Abstract
Activator of G protein signaling 3 (AGS3) is a newly identified protein shown to act at the level of the G protein itself. AGS3 belongs to the GoLoco family of proteins, sharing the 19-aa GoLoco motif that is a Gαi/o binding motif. AGS3 interacts only with members of the Gαi/o subfamily. By surface plasmon resonance, we found that AGS3 binds exclusively to the GDP-bound form of Gαi3. In GTPγS binding assays, AGS3 behaves as a guanine dissociation inhibitor (GDI), inhibiting the rate of exchange of GDP for GTP by Gαi3. AGS3 interacts with both Gαi3 and Gαo subunits, but has GDI activity only on Gαi3, not on Gαo. The fourth GoLoco motif of AGS3 is a major contributor to this activity. AGS3 stabilizes Gαi3 in its GDP-bound form, as it inhibits the increase in tryptophan fluorescence of the Gαi3-GDP subunit stimulated by AlF4−. AGS3 is widely expressed as it is detected by immunoblotting in brain, testis, liver, kidney, heart, pancreas, and in PC-12 cells. Several different sizes of the protein are detected. By Northern blotting, AGS3 shows 2.3-kb and 3.5-kb mRNAs in heart and brain, respectively, suggesting tissue-specific alternative splicing. Taken together, our results demonstrate that AGS3 is a GDI. To the best of our knowledge, no other GDI has been described for heterotrimeric G proteins. Inhibition of the Gα subunit and stimulation of heterotrimeric G protein signaling, presumably by stimulating Gβγ, extend the possibilities for modulating signal transduction through heterotrimeric G proteins.
Heterotrimeric G proteins (G proteins), consisting of an α subunit (Gα) with GTPase activity and a βγ dimer (Gβγ), act as guanine nucleotide-dependent molecular switches in signaling pathways that connect transmembrane receptors with downstream effectors (1, 2). In the classical paradigm at the plasma membrane, the liganded transmembrane receptor activates the G protein by stimulation of GDP dissociation from Gα and acts as a guanine exchange factor (GEF), thereby enhancing GTP binding and releasing free Gα and Gβγ subunits to interact with their respective effectors (3). Inactivation of G protein signaling takes place by inhibiting G protein activation or by GTP hydrolysis, which leads to reformation of the heterotrimer. Precisely timed activation and inactivation of the G protein, dependent on regulatory factors, is crucial in signal transduction. In the case of the small G proteins, two classes of intracellular proteins can act as inhibitors of G protein activation: GTPase activating proteins (GAPs), which enhance GTP hydrolysis, and guanine dissociation inhibitors (GDIs), which inhibit GDP dissociation (4). GAPs for heterotrimeric G protein α subunits have only recently been discovered and for the most part belong to the RGS (regulator of G protein signaling) protein family (5–7). Until now, GDIs acting on heterotrimeric G proteins have remained elusive. However, several additional Gα-interacting proteins, most of them displaying regulatory- or effector-like functions, have recently been identified. PCP2 and activator of G protein signaling (AGS) 1 are novel GEFs (8, 9) and Rap1GAP is a novel effector (10, 11). AGS3, identified in a functional screen based on G protein signaling in yeast but unrelated to AGS1, was recently shown to bind to Gαi-GDP and act as an activator of heterotrimeric G protein signaling (12), possibly through effectors of Gβγ. In contrast to G protein coupled receptors (the classical G protein activators), AGS3 did not enhance GTPγS binding to the Gα subunit. Thus, it apparently acts through a different, yet to be elucidated, molecular mechanism (12). Here, we have further characterized AGS3 and have demonstrated that it acts as a GDI for Gαi3.
Materials and Methods
Isolation of AGS3 cDNA.
For two-hybrid interaction screening, 50 μg of a rat GC cell (pituitary) cDNA library in pACT2 was transformed into yeast HF7c(pGBT9Gαi3) as described (13). Twenty-four positive clones, grouped based on insert size and restriction pattern, were sequenced from the 5′ or 3′ end by automated sequencing. One of these was a partial clone for AGS3, encoding the C-terminal half of the molecule (amino acids 361–590), truncated by its last 60 aa. Full length AGS3 (650 aa) cDNA was obtained by reverse transcription (RT)-PCR on rat brain cDNA (kind gift of Dr. E. Masliah, Department of Pathology, University of California at San Diego), based on the reported sequence (GenBank no. AF107723).
Online BLAST searches were performed via the website of the National Center for Biotechnology Information (NCBI), Bethesda, MD (14). PROSITE was used for searching motifs, and protein structure analysis (PSA) (BMERC, Boston, MA) was used for secondary structure analysis.
Northern Blot Analysis.
A multiple tissue blot of poly(A)+ RNA from rat tissues (CLONTECH) was hybridized to a 200-bp cDNA fragment (corresponding to AGS3591–650 cDNA). The probe was labeled by random priming with [32P]dCTP (3000 Ci/mmol) (Amersham). Quickhyb solution (Stratagene) was used under high-stringency conditions for hybridization (68°C), and high-stringency washes were performed in 0.1× SSC [150 mM NaCl/15 mM sodium citrate (pH 7)] plus 0.1% SDS, at 60°C. Autoradiographs were exposed for 3 days at −70°C with intensifying screens.
Antibody Production.
Anti-AGS3 antibody was raised in rabbits (Covance, Richmond, CA) against the last 14 aa (KGPDPRQQSPPGAS) at the C terminus of AGS3, which has no homologies to other known mammalian proteins. IgG was affinity purified on the same peptide coupled to Affigel 10 (Bio-Rad). The affinity-purified IgG (8.5 μg/ml) recognized 1 ng purified recombinant AGS3 protein by immunoblotting.
Immunoblotting Analysis.
A rat multiple tissue Western blot (Gene Technology, St. Louis, MO) containing lysates (75 μg) of each of nine different tissues (prepared in the presence of protease inhibitors) or immunoblots prepared from PC-12 cells were incubated in TBS [10 mM Tris (pH 7.5)/100 mM NaCl/5 mM KCl] supplemented with 0.1% Tween 20, 5% FCS, and 8.5 μg/ml affinity-purified anti-AGS3 IgG. Detection was performed by enhanced chemiluminescence (ECL) using Supersignal West Pico substrate (Pierce).
Preparation of Cell Fractions.
PC-12 cells were maintained in DMEM (high glucose) medium supplemented with 10% horse serum and 5% FBS. Postnuclear supernatants were prepared and separated into particulate and cytosolic fractions by centrifugation (100,000 × g for 1 h) as described (15). Fractions (normalized by volume) were loaded on 10% SDS/PAGE gels for protein separation, transferred onto poly(vinylidene difluoride) (PVDF) membranes, and immunoblotted with affinity-purified anti-AGS3 antibody, and detection was performed by ECL.
Two Hybrid Interactions.
AGS3361–590 or AGS3361–650 in pACT2 vector were cotransformed in yeast strain SFY526 (CLONTECH) with the following Gα subunits subcloned into pGBT9 vector: rat Gαi3 (16), rat Gαi2, mouse Gα12, and mouse Gαq from P. Insel (University of California at San Diego), rat Gαi1 from T. Kosasa (University of Texas, Southwestern Medical Center), rat Gαs from H. Bourne (University of California at San Francisco), rat Gαo1 from E. Neer (Brigham and Women's Hospital, Boston), rat Gαz from E. Ross (University of Texas, Southwestern Medical Center), and Saccharomyces cerevisiae GPA1 from J. Noel (Salk Institute). Interactions were analyzed qualitatively by a colony lift assay for β-gal using 5-bromo-4-chloro-3-indolyl β-D-galactoside (17), and the appearance of blue colonies was assessed (− to +++) after 2, 4, and 8 h. No background color was detected after 20 h.
Preparation of Recombinant Fusion Proteins.
A cDNA fragment coding for AGS3424–650 was cloned into pGEX-KG vector (Pharmacia) and expressed in Escherichia coli DH5α. The protein was purified according to the manufacturer's instructions and used for surface plasmon resonance (BIAcore) assays (see below). AGS3424–650 and AGS3591–650 cDNAs were subcloned into pET28a vector (Novagen), and hexahistidine (His) fusion proteins were expressed in E. coli BL21(DE3) strain. Bacteria were induced at room temperature with 0.4 mM isopropyl β-D-thiogalactoside (IPTG). Bacterial pellets were resuspended in lysis buffer [1% Tween 20/25 mM Tris (pH 8)/500 mM NaCl/5 mM imidazole] supplemented with 200 μg/ml lysozyme. His-AGS3 proteins were purified by affinity chromatography on Ni2+-NTA resin (Qiagen, Chatsworth, CA) and eluted with lysis buffer containing 250 mM imidazole. His-Gαi3, His-Gαo1, and His-Gαs subunits were purified as described (18). Concentrations of recombinant proteins were estimated by SDS/PAGE after Coomassie staining with known amounts of BSA as standards. Purified His-AGS3 proteins were dialyzed overnight in 10 mM Hepes (pH 7.5), 1 mM DTT, and 0.05% polyoxyethylene 10 lauryl ether (C12E10, Sigma). Aliquots were kept frozen at −80°C until used for in vitro interactions or GTPγS binding assays.
In Vitro Interactions by Surface Plasmon Resonance.
Binding measurements were performed on a BIAcore 2000 (Biacore, Piscataway, NJ) at the University of North Carolina, Chapel Hill Macromolecular Interactions Facility. Goat anti-GST IgG (affinity purified, Biacore) was coupled (1 μg per coupling) to separate carboxymethylated, dextran-coated sensor surfaces (Sensor Chip CM5) that had been activated by using N-hydroxysuccinimide (NHS) and N-ethyl-N′-(dimethylaminopropyl)carbodiimide (EDC) according to the manufacturer's instructions. Antibody coupling was followed by injection of 1 M ethanolamine hydrochloride to inactivate the remaining NHS groups. Recombinant GST and GST-AGS3424–650 were bound to the anti-GST IgG and gave 4400 and 6500 response units (RU), respectively.
Binding analyses were performed at 25°C in HBS-P/M running buffer [10 mM Hepes (pH 7.4)/150 mM NaCl/16 mM MgCl2/0.005% (vol/vol) Nonidet P-40]. Myristoylated, recombinant rat Gαi3 protein (purchased from Calbiochem) was diluted to 0.8 μM in HBS-P/M buffer containing either 32 μM GDP, 32 μM GDP plus 32 μM AlCl3 and 10 mM NaF, or 32 μM GTPγS. Gαi3 protein aliquots containing GDP or GDP plus AlF4− were incubated for 30 min at 30°C before injection. The Gαi3 protein aliquot containing GTPγS was preincubated overnight at room temperature to allow binding of GTPγS. Each preincubated Gαi3 sample with corresponding guanine nucleotide dilution buffer (25 μl) was injected into three different channels of both the GST-AGS3 and GST chips at a flow rate of 5 μl/min. Background binding to the GST surface was subtracted from binding curves of the GST-AGS3 surface by using BIAevaluation software version 3.0 (Biacore). Surface regeneration was performed by 10-μl injections of 1 M NaCl in 10 mM NaOH at a 30 μl/min flow rate.
GTPγS Binding Assay.
GTPγS binding experiments were performed with 2 μM [35S]GTPγS (1000 Ci/mmol; 2000 cpm/pmol) and 1 μM His-AGS3424–650 or His-AGS3591–650 proteins in binding buffer [50 mM Tris (pH 8.0)/1 mM DTT/1 mM EDTA/10 mM MgSO4). Reactions (50 μl) were started by addition of His-Gαi3-GDP, His-Gαo-GDP, or His-Gαs-GDP (2.5 pmols/assay), incubated at 30°C and terminated by rapid filtration through nitrocellulose filters followed by washing with ice cold binding buffer (3 times; 4 ml). Bound radioactivity was determined by scintillation counting. Specific binding was <5% of the total radioactivity, and GTPγS binding to AGS3 was <2% of the binding to the Gα subunits.
Spectrofluorometric Analysis of the AGS3/Gαi3 Interaction.
The increase in intensity of fluorescence of the tryptophan residues in Gα is an indicator for its structural change from the inactive to the active state (19–21) and can be monitored by spectrofluorometry. Fluorescence measurements were performed with a Shimadzu RF 5000 fluorometer with excitation at 292 nm and emission at 342 nm (bandwidths 5 and 30 nm, respectively). For activation of Gα, 300 nM His-Gαi3-GDP in 20 mM Tris⋅HCl, 120 mM KCl, 2 mM MgCl2, 1 mM DTT, 20 μM GDP, was equilibrated at 25°C in the cuvette, and NaF (2 mM) and AlCl3 (30 μM) (final concentrations) were added after 10 and 11 min, respectively. To determine the effect of AGS3 on Gα activation by AlF4−, a complex of 300 nM GST-AGS3424–650 and 300 nM Gαi3-GDP was preformed (15 min at 25°C) in the same buffer and then activated as described above. AGS3424–650 itself contains no tryptophan residues. The signal generated from the GST moiety of GST-AGS3424–650 (which contains 4 tryptophans) was deducted from the total signal.
Results
AGS3 Belongs to a Family of Proteins with Conserved Motifs.
To search for proteins that can interact with Gαi3, we screened a yeast two-hybrid library from rat GC (pituitary) cells as previously described (13). One of the positive clones coded for residues 361 to 590 of the AGS3 protein (Fig. 1A), whose full-length, 650-aa sequence was recently described by Takesono et al. (12). Other positive clones coded for GAIP (16), Calnuc (nucleobindin) (13), and LGN (22).
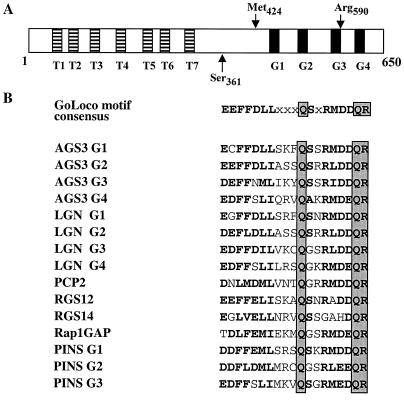
Figure 1.
Schematic representation of the AGS3 protein and alignment of GoLoco motifs. (A) AGS3 (650 aa) contains 7 N-terminal tetratricopeptide (TPR) motifs (T1 to T7) and 4 C-terminal GoLoco motifs (G1 to G4). Important landmark amino acids for AGS3 constructs used in our experiments are shown by arrows. AGS3361–590 is sufficient for interaction with Gαi/o subunits. (B) Alignment showing 15 GoLoco motifs (19 aa) from 7 proteins with identical residues, boxed and conserved substitutions shown in bold. The consensus sequence was defined by the amino acid most represented in each position.
The C-terminal half of AGS3 shows homologies to the following proteins: LGN (22), PCP2 (8), RGS12, RGS14 (23), Rap1GAP (11), Loco (24), and partner of inscuteable (PINS) (25, 26). All of these proteins have in common one or several 19-aa GoLoco motifs (Fig. 1B) (27), which have also been called GPR motifs (12). The GoLoco motif is repeated four times in AGS3 and LGN and three times in PINS. Each of the four GoLoco motifs of AGS3 corresponds to a predicted α-helix. The N-terminal half of AGS3 contains 7 tetratricopeptide (TPR, 34 aa) motifs (Fig. 1A) that in other proteins have been implicated in protein–protein interactions (28). Seven TPR motifs are also present in LGN and in PINS. Each TPR motif also corresponds to a predicted α-helix. Because the C-terminal half of AGS3 interacts with Gα, we have restricted our study to this region of the molecule in subsequent assays.
AGS3 Is Expressed in Multiple Tissues.
When a fragment coding for the C-terminus (amino acids 591–650) of rat AGS3 cDNA was used as a probe in a rat tissue Northern blot, mRNAs of 2.3 kb and 3.5 kb were detected in heart and brain, respectively (Fig. 2A). No detectable mRNAs were found in other tissues with this probe, probably because of the limited sensitivity of the Northern blotting technique or to mRNA instability.
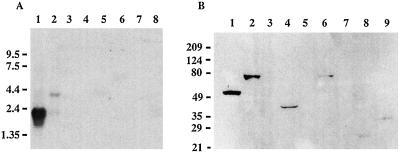
Figure 2.
(A) Expression of AGS3 mRNA. Analysis of a rat multiple tissue Northern blot with an AGS3 probe shows a 2.3-kb mRNA in heart (lane 1) and a 3.5-kb mRNA in brain (lane 2). No mRNAs were detected in spleen (lane 3), liver (lane 4), lung (lane 5), skeletal muscle (lane 6), kidney (lane 7), or testis (lane 8) under these conditions. (B) Expression of AGS3 protein. Analysis of a rat multiple tissue Western blot (75 μg protein/lane) with affinity-purified AGS3 IgG (8.5 μg/ml in TBS, 0.1% Tween 20, 5% FCS) showing that AGS3 protein is widely expressed. A 75-kDa protein is detected in brain (lane 2) and testis (lane 6). Liver (lane 1), kidney (lane 4), heart (lane 8), and pancreas (lane 9) showed a single protein band at 53, 42, 26, and 35 kDa, respectively. No AGS3 immunoreactivity was detected in lung (lane 3), spleen (lane 5), and ovary (lane 7) under these conditions.
To determine the sites of expression of the AGS3 protein, we performed Western blotting with an affinity-purified AGS3-specific antibody (directed to the C terminus of AGS3) on a multiple rat tissue Western blot. Immunodetection revealed the presence of single bands with different sizes in several tissues. We detected a 75-kDa protein corresponding to the deduced full-length AGS3 protein described by Takesono et al. (12) in brain and testis (Fig. 2B) and specific bands of smaller size in liver (53 kDa), kidney (42 kDa), heart (26 kDa), and pancreas (35 kDa). No bands were detected in lung, spleen, and ovary under these conditions. Brain and liver lysates showed the highest immunoreactivity, and heart and pancreas the lowest. By RT-PCR (primers in the region coding for amino acids 424 to 430 and 645 to 650), we detected a transcript in kidney (data not shown). We also identified a rat expressed sequence tag (EST) sequence (GenBank no. C06699) from pancreas and a human EST sequence (different splice form) from an oligodendroglioma (GenBank no. AI272212) by BLAST homology searches (GenBank).
Our Western blot and RT-PCR data together with the EST data indicate that expression of AGS3 is more widespread than anticipated by mRNA expression levels on Northern blots and suggest that there may be alternatively spliced forms of AGS3 in different tissues.
Distribution of AGS3 in Cell Fractions.
As a first approach to determine the cellular localization of AGS3, we surveyed its expression in several cell lines (AtT-20 and GC pituitary cell lines, HEK-293, MDCK, PC-12, and REF52 cells). Because nondifferentiated PC-12 cells showed the highest levels of expression of the 75-kDa form, we determined the distribution of AGS3 by immunoblotting in cytosolic (100,000 × g supernatant) and particulate (100,000 × g pellet) fractions from these cells. The majority (95%) of the protein was found in the cytosolic fraction (Fig. 3), but 5% sedimented with the particulate fraction, indicating its association with membranes or cytoskeletal elements. We therefore conclude that the 75-kDa form of AGS3 is mainly a cytosolic protein in nondifferentiated PC-12 cells.
Figure 3.
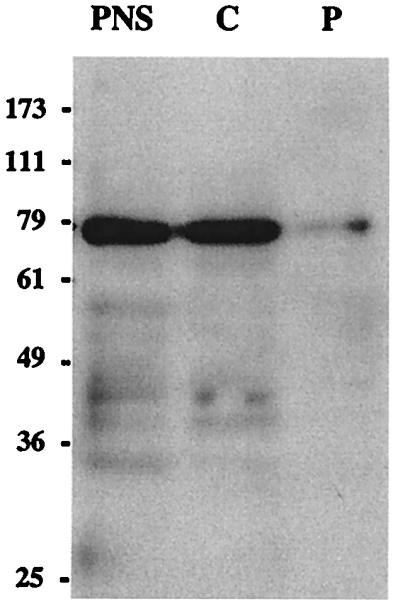
Distribution of AGS3 protein in membrane and cytosolic fractions of PC-12 cells. A postnuclear supernatant (PNS) was centrifuged at 100,000 × g to yield particulate (P) and cytosolic (C) fractions. These fractions (normalized by volume) were immunoblotted with affinity-purified AGS3 IgG and detected by ECL, and the amount found in each fraction was quantified by densitometry. The majority of AGS3 (>95%) is found in the cytosolic fraction, but a small amount (5%) is found in the particulate fraction.
AGS3 Interacts with Gαi/o Subunits.
To determine the specificity of AGS3 for different Gα subunits, we used the two-hybrid system. Results of the qualitative filter lift assay (Table 1) showed that AGS3 interacts with Gαi1, Gαi2, Gαi3, and Gαo, but no interaction was detected with Gαz, Gαs, Gαq, or Gα12 in this assay. Interaction with Gαo and Gαi2 was slightly weaker than with Gαi1 and Gαi3. Interestingly, AGS3 did not interact with Gpa1, the yeast Gα subunit that is most similar to the mammalian Gαi subfamily (29). For unknown reasons, AGS3361–590, the truncated form we originally isolated, showed a stronger interaction with Gαi3 than AGS3361–650.
Table 1.
AGS3 interacts with Gαi subfamily members
| Bait | β-gal | Prey |
|---|---|---|
| Gαi1 | +++ | AGS3361–650 |
| Gαi2 | ++ | AGS3361–650 |
| Gαi3 | +++ | AGS3361–650 |
| Gαi3 | ++++ | AGS3361–590 |
| Gαo | ++ | AGS3361–650 |
| Gαz | − | AGS3361–650 |
| Gαs | − | AGS3361–650 |
| Gαq | − | AGS3361–650 |
| Gα12 | − | AGS3361–650 |
| Gpa1 | − | AGS3361–650 |
The β-gal filter assay was performed on (Leu−, Trp−) plates, and intensity of color was scored after 8 h. −, no color; ++, moderate color; +++, strong color; ++++, very strong color. Yeast cotransformed with void bait and prey vectors were taken as background (none detected after 20 h). Baits were constructed in pGBT9, and AGS3 preys were in pACT2. Three independent experiments were performed with the same results.
AGS3 Interacts with the GDP-Bound Form of Gαi3.
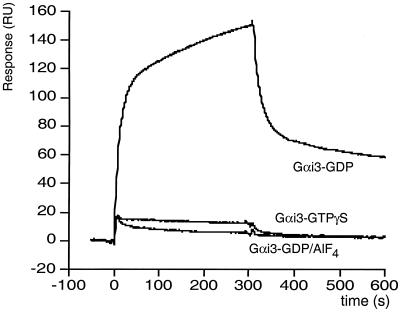
We investigated the nucleotide dependence of the binding of recombinant Gαi3 to AGS3 by surface plasmon resonance (BIAcore) analysis. GST-AGS3424–650 and GST control proteins were immobilized with anti-GST IgG that had been covalently coupled to a dextran-coated biosensor surface. Recombinant Gαi3 was preincubated with GDP, GDP + AlF4−, or GTPγS and passed over these surfaces. In this assay, AGS3 clearly interacted exclusively with the inactive (GDP-bound) form of Gαi3 (Fig. 4), suggesting it could be either a GEF or a GDI.
Figure 4.
In vitro interactions of AGS3 with Gαi3 assessed by surface plasmon resonance. AGS3 interacts with Gαi3-GDP but not with Gαi3-GTPγS or Gαi3-GDP-AlF4−. GST-AGS3424–650 fusion protein or GST protein were bound to anti-GST IgG covalently coupled to separate Biacore chips. Gαi3 was preincubated with GDP, AlF4−, or GTPγS as described in Materials and Methods and then injected in the flow buffer (simultaneous coinjection over both chips) into a BIAcore 2000. The signal generated by GST alone was deducted from the GST-AGS3424–650 signal.
AGS3 is a GDI.
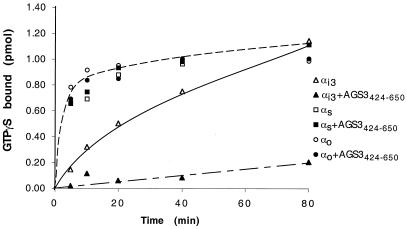
To determine whether AGS3 functions as a GEF or a GDI, we performed GTPγS (a nonhydrolyzable analog of GTP) binding experiments. Increased GTPγS binding to Gαi3 would imply AGS3 is a GEF, whereas decreased binding would imply that AGS3 is a GDI. Addition of 1 μM AGS3424–650 decreased the binding of GTPγS to 0.05 μM Gαi3 by 5 min, and after 80 min incubation binding was reduced by 85% (Fig. 5). By contrast, no inhibitory effect on GTPγS binding to Gαo and Gαs (Fig. 5) was found in the presence of AGS3424–650. Based on this assay, we conclude that AGS3 is a specific GDI for Gαi3. This specificity toward Gαi3 is surprising because AGS3 interacts with Gαo, a member of the Gαi subfamily, in the two-hybrid assay.
Figure 5.
AGS3 is a GDI for Gαi3. Shown is the time course of GTPγ35S binding by Gαi3-, Gαo-, and Gαs-GDP in the absence or presence of AGS3424–650. AGS3 inhibits (up to 85%) GTPγS binding to Gαi3. The effect is observed as early as 5 min and lasts over the entire 80-min time period. No inhibition of GTPγS binding to Gαo or Gαs is observed in the presence of AGS3424–650. GTPγ35S was incubated in the absence or presence of 1 μM His-AGS3424–650. Gα-GDP (50 nM final) subunits were added to start the reaction, and the reaction was stopped at different times by filtration. Results represent the mean of three (Gαs, Gαo) or four (Gαi3) experiments.
Further analysis showed that AGS3591–650 (the last 60 aa of AGS3), containing GoLoco 4 also significantly reduced GTPγS binding to Gαi3 (≈75%) (Table 2). We conclude that the C-terminal half of AGS3 containing the GoLoco motifs is responsible for the GDI effect on Gαi3 and that the fourth GoLoco motif in particular is the major contributor to this effect. However, further studies are needed to determine the importance of each separate GoLoco domain.
Table 2.
The C-terminus of AGS3 inhibits GTPγS binding to Gαi3
| Time, min | GTPγS bound, % of total binding
|
||
|---|---|---|---|
| — | +AGS3424–650 | +AGS3591–650 | |
| 10 | 100 | 16.6 | 28.9 |
| 80 | 100 | 15.7 | 26.2 |
GTPγS was incubated in the absence or presence of 1 μM each of His-AGS3424–650 or His-AGS3591–650. Reactions were started by addition of 10 μl of Gαi3-GDP (250 nM). Results represent the percentage of GTPγS binding remaining after 10 and 80 min of incubation. Binding of Gαi3, defined as 100%, was 0.27 ± 0.01 after 10 min and 1.15 ± 0.04 pmol after 80 min. AGS3424–650 (4 GoLocos) and AGS3591–650 (last GoLoco) inhibit (84% and 74%, respectively) the binding of GTPγS to Gαi3. n = 3.
AGS3 Inhibits the Activation of Gαi3 by AlF4−.
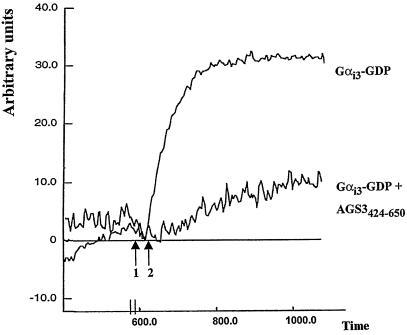
To confirm the inhibitory effect of AGS3 on Gαi3 activation, we determined the effect of AGS3 on the activation of Gαi3-GDP by analyzing the intrinsic tryptophan fluorescence of the Gα subunit in the presence or absence of AlF4−. It has been shown that the intensity of tryptophan fluorescence increases significantly on activation of Gαi3-GDP by AlF4− (19–21) as a consequence of the conformational change of the Gα subunit. The binding of AGS3424–650 to Gαi3-GDP significantly inhibited Gα activation by AlF4− (Fig. 6). The fluorescence signal for Gαi3 never reached the level of the AlF4−-activated Gαi3 in presence of AGS3424–650, even after longer measurements (15 min, data not shown). These results indicate that AGS3 inhibits the conformational change in Gαi3 from the inactivated to the activated state by stabilizing it in its GDP-bound form. These findings also define AGS3 as a GDI.
Figure 6.
AGS3 stabilizes Gαi3 in its inactive GDP-bound form. Activation of Gαi3 by AlF4− is visualized by a dramatic enhancement of the fluorescence of Gαi3 tryptophans. The amplitude of activation of Gαi3 is significantly decreased in the presence of AGS3424–650. Gαi3-GDP was preincubated in the absence or presence of AGS3424–650 before addition of NaF (arrow 1) and AlCl3 (arrow 2). Tryptophan fluorescence was monitored by spectrofluorometry as described in Materials and Methods. Time is expressed in seconds and fluorescence in arbitrary units.
Discussion
AGS3 was recently isolated and characterized by Takesono and coworkers as a receptor-independent activator of G protein signaling through a functional screen in yeast and was shown to preferentially bind to the GDP-bound form of Gαi2 and Gαi3 in GST pulldown assays (12). This suggested that AGS3 might act as either a GEF or a GDI, stimulating or inhibiting, respectively, the exchange of GDP for GTP. Because these workers found no change in GTPγS binding to Gαi2 and to trimeric G proteins purified from brain (mainly Gαo) in the presence of AGS3, they were not able to conclude whether AGS3 is a GEF or a GDI (12). We confirmed AGS3's specific interaction with the GDP-bound form by surface plasmon resonance (BIAcore) where AGS3 binds exclusively to Gαi3-GDP. In addition, we demonstrated that AGS3 behaves as a GDI by showing that GTPγS binding by Gαi3 was decreased 6-fold in the presence of AGS3424–650. AGS3591–650, containing only GoLoco 4 (14 aa shorter than the protein used by Takesono et al. (12)), also showed potent GDI activity in this assay. We confirmed that AGS3 behaves as a GDI by using spectrofluorometry, a classical technique that measures the increase in fluorescence of Gα tryptophan residues after the switch from the inactive (GDP-bound) state to the active (GTP-bound) state. Preincubation of Gαi3 with AGS3424–650 significantly inhibited the activation of Gαi3 by AlF4−, indicating that it stabilizes Gαi3 in its GDP-bound state.
Takesono et al. suggested that AGS3 and Gβγ can compete for binding to the Gα-GDP subunit (12), and we have found in this work that AGS3 is a GDI that keeps the Gα subunit in its inactive state. These observations suggest that the AGS3/Gαi3 interaction might prevent Gβγ from returning to Gα and thus leads to enhanced Gβγ-dependent signaling, which corresponds to what Takesono et al. (12) observed when isolating AGS3 in their functional screen in yeast, as the signaling pathway leading to proliferation and survival in yeast depends on Gβγ signaling. AGS3 would thus selectively shut off signaling pathways linked to Gα effectors and favor Gβγ effector pathways.
It is interesting to note that AGS3 could still activate G protein signaling through a G204A mutant (mimicking the GDP-bound form) of Gαi2 (12), which is incapable of releasing Gβγ after activation by a receptor (30). These findings suggest that AGS3 activates G protein signaling via a different mechanism than a receptor does.
An as yet unknown intracellular regulatory mechanism must be invoked for AGS3 to act on Gα, maybe by translocation or by interaction with other signaling partners. The GDIs for small G proteins generally prevent translocation of the small G protein to their membrane-bound activators (GEFs) by keeping the GDI/small G protein complex in the cytosol (4). A similar mechanism might take place in the case of AGS3, preventing Gαi activation by keeping Gα in the GDP-bound state and by keeping Gα away from membranes.
The results of our two-hybrid interaction assay with a panel of Gα subunits demonstrates that AGS3 binds to all members of the Gαi/o subfamily with the exception of Gαz, but does not bind to Gαs, Gαq, or Gα12. Data from GTPγS binding assays show that AGS3 is a GDI for Gαi3, but surprisingly not for Gαo. Thus, AGS3 is capable of binding to Gαo, but its GDI activity seems restricted to Gαi3, although we have not tested GDI activity of AGS3 on Gαi1 and Gαi2. This specificity is also surprising because AGS3 and Gαo are both highly expressed in brain. Additional research is needed to address the in vivo specificity of AGS3.
BLAST searches with AGS3 showed strong local homologies to the following mammalian proteins: LGN (22), PCP2 (8), RGS12 and RGS14 (23), Rap1GAP (11), Loco (24), and PINS (25, 26). The common sequence in these proteins is restricted to a 19-aa motif, recently named GoLoco (27) or GPR (12), which defines a newly recognized family of proteins. AGS3 and LGN contain four GoLoco motifs, PINS contains three and PCP2, RGS12, RGS14, Rap1GAP, and Loco only one. All these proteins have in common the ability to bind to Gαi/o subunits (27). The fact that both PCP2 and Rap1GAP, each with only one GoLoco repeat, were isolated in two-hybrid screens with Gαo indicates that the presence of one such motif is sufficient for interaction with Gα. The single GoLoco motifs of RGS12 and RGS14 (RGS domains deleted) were also found to be sufficient for binding to Gαi subunits (R. Kimple and D.P.S., unpublished data).
At this time, the role of each GoLoco motif in AGS3 is not clear. The AGS3 cDNA fragment we originally isolated based on its ability to bind Gαi3 coded for only the first 3 GoLoco motifs, suggesting that the 4th (C-terminal) GoLoco motif is not essential for the interaction with Gαi3. However, we found GoLoco 4 to be an important component for the GDI activity of AGS3.
GoLoco motifs functionally act as general binding motifs to Gαi/o subunits. However, the structural requirements for nucleotide-specific binding (to GDP- or GTP-bound Gα) and for specific interactions within the Gαi/o subfamily have not been extensively addressed. Most GoLoco proteins that have been studied interact with the GDP-bound forms of Gαi/o subunits. LGN, the closest homolog of AGS3, preferentially interacted with the GDP-bound form of Gαi2 (22), and PCP2 preferentially interacted with the GDP-bound form of Gαo. The nucleotide preference of Rap1GAP is unclear as one report indicates it interacts with the GDP-bound form of Gαo (10) and another with the GTP-bound form of Gαi1 and Gαi2 (11). A third study reported interaction of Rap1GAP with GTP-bound Gαz and Gαi2, but not with GTP-bound Gαo (31). Preferential binding of a protein to GDP-bound Gα subunits indicates that it functions either as a GEF or as a GDI. Based on the ability of PCP2 to stimulate GDP release from Gαo, it was suggested that proteins with GoLoco domains are GEFs for Gαi/o subunits (8, 27). However, here we show that the GoLoco motifs of AGS3, all four together as well as the C-terminal GoLoco alone, behave as a GDI. Thus, specific residues within or the structural context surrounding each GoLoco motif might dictate whether the protein behaves as a GEF or a GDI, respectively. Mutational analysis of GoLoco motifs and their amino acid context should shed light on these issues.
Although we have attributed a GDI function to the GoLoco motifs of AGS3, no role yet has been found for its N-terminal TPR motifs. TPR motifs are found in a variety of functionally unrelated molecules and have been implicated in protein–protein interactions (28). PINS was originally isolated by interaction of its N-terminal 7 TPR motifs with the Drosophila Inscuteable protein (25). The third TPR motif in p67phox, a subunit of the NADPH oxidase complex that has four TPRs, has been implicated in the binding of GTP-bound Rac, a small GTPase (32). These results raise the possibility that AGS3 might function as a connector molecule that links GDP-Gαi/o (via its GoLoco motifs) to other signaling pathways via its TPR motifs. It is our goal to identify proteins that interact with the N-terminal TPR domains of AGS3 as they may reveal additional clues to AGS3's function, its exact moment of action, and its effects on G protein signaling.
We have found that AGS3 is widely expressed, but different isoforms may be expressed in different tissues. Using a specific affinity-purified anti-peptide antibody directed against the extreme C terminus of AGS3, we detected several forms of AGS3 by immunoblotting. Each organ showed only one immunoreactive band, but their size varied. The 75-kDa form detected in brain, testis, and PC-12 cells corresponds to the size of the AGS3 protein deduced from its cDNA isolated from rat brain (12). Additional isoforms were detected in liver (53 kDa), kidney (42 kDa), heart (26 kDa), and pancreas (35 kDa). The existence of alternative spliced forms of AGS3 is suggested by our Northern blot analysis, showing two different sizes of mRNA for heart and brain, and by the cDNA sequence of a human EST isolated from an oligodendroglioma. Although AGS3 mRNA was detected only in heart and brain by Northern blotting, RT-PCR performed on kidney RNA showed the expression of an mRNA that codes for AGS3. Furthermore we have isolated an AGS3 cDNA from rat pituitary, and the existence of a rat EST from pancreatic islets has been documented. The relationship between these different mRNAs remains to be established; however, our collective evidence suggests the existence of multiple isoforms of AGS3 probably produced by alternative splicing. Further molecular characterization of the liver, kidney, heart, and pancreas forms should clarify the significance of these different forms.
In summary, in this study we have described the molecular mechanism whereby AGS3 acts on the Gα subunit and found that it is a GDI. Crystallographic data of the AGS3/Gα-GDP complex will possibly refine the molecular details of this interaction, and localization of this complex within the cell may help to define its function. It is already clear that, with the discovery of AGS3 and its GDI activity, an interesting complexity has been added to the classical G protein signaling paradigm.
Acknowledgments
We thank Marc Chabre (Centre National de la Recherche Scientifique, Sophia Antipolis, France) and Maria Diversé-Pierluissi (Mount Sinai, New York) for helpful discussions. This research was supported by National Institutes of Heath Grants CA 58689 and DK17780 to M.G.F. and GM62338 to D.P.S.
Abbreviations
- AGS
activator of G protein signaling
- RGS
regulator of G protein signaling
- EST
expressed sequence tag
- GEF
guanine exchange factor
- GAP
GTPase activating protein
- GDI
guanine dissociation inhibitor
- PINS
partner of inscuteable
References
- 1.Gilman A G. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Morris A J, Malbon C C. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 3.Neer E J. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 4.Geyer M, Wittinghofer A. Curr Opin Struct Biol. 1997;7:786–792. doi: 10.1016/s0959-440x(97)80147-9. [DOI] [PubMed] [Google Scholar]
- 5.De Vries L, Zheng B, Fischer T, Elenko E, Farquhar M G. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 6.Siderovski D, Strockbine B, Behe C. Crit Rev Biochem Mol Biol. 1999;34:215–251. doi: 10.1080/10409239991209273. [DOI] [PubMed] [Google Scholar]
- 7.Hepler J R. Trends Pharmacol Sci. 1999;20:376–382. doi: 10.1016/s0165-6147(99)01369-3. [DOI] [PubMed] [Google Scholar]
- 8.Luo Y, Denker B M. J Biol Chem. 1999;274:10685–10688. doi: 10.1074/jbc.274.16.10685. [DOI] [PubMed] [Google Scholar]
- 9.Cismowski M J, Ma C, Ribas C, Xie X, Spruyt M, Lizano J S, Lanier S M, Duzic E. J Biol Chem. 2000;275:23421–23424. doi: 10.1074/jbc.C000322200. [DOI] [PubMed] [Google Scholar]
- 10.Jordan J D, Carey K D, Stork P J, Iyengar R. J Biol Chem. 1999;274:21507–21510. doi: 10.1074/jbc.274.31.21507. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Nature (London) 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- 12.Takesono A, Cismowski M J, Ribas C, Bernard M, Chung P, Hazard S, 3rd, Duzic E, Lanier S M. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- 13.Lin P, Le-Niculescu H, Hofmeister R, McCaffery J M, Jin M, Hennemann H, McQuistan T, De Vries L, Farquhar M G. J Cell Biol. 1998;141:1515–1527. doi: 10.1083/jcb.141.7.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madden T L, Tatusov R L, Zhang J. Methods Enzymol. 1996;266:131–141. doi: 10.1016/s0076-6879(96)66011-x. [DOI] [PubMed] [Google Scholar]
- 15.De Vries L, Elenko E, Hubler L, Jones T L, Farquhar M G. Proc Natl Acad Sci USA. 1996;93:15203–15208. doi: 10.1073/pnas.93.26.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vries L, Mousli M, Wurmser A, Farquhar M G. Proc Natl Acad Sci USA. 1995;92:11916–11920. doi: 10.1073/pnas.92.25.11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guarente L. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- 18.Fischer T, Elenko E, McCaffery J M, DeVries L, Farquhar M G. Proc Natl Acad Sci USA. 1999;96:6722–6727. doi: 10.1073/pnas.96.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antonny B, Bigay J, Chabre M. FEBS Lett. 1990;268:277–280. doi: 10.1016/0014-5793(90)81027-l. [DOI] [PubMed] [Google Scholar]
- 20.Higashijima T, Ferguson K M, Smigel M D, Gilman A G. J Biol Chem. 1987;262:757–761. [PubMed] [Google Scholar]
- 21.Phillips W J, Cerione R A. J Biol Chem. 1988;263:15498–15505. [PubMed] [Google Scholar]
- 22.Mochizuki N, Cho G, Wen B, Insel P A. Gene. 1996;181:39–43. doi: 10.1016/s0378-1119(96)00456-8. [DOI] [PubMed] [Google Scholar]
- 23.Snow B E, Antonio L, Suggs S, Gutstein H B, Siderovski D P. Biochem Biophys Res Commun. 1997;233:770–777. doi: 10.1006/bbrc.1997.6537. [DOI] [PubMed] [Google Scholar]
- 24.Granderath S, Stollewerk A, Greig S, Goodman C S, O'Kane C J, Klambt C. Development. 1999;126:1781–1791. doi: 10.1242/dev.126.8.1781. [DOI] [PubMed] [Google Scholar]
- 25.Yu F, Morin X, Cai Y, Yang X, Chia W. Cell. 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer M, Shevchenko A, Knoblich J A. Curr Biol. 2000;10:353–362. doi: 10.1016/s0960-9822(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 27.Siderovski D P, Diverse-Pierluissi M, De Vries L. Trends Biochem Sci. 1999;24:340–341. doi: 10.1016/s0968-0004(99)01441-3. [DOI] [PubMed] [Google Scholar]
- 28.Blatch G L, Lassle M. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Dietzel C, Kurjan J. Cell. 1987;50:1001–1010. doi: 10.1016/0092-8674(87)90166-8. [DOI] [PubMed] [Google Scholar]
- 30.Wall M A, Posner B A, Sprang S R. Structure. 1998;6:1169–1183. doi: 10.1016/s0969-2126(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 31.Meng J, Glick J L, Polakis P, Casey P J. J Biol Chem. 1999;274:36663–36669. doi: 10.1074/jbc.274.51.36663. [DOI] [PubMed] [Google Scholar]
- 32.Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, Sumimoto H. J Biol Chem. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]