Abstract
Background
The effects of hypercapnia on coronary arteries in humans are not known. We used transthoracic Doppler echocardiography (TTDE) to evaluate coronary blood flow velocity (CFV) changes in response to hypercapnia in healthy adults.
Methods and Results
Twenty adults underwent TTDE of the left anterior descending coronary artery while breathing room air, 40% FiO2, and 40% FiO2 with CO2 supplemented to end-tidal tensions of +5, +7.5, and +10 mmHg above baseline. Mean (standard deviation) diastolic peak CFV values for these conditions were 23.1(9.1), 23.0(9.0), 25.5(9.3), 27.9(11.5), and 31.5(13.0) cm/s. Significant overall differences between conditions (p<0.001) and progressive levels of hypercapnia (p≤0.01) were observed. CFV increases remained significant after adjusting for increases in cardiac output (p=0.038).
Conclusions
CFV increases with hypercapnia. This is the first report of human coronary artery flow responses to hypercapnia. TTDE methodology is feasible for measuring CFV and the effects of hypercapnia on the coronary circulation.
Keywords: Blood flow, Coronary arteries, Carbon dioxide, Echocardiography
Coronary flow reserve (CFR) is defined as the maximal increase in coronary blood flow, relative to baseline flow, that occurs when the coronary microcirculation is maximally dilated. CFR is impaired in the setting of epicardial coronary artery disease, as well as in disorders of the coronary microcirculation, such as diabetes mellitus, hypertension, hypercholesterolemia, cardiomyopathy, and syndrome X, in which epicardial vessels are angiographically normal.1-4 Improvement of CFR in some of these disorders has been observed with treatment.1-3,5,6 Blunted coronary flow velocity (CFV) responses to vasodilators also have been observed in disorders of the coronary microcirculation, in the presence and absence of angiographic coronary artery disease.5,7,8
Although coronary blood flow and CFR traditionally have been studied using an Doppler flow wire with intracoronary or intravenous administration of vasodilators during coronary artery catheterization,2,3,9-13 this method is invasive and entails potentially harmful exposures which limit its routine clinical or experimental use. There are less invasive imaging methods, including myocardial scintigraphy, magnetic resonance imaging, and transesophageal echocardiography, but their cost, radiation exposure, availability, and/or incomplete ability to localize abnormalities limits their application for serial studies of experimental or clinical conditions.3 Transthoracic Doppler echocardiography (TTDE) is an emerging noninvasive method to measure CFV.3,4,14-16 The proximity of the distal left anterior coronary artery (LAD) to the chest wall makes transthoracic CFV and CFR evaluations in this vessel segment possible, thus decreasing the potential for more distal stenoses and imaging artifacts that could affect interpretation. Distal LAD CFV and CFR measured by TTDE have been shown to be comparable to those obtained using the gold-standard Doppler flow wire technique.17-19
Hypercapnia is present in several disease states associated with increased cardiovascular risk, including obstructive sleep apnea. In animal models, hypercapnia causes coronary vasodilation and increases coronary arterial blood flow.20 In humans, hypercapnia increases flow in peripheral conduit arteries, a response that is blunted in the presence of cardiovascular risk factors through mechanisms that appear to be mediated at least in part by the endothelium.21-24 The effect of hypercapnia on the coronary arteries in humans, however, is not known. This study used TTDE to evaluate CFV changes in response to hypercapnia in healthy adults.
METHODS
Subject Characteristics
This study was approved by the Institutional Review Boards of the University of Wisconsin Medical School and the William S. Middleton Veterans Administration Hospital (Madison, WI). Subjects were healthy men (18–45 years old) and women (18–50 years old) with no known cardiovascular risk factors (including dyslipidemia, diabetes mellitus, hypertension, and current cigarette smoking) or disease or active obstructive pulmonary disease. All provided informed consent prior to study procedures.
General Procedures
Prior to the hypercapnic interventions, 12-hour fasting blood samples were collected by antecubital venipuncture for determination of complete blood count, serum electrolytes, creatinine, blood urea nitrogen, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), high-sensitivity C-reactive protein (hsCRP), carbon monoxide, and plasma glucose. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. Height and weight were measured. Waist circumference was measured at the midpoint between the inferior margin of the ribcage and the superior border of the iliac crest.
Experimental Procedures
Subjects were positioned in the left-lateral decubitus position. Heart rate was monitored by continuous electrocardiography. Blood pressure was monitored non-invasively by automated upper arm sphygmomanometer (Dinamap, Critikon). Arterial oxygen saturation was measured non-invasively by continuous pulse oximetry (Model 3740, Ohmeda). Ventilation was measured with a pneumotachograph attached to a mouthpiece (Model 3700; Hans Rudolph) from which PETCO2 was sampled continuously (Model CD3A, Ametek). Subjects inspired controlled mixtures of air through the mouthpiece with a nose-clip in place, while simultaneously undergoing echocardiography. CFV values were obtained at 5 stages: Baseline 1, as subjects breathed room air; Baseline 2, as subjects breathed room air supplemented with oxygen (FIO2=40%); and Stages 1–3, as subjects inspired air supplemented with oxygen and CO2 concentrations titrated to produce increases in PETCO2 of +5, +7.5, and +10 mmHg above the eupneic baseline level.
Echocardiography
A trained sonographer obtained echocardiographic images using a digital ultrasound system (Acuson Sequoia, Siemens Medical Solutions) and 4–7 MHz transducer at a Doppler frequency of 4 MHz. The acoustic window was localized to the 4th or 5th left intercostal space in the mid-clavicular line, depending on where the sharpest spectral Doppler envelope representing the LAD flow signal could be obtained. Color Doppler mapping was used to identify the LAD position with velocity ranges of ±12 cm/s to ±24 cm/s and pulsed-wave Doppler spectral tracings of CFV were recorded digitally. Additional echocardiographic images were recorded at both baseline stages and Stage 3 to permit measurement of the left ventricular outflow tract (LVOT) diameter, velocity-time integral (VTI) of pulsed-wave Doppler signals from the LVOT, as well as peak E and A velocities and deceleration times from the transmitral diastolic spectral Doppler signal. All studies were analyzed off-line using Access Point 2000 software (Freeland Systems). Measurements were performed by a single reader blinded to study stage in triplicate and averaged to determine peak diastolic and systolic CFV. Cardiac output was calculated as 0.785 * (LVOT diameter)2 * VTILVOT * heart rate.25
Data Analysis
SigmaStat for Windows 3.0 (SPSS, Inc.) was used for analyses. Continuous data were described by means (standard deviation), and categorical data were described using proportions. Comparisons between experimental stages were performed using one-way analysis of variance of repeated measures (ANOVA) for the following parameters: peak diastolic CFV, heart rate, systolic and diastolic blood pressures, VTI, cardiac output, and diastolic filling parameters. Adjustments for multiple pair-wise comparisons were performed using the Holm-Sidak method. The general linear model was used to adjust observed changes in CFV with inspired CO2 for changes in cardiac output, heart rate, and diastolic filling parameters.
RESULTS
Baseline Characteristics
For the 20 subjects, the mean age was 33 (7) years, 90% were white, and 52% were female (Table 1). A full set of images at all stages could be acquired from 17 of the subjects. Two subjects had adequate images at both baseline states but only the first level of hypercapnia. No images could be obtained in 1 subject. Compared to the others, the 3 with incomplete images were older (44[3] versus 32[5] years, p<0.001), more tachycardic (79[16] versus 67[8] beats per minute, p=0.04), and had higher body-mass index (29[5] versus 22[3] kg/m2, p=0.004).
Table 1.
Baseline Characteristics, n=20
| Age, years | 33 (7) |
| Male, n (%) | 9 (48) |
| White race, n (%) | 18 (90) |
| Body-mass index, kg/m2 | 23 (4) |
| Weight, kg | 68 (15) |
| Waist circumference, cm | 77 (12) |
| Heart Rate, beats/min | 63 (12) |
| Systolic blood pressure, mmHg | 107 (16) |
| Diastolic blood pressure, mmHg | 60 (10) |
| Respiratory rate, breaths/min | 13 (3) |
| Oxygen saturation at room air, % | 98 (2) |
| Hemoglobin, g/dL | 14.3 (1.5) |
| CO2, mmol/L | 26.1 (1.9) |
| Carbon monoxide, % | 1.0 (0.0) |
| Creatinine, mg/dL | 1.1 (0.2) |
| C-reactive protein, mg/L | 1.5 (1.6) |
| Glucose, mg/dL | 91.2 (7.7) [80–105] |
| Total cholesterol, mg/dL | 165 (26) [108–221] |
| High-density lipoprotein cholesterol, mg/dL | 68 (20) [37–112] |
| Triglycerides, mg/dL | 70 (43) [36–152] |
| Low-density lipoprotein cholesterol, mg/dL | 83 (22) [31–128] |
Continuous values reported as mean (standard deviation)
Ranges reported in square brackets
Effects of Hypercapnia
Physiological changes with hypercapnia are reported in Table 2. Representative spectral Doppler tracings from a subject at baseline and under maximal hypercapneic conditions are depicted in Figure 1. With increasing levels of inspired CO2, peak diastolic CFV increased in a dose-response manner (pANOVA<0.001), with significant increases observed with progressive levels of hypercapnia (p≤0.010 for each stage) (Figure 1). Heart rate and cardiac output also increased (both pANOVA<0.001), along with systolic blood pressure (pANOVA=0.028); however, stroke volume and diastolic blood pressure did not change significantly. Significant increases in velocities during early (E) and late diastole (A) were observed, as well as a decrease in deceleration time (p<0.03), but the overall E/A ratio did not change with inspired CO2 (p=0.18). The hypercapnia-induced changes observed in peak diastolic CFV remained significant even after adjusting for the change in cardiac output (p=0.038), but not heart rate (p=0.192). The relationships between CFV and the transmitral peak E-wave (p=0.072) and E-wave deceleration time (p=0.794) were not statistically significant after the effects of heart rate were considered. The respiratory rate increased slightly but significantly with increasing hypercapnia (from baseline 13 [3] to 16 [4] breaths/minute with maximal CO2 inhalation, p<0.001); the oxygen saturation did not change significantly.
Table 2.
Changes in Peak Diastolic CFV and Other Physiologic Parameters With Increasing Levels of Inspired CO2
| Experimental Stages | ||||||
|---|---|---|---|---|---|---|
| Baseline 1 | Baseline 2 | +5 mm Hg PETCO2 | +7.5 mm Hg PETCO2 | +10 mm Hg PETCO2 | PANOVA | |
| CFV, cm/s | 23.1 (9.1) | 23.0 (9.0) | 25.0 (9.3) | 27.9 (11.2) | 31.5 (13.0) | <0.001 |
| VTILVOT, cm | 22.8 (3.0) | 21.6 (1.9) | -- | -- | 23.3 (2.6) | 0.417 |
| Stroke volume, cm3 | 80.4 (16.8) | 76.3 (15.8) | -- | -- | 81.8 (18.0) | 0.337 |
| Heart rate, bpm | 61 (10) | 62 (8) | 63 (9) | 67 (7) | 67 (11) | <0.001 |
| Cardiac Output, L/min | 4.9 (1.0) | 4.7 (0.8) | 5.5 (1.3) | <0.001 | ||
| Systolic blood pressure, mmHg | 107 (16) | 103 (13) | 104 (14) | 106 (14) | 108 (15) | 0.028 |
| Diastolic blood pressure, mmHg | 60 (10) | 59 (8) | 59 (7) | 61 (7) | 62 (7) | 0.126 |
| Diastolic function parameters | ||||||
| E, cm/s | 87.9 (11.1) | 90.8 (12.6) | -- | -- | 101.4 (17.0) | 0.026 |
| Deceleration time, ms | 199.2 (2.4) | 190.2 (30.3) | -- | -- | 173.2 (27.7) | 0.002 |
| A, cm/s | 52.5 (8.9) | 53.2 (7.5) | -- | -- | 62.3 (12.1) | <0.001 |
| E/A ratio | 1.7 (0.3) | 1.7 (0.3) | -- | -- | 1.7 (0.3) | 0.180 |
All values reported as mean (standard deviation)
Baseline 1=room air
Baseline 2 and all subsequent stages =room air supplemented with 40% FiO2
CFV = coronary flow velocity
VTILVOT = velocity time-integral of flow in left ventricular outflow tract
ANOVA = analysis of variance
Figure 1.
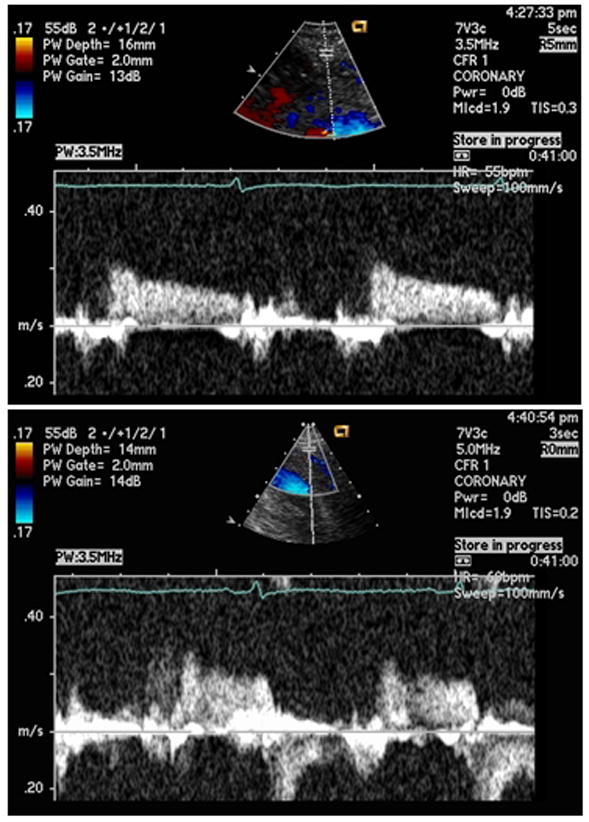
Representative Peak Diastolic Coronary Artery Blood Flow Velocities. Top panel = baseline conditions. Bottom panel = maximal experimental hypercapnia (+10mmHg PETCO2)
DISCUSSION
This study demonstrated that CFV increases with hypercapnia and that changes in CFV can be measured with transthoracic echocardiography. Among healthy adults, progressive hypercapnia increased peak diastolic CFV in dose-dependent fashion, independent of the concurrent increase in cardiac output. The increased cardiac output with inspired CO2 most likely was due to an increase in heart rate, as no significant change was observed in stroke volume. Finally, although significant changes were noted with hypercapnia among individual diastolic parameters, overall diastolic function as assessed by the transmitral E/A ratio did not change.
These findings are interesting for several reasons. This is the first study to report effects of hypercapnia on the human coronary circulation. It adds to previous findings in animal models showing hypercapnia-induced increases in coronary blood flow20 and in human studies showing increases in middle cerebral artery and internal thoracic artery flow velocities with inspired CO2.21-24 The observed arterial effect of inhaled CO2 may be mediated, at least in part by the endothelium-derived nitric oxide,23 and it is feasible that similar mechanisms may play a role in the response of the coronary arteries to CO2 shown in the present study. These considerations have particular relevance when addressing disease states such as obstructive sleep apnea (OSA), which is marked by both chronic intermittent hypercapnia and increased CVD risk. The reasons behind the latter are uncertain, but peripheral arterial endothelial dysfunction has been demonstrated among such cohorts so it is likely that coronary arterial endothelial dysfunction co-exists. 26-30 It would be interesting to determine whether those with OSA have a different CFV response to hypercapnia compared to healthy individuals without OSA, and whether chronically increased CO2 exposures induce a compensatory response that leads to abnormal endothelial function. However, a deeper understanding of the mechanisms of the CFV response to hypercapnia is needed before the latter inference can be made.
The study also is interesting because of the use of TTDE to assess changes in coronary flow in response to a non-pharmacologic, physiological intervention. Routine use of TTDE for the evaluation of coronary blood flow to-date has been an emerging technique limited to selected research facilities. These studies usually have used non-physiological exogenous vasodilators such as adenosine.2-4,9-11 While a fair amount of technical skill still is required, this study showed that appropriately trained echocardiographers can perform this technique in response to hypercapnia, which is a physiological intervention relevant to certain disease states. These combined techniques may offer a way to non-invasively and safely assess functional aspects of the coronary arteries among healthy and diseased cohorts, given its relatively minor effects on heart rate and blood pressure, and its coronary vasodilator effects.
Limitations
Considerable technical skills are required to perform TTDE evaluations of CFV consistently, and patient-specific characteristics can make image acquisition more difficult, particularly for those without extensive previous experience with the technique. Presence of any conditions that either interfere with echo transmission or promote increased coronary artery motion, including large body habitus, emphysema, tachycardia, or tachypnea, are examples. In the present study, the 3 subjects with incomplete or no images did have larger BMI; however, the one without any images was not overweight (BMI=23.1 kg/m2) but was simply more tachycardic. Lack of a complete set of images in the other two subjects was due to a combination of both tachypnea and tachycardia with higher levels of hypercapnia, since acquisition of images at both baselines and Stage 1 was possible. Thus while the technique is feasible and appears safe, it does require a skilled technician and may not be ideal for use in all populations. Use of echo contrast may have eased image acquisition in the more difficult cases; however, it was not tested in this study. Along with the technical challenges of Doppler signal acquisition, capturing a consistent two-dimensional image of the LAD sufficient for accurately measuring its diameter and changes in diameter with interventions, was not possible. Thus we could not directly determine if the observed changes in CFV values were due to changes in arterial diameter. In animal models, however, hypercapnia causes coronary vasodilation and increases coronary arterial blood flow.20
The etiology of increased CFV with inhaled CO2 could not be determined in this pilot study. Previous work has suggested that the endothelium could be involved, as has been demonstrated in the cerebral vasculature.23 However, changes in pH, the effect of sympathetic activation on myocardial oxygen consumption, or activation of opiate receptors could have played a role.20,23,31 This pilot study did indicate some adrenergic stimulation with hypercapnia, given that heart rate and cardiac output increased. The increase in CFV was independent of cardiac output, but not heart rate. Although this may be due to a lack of statistical power and a stronger association between heart rate and CFV than between hypercapneic stage and CFV, it also may suggest that increased myocardial oxygen consumption contributed to the increased coronary flow velocities we observed. The peak transmitral E-wave velocity and deceleration time also did not independently predict CFV when heart rate was considered, and the E/A ratio did not change, suggesting that the observed changes in CFV were not due to intrinsic changes in left ventricular diastolic function or left heart filling pressures. It is likely that several factors affect CFV during hypercapnia. Future studies of this technique incorporating more specific markers of endothelial function and/or adrenergic pathways could further clarify these issues.
Conclusions
In healthy individuals, CFV increases with hypercapnia. This is the first report of human coronary artery flow responses to hypercapnia. These combined techniques offer a feasible, non-invasive method for studying functional aspects of coronary arteries.
Figure 2.
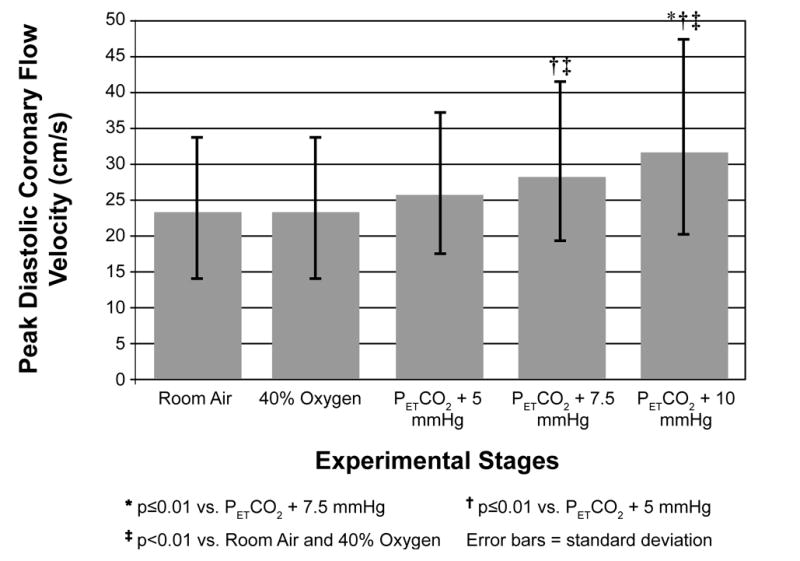
Change in Peak Diastolic Coronary Blood Flow Velocity With Increasing Levels of Inspired CO2
Footnotes
Financial Support:
Funded in part by the National Heart, Lung, and Blood Institute (HL-07936, HL-07654, HL-015469), the National Center for Research Resources (RR-03186, RR-16176, and RR-021086), and the Medical Research Service of the United States Department of Veterans Affairs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ofili EO, Kern MJ, Labovitz AJ, St Vrain JA, Segal J, Aguirre FV, Castello R. Analysis of coronary blood flow velocity dynamics in angiographically normal and stenosed arteries before and after endolumen enlargement by angioplasty. J Am Coll Cardiol. 1993;21:308–316. doi: 10.1016/0735-1097(93)90668-q. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, di Mario C, Piek J, Schroeder E, Vrints C, Probst P, de Bruyne B, Hanet C, Fleck E, Haude M, Verna E, Voudris V, Geschwind H, Emanuelsson H, Muhlberger V, Danzi G, Peels HO, Ford AJ, Jr, Boersma E. Prognostic value of intracoronary flow velocity and diameter stenosis in assessing the short- and long-term outcomes of coronary balloon angioplasty: the DEBATE Study (Doppler Endpoints Balloon Angioplasty Trial Europe) Circulation. 1997;96:3369–3377. doi: 10.1161/01.cir.96.10.3369. [DOI] [PubMed] [Google Scholar]
- 3.Tries H-P, Lethen H, Lambertz H. Coronary Flow Reserve: A Practical Echocardiographic Approach. Hounslow, Middlesex UK: Acuson Limited, Siemens House; 2001. [Google Scholar]
- 4.Dimitrow PP. Transthoracic Doppler echocardiography - noninvasive diagnostic window for coronary flow reserve assessment. Cardiovasc Ultrasound. 2003;1:4. doi: 10.1186/1476-7120-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwakura K, Ito H, Kawano S, Okamura A, Tanaka K, Nishida Y, Maekawa Y, Fujii K. Assessing myocardial perfusion with the transthoracic Doppler technique in patients with reperfused anterior myocardial infarction: comparison with angiographic, enzymatic and electrocardiographic indices. Eur Heart J. 2004;25:1526–1533. doi: 10.1016/j.ehj.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259–264. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 7.Kyriakidis MK, Dernellis JM, Androulakis AE, Kelepeshis GA, Barbetseas J, Anastasakis AN, Trikas AG, Tentolouris CA, Gialafos JE, Toutouzas PK. Changes in phasic coronary blood flow velocity profile and relative coronary flow reserve in patients with hypertrophic obstructive cardiomyopathy. Circulation. 1997;96:834–841. doi: 10.1161/01.cir.96.3.834. [DOI] [PubMed] [Google Scholar]
- 8.Misawa K, Nitta Y, Matsubara T, Oe K, Kiyama M, Shimizu M, Mabuchi H. Difference in coronary blood flow dynamics between patients with hypertension and those with hypertrophic cardiomyopathy. Hypertens Res. 2002;25:711–716. doi: 10.1291/hypres.25.711. [DOI] [PubMed] [Google Scholar]
- 9.Pizzuto F, Voci P, Mariano E, Puddu PE, Sardella G, Nigri A. Assessment of flow velocity reserve by transthoracic Doppler echocardiography and venous adenosine infusion before and after left anterior descending coronary artery stenting. J Am Coll Cardiol. 2001;38:155–162. doi: 10.1016/s0735-1097(01)01333-x. [DOI] [PubMed] [Google Scholar]
- 10.Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, Morioka S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation. 1998;97:1557–1562. doi: 10.1161/01.cir.97.16.1557. [DOI] [PubMed] [Google Scholar]
- 11.Winter R, Gudmundsson P, Willenheimer R. Feasibility of noninvasive transthoracic echocardiography/doppler measurement of coronary flow reserve in left anterior descending coronary artery in patients with acute coronary syndrome: A new technique tested in clinical practice. J Am Soc Echocardiogr. 2003;16:464–468. doi: 10.1016/s0894-7317(03)00112-3. [DOI] [PubMed] [Google Scholar]
- 12.Caiati C, Montaldo C, Zedda N, Bina A, Iliceto S. New noninvasive method for coronary flow reserve assessment: contrast-enhanced transthoracic second harmonic echo Doppler. Circulation. 1999;99:771–778. doi: 10.1161/01.cir.99.6.771. [DOI] [PubMed] [Google Scholar]
- 13.Lowenstein J, Tiano C, Marquez G, Presti C, Quiroz C. Simultaneous analysis of wall motion and coronary flow reserve of the left anterior descending coronary artery by transthoracic doppler echocardiography during dipyridamole stress echocardiography. J Am Soc Echocardiogr. 2003;16:607–613. doi: 10.1016/s0894-7317(03)00281-5. [DOI] [PubMed] [Google Scholar]
- 14.Crowley JJ, Shapiro LM. Transthoracic echocardiographic measurement of coronary blood flow and reserve. J Am Soc Echocardiogr. 1997;10:337–343. doi: 10.1016/s0894-7317(97)70070-1. [DOI] [PubMed] [Google Scholar]
- 15.Lambertz H, Tries HP, Stein T, Lethen H. Noninvasive assessment of coronary flow reserve with transthoracic signal-enhanced Doppler echocardiography. J Am Soc Echocardiogr. 1999;12:186–195. doi: 10.1016/s0894-7317(99)70134-3. [DOI] [PubMed] [Google Scholar]
- 16.Nohtomi Y, Takeuchi M, Nagasawa K, Arimura K, Miyata K, Kuwata K, Yamawaki T, Kondo S, Yamada A, Okamatsu S. Simultaneous assessment of wall motion and coronary flow velocity in the left anterior descending coronary artery during dipyridamole stress echocardiography. J Am Soc Echocardiogr. 2003;16:457–463. doi: 10.1016/s0894-7317(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 17.Caiati C, Montaldo C, Zedda N, Montisci R, Ruscazio M, Lai G, Cadeddu M, Meloni L, Iliceto S. Validation of a new noninvasive method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve: comparison with intracoronary Doppler flow wire. J Am Coll Cardiol. 1999;34:1193–1200. doi: 10.1016/s0735-1097(99)00342-3. [DOI] [PubMed] [Google Scholar]
- 18.Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, Kaji S, Kawamoto T, Ueda Y, Morioka S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32:1251–1259. doi: 10.1016/s0735-1097(98)00389-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Otsuji Y, Minagoe S, Hamasaki S, Toyonaga K, Negishi M, Tsurugida M, Toda H, Tei C. Noninvasive evaluation of coronary reperfusion by transthoracic Doppler echocardiography in patients with anterior acute myocardial infarction before coronary intervention. Circulation. 2003;108:2763–2768. doi: 10.1161/01.CIR.0000103625.15944.62. [DOI] [PubMed] [Google Scholar]
- 20.Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Ozkan M, Koramaz I, Ulus AT, Tavil Y, Filizlioglu H, Baykan EC, Eryilmaz S, Inan B, Katircioglu SF, Ozyurda U. Effect of carbon dioxide insufflation on free internal thoracic artery flows: is it a vasodilator? J Thorac Cardiovasc Surg. 2004;128:354–356. doi: 10.1016/j.jtcvs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Przybylowski T, Bangash MF, Reichmuth K, Morgan BJ, Skatrud JB, Dempsey JA. Mechanisms of the Cerebrovascular Response to Apnoea. J Physiol. 2003;548:323–332. doi: 10.1113/jphysiol.2002.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation. 2003;107:1901–1905. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- 24.Rosengarten B, Spiller A, Aldinger C, Kaps M. Control system analysis of visually evoked blood flow regulation in humans under normocapnia and hypercapnia. Eur J Ultrasound. 2003;16:169–175. doi: 10.1016/s0929-8266(02)00070-8. [DOI] [PubMed] [Google Scholar]
- 25.Otto C. Textbook of Clinical Echocardiography. 3. Philadelphia: Elsevier Saunders; 2004. [Google Scholar]
- 26.Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea-induced hypoxemia during sleep. Chest. 2001;119:1085–1091. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 28.Carlson JT, Rangemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens. 1996;14:577–584. doi: 10.1097/00004872-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Diomedi M, Placidi F, Cupini LM, Bernardi G, Silvestrini M. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology. 1998;51:1051–1056. doi: 10.1212/wnl.51.4.1051. [DOI] [PubMed] [Google Scholar]
- 30.Loeppky JA, Voyles WF, Eldridge MW, Sikes CW. Sleep apnea and autonomic cerebrovascular dysfunction. Sleep. 1987;10:25–34. doi: 10.1093/sleep/10.1.25. [DOI] [PubMed] [Google Scholar]
- 31.Komjati K, Greenberg JH, Reivich M, Sandor P. Interactions between the endothelium-derived relaxing factor/nitric oxide system and the endogenous opiate system in the modulation of cerebral and spinal vascular CO2 responsiveness. J Cereb Blood Flow Metab. 2001;21:937–944. doi: 10.1097/00004647-200108000-00006. [DOI] [PubMed] [Google Scholar]


