: Specific detection of metastasized human tumor cells in embryonic chicks by the polymerase chain reaction. Jpn J Cancer Res 1990, 81:723-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, May MJ, Kopp EB: NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998, 16:225-260 [DOI] [PubMed] [Google Scholar]
- 34.Helbig G, Christopherson KW, II, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H: NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 2003, 278:21631-21638 [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Pakneshan P, Gladu J, Slack A, Szyf M, Rabbani SA: Regulation of DNA methylation in human breast cancer. Effect on the urokinase-type plasminogen activator gene production and tumor invasion. J Biol Chem 2002, 277:41571-41579 [DOI] [PubMed] [Google Scholar]
- 36.Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR: MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol 1996, 148:313-319 [PMC free article] [PubMed] [Google Scholar]
- 37.Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, Suva LJ: Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res 2002, 62:5571-5579 [PubMed] [Google Scholar]
- 38.Wiseman BS, Werb Z: Stromal effects on mammary gland development and breast cancer. Science 2002, 296:1046-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee PP, Hwang JJ, Mead L, Ip MM: Functional role of matrix metalloproteinases (MMPs) in mammary epithelial cell development. J Cell Physiol 2001, 188:75-88 [DOI] [PubMed] [Google Scholar]
- 40.Singer CF, Kronsteiner N, Marton E, Kubista M, Cullen KJ, Hirtenlehner K, Seifert M, Kubista E: MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactions. Breast Cancer Res Treat 2002, 72:69-77 [DOI] [PubMed] [Google Scholar]
- 41.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA: p53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415:45-53 [DOI] [PubMed] [Google Scholar]
- 42.Chen HC, Smith SJ, Tow B, Elias PM, Farese RV, Jr: Leptin modulates the effects of acyl CoA: diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest 2002, 109:175-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH: Premature aging in mice deficient in DNA repair and transcription. Science 2002, 296:1276-1279 [DOI] [PubMed] [Google Scholar]
- 44.Briddon S, Beck SA, Tisdale MJ: Changes in activity of lipoprotein lipase, plasma free fatty acids and triglycerides with weight loss in a cachexia model. Cancer Lett 1991, 57:49-53 [DOI] [PubMed] [Google Scholar]
- 45.Gercel-Taylor C, Doering DL, Kraemer FB, Taylor DD: Aberrations in normal systemic lipid metabolism in ovarian cancer patients. Gynecol Oncol 1996, 60:35-41 [DOI] [PubMed] [Google Scholar]
- 46.Tessitore L, Vizio B, Jenkins O, De Stefano I, Ritossa C, Argiles JM, Benedetto C, Mussa A: Leptin expression in colorectal and breast cancer patients. Int J Mol Med 2000, 5:421-426 [DOI] [PubMed] [Google Scholar]
- 47.O’Brien SN, Welter BH, Price TM: Presence of leptin in breast cell lines and breast tumors. Biochem Biophys Res Commun 1999, 259:695-698 [DOI] [PubMed] [Google Scholar]
- 48.Margetic S, Gazzola C, Pegg GG, Hill RA: Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord 2002, 26:1407-1433 [DOI] [PubMed] [Google Scholar]
- 49.Hirai K, Hussey HJ, Barber MD, Price SA, Tisdale MJ: Biological evaluation of a lipid-mobilizing factor isolated from the urine of cancer patients. Cancer Res 1998, 58:2359-2365 [PubMed] [Google Scholar]
- 50.Freije JP, Fueyo A, Uria J, Lopez-Otin C: Human Zn-alpha 2-glycoprotein cDNA cloning and expression analysis in benign and malignant breast tissues. FEBS Lett 1991, 290:247-249 [DOI] [PubMed] [Google Scholar]
- 51.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS, Jr: NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 2000, 289:2363-2366 [DOI] [PubMed] [Google Scholar]
- 52.Lazarus DD, Destree AT, Mazzola LM, McCormack TA, Dick LR, Xu B, Huang JQ, Pierce JW, Read MA, Coggins MB, Solomon V, Goldberg AL, Brand SJ, Elliott PJ: A new model of cancer cachexia: contribution of the ubiquitin-proteasome pathway. Am J Physiol 1999, 277:E332-E341 [DOI] [PubMed] [Google Scholar]
- 53.Lorite MJ, Thompson MG, Drake JL, Carling G, Tisdale MJ: Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br J Cancer 1998, 78:850-856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ: Induction of cachexia in mice by systemically administered myostatin. Science 2002, 296:1486-1488 [DOI] [PubMed] [Google Scholar]
- 55.Argiles JM, Lopez-Soriano FJ: The role of cytokines in cancer cachexia. Med Res Rev 1999, 19:223-248 [DOI] [PubMed] [Google Scholar]
- 56.Baeuerle PA, Henkel T: Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 1994, 12:141-179 [DOI] [PubMed] [Google Scholar]
- 57.Apte RN, Voronov E: Interleukin-1-a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol 2002, 12:277-290 [DOI] [PubMed] [Google Scholar]
- 58.Douvdevani A, Huleihel M, Zoller M, Segal S, Apte RN: Reduced tumorigenicity of fibrosarcomas which constitutively generate IL-1 alpha either spontaneously or following IL-1 alpha gene transfer. Int J Cancer 1992, 51:822-830 [DOI] [PubMed] [Google Scholar]
- 59.Carbone JE, Ohm DP: Immune dysfunction in cancer patients. Oncology (Huntingt) 2002, 16:11-18 [PubMed] [Google Scholar]
- 60.Miller LJ, Kurtzman SH, Anderson K, Wang Y, Stankus M, Renna M, Lindquist R, Barrows G, Kreutzer DL: Interleukin-1 family expression in human breast cancer: interleukin-1 receptor antagonist. Cancer Invest 2000, 18:293-302 [DOI] [PubMed] [Google Scholar]
- 61.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN: IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA 2003, 100:2645-2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dieudonne MN, Machinal-Quelin F, Serazin-Leroy V, Leneveu MC, Pecquery R, Giudicelli Y: Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Commun 2002, 293:622-628 [DOI] [PubMed] [Google Scholar]
- 63.Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS: Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 2001, 33:95-102 [DOI] [PubMed] [Google Scholar]
- 64.McAndrew PF: Fat metabolism and cancer. Surg Clin North Am 1986, 66:1003-1012 [DOI] [PubMed] [Google Scholar]
- 65.Tisdale MJ: Cachexia in cancer patients. Nat Rev Cancer 2002, 2:862-871 [DOI] [PubMed] [Google Scholar]
- 66.Tisdale MJ: Metabolic abnormalities in cachexia and anorexia. Nutrition 2000, 16:1013-1014 [DOI] [PubMed] [Google Scholar]
- 67.Rubin H: Cancer cachexia: its correlations and causes. Proc Natl Acad Sci USA 2003, 100:5384-5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith HJ, Tisdale MJ: Induction of apoptosis by a cachectic-factor in murine myotubes and inhibition by eicosapentaenoic acid. Apoptosis 2003, 8:161-169 [DOI] [PubMed] [Google Scholar]
- 69.Inui A, Meguid MM: Cachexia and obesity: two sides of one coin? Curr Opin Clin Nutr Metab Care 2003, 6:395-399 [DOI] [PubMed] [Google Scholar]
- 70.Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, Erbs S, Moebius-Winkler S, Schuler G: Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail 2003, 5:33-40 [DOI] [PubMed] [Google Scholar]
- 71.Wilding JP: Leptin and the control of obesity. Curr Opin Pharmacol 2001, 1:656-661 [DOI] [PubMed] [Google Scholar]
- 72.Iguchi M, Aiba S, Yoshino Y, Tagami H: Human follicular papilla cells carry out nonadipose tissue production of leptin. J Invest Dermatol 2001, 117:1349-1356 [DOI] [PubMed] [Google Scholar]
- 73.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C: IL-1 beta mediates leptin induction during inflammation. Am J Physiol 1998, 274:R204-R208 [DOI] [PubMed] [Google Scholar]
- 74.William WN, Jr, Ceddia RB, Curi R: Leptin controls the fate of fatty acids in isolated rat white adipocytes. J Endocrinol 2002, 175:735-744 [DOI] [PubMed] [Google Scholar]
- 75.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM: The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419:624-629 [DOI] [PubMed] [Google Scholar]
- 76.Edwards DR, Murphy G: Cancer. Proteases—invasion and more. Nature 1998, 394:527-528 [DOI] [PubMed] [Google Scholar]
- 77.Gabig TG, Crean CD, Klenk A, Long H, Copeland NG, Gilbert DJ, Jenkins NA, Quincey D, Parente F, Lespinasse F, Carle GF, Gaudray P, Zhang CX, Calender A, Hoeppener J, Kas K, Thakker RV, Farnebo F, Teh BT, Larsson C, Piehl F, Lagercrantz J, Khodaei S, Carson E, Weber G: Expression and chromosomal localization of the Requiem gene. Mamm Genome 1998, 9:660-665 [DOI] [PubMed] [Google Scholar]