Abstract
Many strategies for redirecting the tropism of murine Moloney leukemia virus (MMLV) have been described. Preformed virion-liposome complexes, termed virosomes, have been reported to be relatively stable. Virosomes mediate envelope-independent transduction that allows efficient superinfection of resistant cell lines; however, virosome-mediated transduction behaves in a non–target-specific manner. We developed a novel method using antibodies to direct MMLV to vascular endothelium. We have given the term immunovirosomes to the complexes formed between viruses, liposomes, and antibodies. These immunovirosomes improve the transduction efficiency of the viruses and alter their tropism. We have shown improved transduction when immunovirosomes were targeted at the endocytic receptors CD71 and CD62E/P and rather less good delivery when targeted at CD106. The enhancement of the transduction efficiency was transient, however, suggesting that rerouting the entry pathway of viruses alters the expression properties of the viruses.
INTRODUCTION
Viral and nonviral vectors can be manipulated to increase the efficiency and specificity for vascular cell transduction (1,2). This can be achieved either by modifying the cell binding properties of the vectors or by the use of cell-selective promoters (3,4). Altering the tropism of viral vectors can be achieved by genetic modification of the viral envelope (5–8) or by the use of proteins derived from other enveloped viruses (9,10). Alternatively, targeting molecules can be derived from nonretroviral proteins expressed on the packaging cell line (11–13). A further alternative is to use adaptor molecules that retarget the virus to specific cell-surface molecules (14–16). These approaches can be time consuming and often affect the production of the virus. In addition, although in some cases they offer high specificity, they can result in poor transduction efficiency.
In this report, we describe an alternative targeting strategy using immunovirosomes generated by mixing mildly aggregated monoclonal antibodies, liposomes, and viral particles. This strategy is based on the ability of cationic liposomes to form stable complexes with viral vectors (17,18). This interaction has been reported for Moloney murine leukemia virus (MMLV). Here, we have shown that immunovirosomes carrying monoclonal antibodies against endothelial markers can target activated vascular endothelium. The resulting gene transfer efficiency is enhanced if endocytic receptors are chosen. Importantly, our results demonstrate that changing viral tropism can alter the ability of viral transduction to result in long-term expression. These findings will have profound consequences on future viral vector design with respect to improving specificity and efficiency.
MATERIALS AND METHODS
Reagents
RPMI-1640, CD hybridoma medium, hybridoma serum-free medium (SFM), human endothelial basal growth-SFM, l-glutamine, penicillin, streptomycin, and trypsin-EDTA were purchased from Invitrogen (Paisley, UK), and fetal calf serum (FCS) was purchased from Globepharm (Esher, UK). The synthetic liposome Tfx-50 was purchased from Promega (Southampton, UK). Lipofectin and LipofectAMINE were obtained from Invitrogen. Other reagents were purchased from Sigma (Poole, UK), unless stated otherwise.
Preparation of Monoclonal Antibodies
Hybridomas producing the anti-human transferrin receptor (CD71) mAb, OKT9; anti-E/P-selectin (CD62E/P), 1.2B6 (dual-specificity), and anti-VCAM-1 (CD106), 1.4C3, were grown and purified by protein G chromatography, as described (19).
Immunoliposome Preparation
Immunoliposomes were prepared as described (19,20). In brief, heat-aggregated Ab [optimal mAb concentrations; OKT9 (60 μ g/mL), 1.2B6 (30 μ g/mL), and 1.4C3 (60 μ g/mL)] was mixed with liposomes (Tfx-50, Lipofectin, or LipofectAMINE), for 30 min at room temperature (in total volume of 250 μ L in Opti-MEM). For plasmid-mediated transfection, the immunoliposomes were then incubated with DNA at a ratio recommended by the manufacturer for the liposome-DNA complex. The resulting transfection complexes were added to endothelial cells (ECs). As in the case of the immunovirosomes, the retroviral supernatants were mixed with immunoliposomes at equal volumes for 30 min at room temperature before the addition to the cells (1 × 105). In some cases, the retroviral supernatant was treated with DNAse before transduction.
EC Isolation and Culture
ECs were isolated from human saphenous veins and maintained in culture as described (21,22). Briefly, ECs were cultured in EBM-2 (BioWhittaker, Cambridge, UK) and human endothelial-SFM basal growth medium supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μ g/mL streptomycin, 25 μ g/mL fungizone, 0.03 mg/mL endothelial cell growth supplement (ECGS), and 100 U/mL heparin. All cells were used between the 3rd and 4th passages. Human saphenous veins were obtained from patients undergoing varicose vein or coronary artery bypass surgery. Selected patients were either healthy young adults or with coronary artery disease, but without other comorbidity. Patients with diabetes or other autoimmune diseases were excluded from the study. Informed consent was obtained from all patients and approval obtained from the local research ethics committee. To inhibit de novo protein synthesis, the cells were treated with 50 μ g/mL cycloheximide.
Vascular Samples
Vascular tissues were obtained from the Cardiothoracic Department of Hammer-smith Hospital, UK. The tissues were obtained as a surplus product from coronary bypass surgery, after local research ethics approval, of patients ages 45 to 85 years.
MMLV Production and Titration: Retroviral Vectors and Packaging Cell Lines
The plasmids—pHIT60 containing gag-pol genes (23), pCL-Eco env containing ecotropic envelope (24), pCOLT-GALV env containing gibbon ape leukemia virus envelope (GALV env) protein (25), or pRV67 env containing vesicular stomatitis virus G envelope (VSV-G env) protein (22,26)—and the retroviral constructs—pBullet-EGFP (22,26,27), LZRS-EGFP (22,26,28,29), pFB-rhGFP (Stratagene, Cambridge, UK) (22,26), or Pinco-EGFP (30)—were used to generate retro-viral particles as described below.
Phoenix ecotropic (30) and amphotropic (23) packaging lines (kind gifts from Dr Gary Nolan’s laboratory, Stanford University) and 293T cells (22,26) were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FCS.
Ecotropic MMLV Production
The ecotropic MMLV particles were generated as described (26). Briefly, the Phoenix ecotropic cells and 293T cells were cocultured in DMEM at a ratio of 1:1 (1.5 × 106 cells seeded in 25-cm2 flask on the day before transfection). Twenty-four hours later, the retroviral-GFP construct (Pinco-EGFP) together with pHIT60 and pCL-Eco were cotransfected into the seeded packaging cells using a calcium phosphate transfection kit (Invitrogen) according to the manufacturer’s instructions. Sixteen hours after transfection, cells were washed twice with warm PBS and the DMEM was replaced with RPMI-1640. Forty-eight hours after transfection, the viral supernatants were harvested and filtered through a 0.45-μ m pore size filter and stored at –80°C. Retroviral titers of the PINCO vector were determined on NIH 3T3 cells as described (31). The cells were analyzed by flow cytometry to determine the proportion of NIH 3T3 cells expressing EGFP.
VSV-G–Pseudotyped and GALV-Pseudotyped MMLV Production and Titration
The MMLV-based constructs were propagated by a 3-plasmid cotransfection technique in 293T cells as described (22,26). Transfections were performed using polyethylenimine (PEI) (Sigma) on 9-cm tissue culture plates as described (26). In the case, of GALV-pseudotyping, 2 × 106 293T were seeded into a T25 tissue culture flask and 24 hours later transiently transfected with retroviral-EGFP constructs with pCOLT-GALV using a calcium phosphate transfection kit (Invitrogen) according to the manufacturer’s instructions.
MMLV were titred on either D17 cell (VSV-G pseudotyped) or 293 and/or Hela cells (GALV pseudotyped) as previously described (26). In brief cells were seeded on 24 well plates at 3 x 104 cells per well, infected with serial 1:10 dilutions of virus and analyzed for EGFP expression at 3 to 5 days post-infection.
Amphotropic MMLV Production and Titration
The amphotropic MMLV particles were generated as described (23). Briefly, 2 × 106 amphotropic packaging cells were seeded into a T25 tissue culture flask and 24 hours later transiently transfected with retroviral-EGFP constructs using a calcium phosphate transfection kit (Invitrogen) according to the manufacturer’s instructions. Sixteen hours after transfection, cells were washed twice with warm PBS, and the DMEM was replaced with RPMI-1640. Forty-eight hours after transfection, the viral supernatants were harvested and filtered through a 0.45-μ m pore size filter and stored at –80°C. As described (26), stocks of vectors were titered by infecting 293T and/or HeLa cells with serial 1:10 dilutions of virus and analyzing for EGFP expression at 3 to 5 days postinfection.
Transfection with Virosomes and Immunovirosomes
For transfection, virosomes and immunovirosomes were generated as described above and cultured with 105 EC cells. The MMLV [with or without polybrene (PB)], virosomes, or immunovirosomes were exposed to cells for 2 h at 37°C. To block targeted endothelial receptors of ECs before transduction, ECs were preincubated with 200 μ g/mL of appropriate antibody at 4°C, then transfected. In some cases, the viral particles were exposed to 50 kGy gamma irradiation.
Flow Cytometry
The phenotype of transfected or untransfected ECs was assessed by flow cytometry 48 h after transfection. Cell staining was performed using mouse antibodies conjugated to APC or primary antibodies followed by goat anti-mouse-APC, as described (19,26). Flow cytometric analysis of all cells was performed using the following mouse monoclonal antibodies: 1.2B6 (anti-CD62E/P) (21), 1.4C3 (anti-CD106) (21), and 6.5B5 (anti-CD54) (21). All antibodies raised from hybridomas were kindly given by Prof. D Haskard, Imperial College London, UK, unless stated otherwise (21).
Assessment of EGFP Reporter Gene Expression
After transduction, EGFP reporter gene expression was determined using flow cytometry or an inverted fluorescent microscope, as described (19,32).
Labeling of YOYO-1 and Determination of Internalization
YOYO-1 stock solution (1 × 10−3 M) (Molecular Probe, Cambridge, UK) was initially diluted 7.5 times in DMSO to a concentration of 1.33 × 10−4 M. The working concentration of YOYO-1 in all samples was 6.3 × 10−6 M. To prevent photo-bleaching of YOYO-1, its solutions were kept in the dark and all experiments were protected from light. The viral particles were labelled with YOYO-1 and visualisation of these labelled particles using flow cytometry was carried out as previously described (33, 35). Uninternalized and surface-bound particles were removed as described (32). Internalization of viral particles was assessed using flow cytometry. In some cases, the cells were treated with chloroquine as described (35).
RT-PCR and Southern Blots
RT-PCR analysis for EGFP gene expression was carried out as described (36) with the following primers; 5 ′ -GAT CTT GAA GTT CAC CTT GAT GCC (sense) and 5 ′ -AGC TGA CCC TGA AGT TCA TCT GC (antisense) at 60°C annealing temperature. To detect the level of integration of EGFP, we carried out Southern blot hybridization for EGFP DNA in the genomic DNA of transduced cells. Genomic DNA was extracted in accordance with manufacturer’s instructions (Qiagen, Crawley, UK). It was then digested with NcoI (position 4137) and NotI (position 4870) (pBullet-EGFP). Gel electrophoresis and Southern blotting were performed as described (37). The blot was hybridized with [32P]-labeled probe using EGFP fragment released from pBullet-EGFP vector. After 12 h hybridization at 68°C, the blot was washed 3 times in 0.3 × SSC, 0.1% SDS at 68°C before exposing to X-ray film at –80°C for 2 h. The intensity of Southern blot was analyzed as previously described (38).
Reproducibility and Statistical Analysis
For multiple comparisons, results were analyzed by ANOVA, whereas for comparison of 2 means, results were analyzed by Student t test where P < 0.05 was considered statistically significant. All data shown are representative of at least three experiments, unless stated otherwise.
RESULTS
Expression of Adhesion Molecules by Activated Endothelial Cells
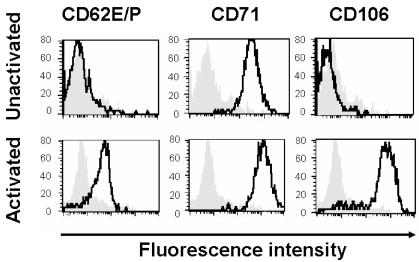
As previously reported (19), HSVECs constitutively express CD71 (Figure 1). However, after activation with proinflammatory cytokines (80 ng/mL TNF- α, 80 ng/mL IFN-γ , and 80 ng/mL IL-1β ) for 8 h, the cells upregulate the expression of adhesion molecules (CD62E/P and CD106) and CD71 (21) (Figure 1).
Figure 1.
Expression of surface receptors. HSVECs were cultured and the surface expression of CD62E/P, CD71, and CD106 were analyzed using flow cytometry. Where indicated, the cells were stimulated with proinflammatory cytokines (80 ng/mL TNF- α , 80 ng/mL IFN-γ , and 80 ng/mL IL-1β ) for 8 h. The gray backgrounds show staining with isotype-control antibody, and the dark black lines are specific antibodies against CD62E/P, CD71, and CD106. These results are representative of at least three experiments.
Immunoliposomes Enhance Gene Expression by MMLV and Alter Viral Tropism
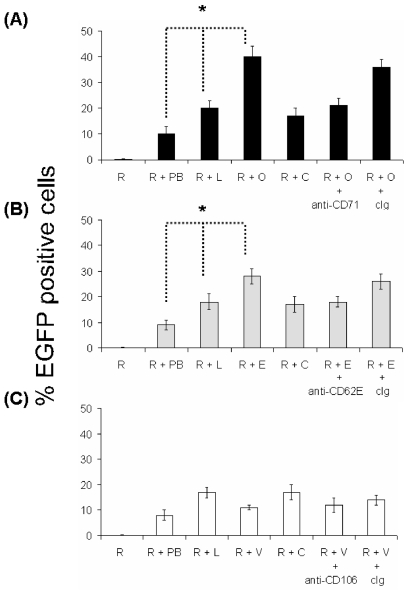
In our experience, MMLV inefficiently transduces HSVECs, in both the presence or absence of polybrene. The addition of liposomes to MMLV has been shown to enhance transduction efficiency (17,18). Using liposomes [Tfx-50 or lipofectin (data not shown) or lipofectAMINE (data not shown)] and amphotropic MMLV [LZRS-EGFP (data not shown), pFB-rhGFP (data not shown), or pBullet-EGFP (Figure 2 with Tfx50 liposomes)], we demonstrated an approximately 10-fold increase in cells expressing EGFP compared with MMLV alone. We investigated whether this could be further increased by incorporation of antibodies. An approximately two-fold increase in expression efficiency was seen with anti-CD71 immunovirosomes compared with virosomes (Figure 2). Similarly, anti-CD62E/P could increase transduction in stimulated HSVECs expressing CD62E/P (Figure 2b). However, as expected from a previous study with immunoliposomes (19), anti-CD106 (Figure 2c) showed only minor or no augmentation. The expression levels of CD106 as determined by flow cytometry were more than CD62E/P on the endothelial cells following 8-h activation (Figure 1) (21). In all cases, the enhanced expression efficiency mediated by an antibody could be blocked with free specific antibody, but not with control antibodies.
Figure 2.
Transducibility of ECs by retroviruses. pBullet-EGFP was used to generate amphotropic MMLV. HSVECs (105) were cultured until confluent in 24-well plates. HSVECs were left unstimulated (A) or activated with proinflammatory cytokines (80 ng/mL TNF- α , 80 ng/mL IFN-γ , and 80 ng/mL IL-1β ) for 8 h (B and C). The cells (105) were treated with retroviral supernatants only (R), retroviral supernatant plus 8 μ g/mL Polybrene (R + PB), retroviral supernatants in combination with liposomes (R + L), retroviral supernatants together with liposomes and antibodies [anti-CD71, OKT9 (R + O) (A), anti-CD62, 1.2B6 (R + E) (B), anti-CD106, 1G11 (R + V) (C), or isotype control (R + C) (A–C) mAbs]. For some experiments (as indicated), the cells were preincubated with 200 μ g/mL OKT9 (+ anti-CD71) (A), 1.2B6 (+ anti-CD62) (B), 1G11 (+ anti-CD106) (C), or isotype control Ig (+ cIg) (A–C). The expression of EGFP after transduction was determined by flow cytometry. The results are expressed as percentage of EGFP-positive cells (transfection efficiency) ± SD of triplicates. These are representative of three independent experiments. Results were analyzed with ANOVA. *P < 0.05.
Similar augmentation of expression was seen whether the immunovirosomes were made with amphotropic, GALV, ecotropic, or VSV-G coat proteins. In all cases, the presence of liposomes and anti-CD71 antibody enhanced expression efficiency (Table 1). These effects were seen not only on human cells; a specific enhancement of expression efficiency in primary mouse cardiac ECs was also observed using transferrin (in nonactivated ECs) or anti-CD62E (in activated ECs) (data not shown).
Table 1.
Gene transfer with different pseudotyped MMLV, virosomes, and immunovirosomes.
| Viral coat proteins
|
||||
|---|---|---|---|---|
| Amphotropic | Ecotropic | GALV | VSV-G | |
| Unstimulated HSVECs | ||||
| MMLV | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.7 ± 0.5 | 0.4 ± 0.8 |
| MMLV + polybrene | 9.2 ± 1.7 | 3.1 ± 2.9 | 10.2 ± 2.8 | 10.1 ± 2.3 |
| Virosomes | 19.1 ± 3.2 | 20.8 ± 2.4 | 22.8 ± 3.1 | 21.8 ± 1.7 |
| Anti-CD71 immunovirosomes | 39.3 ± 3.4 | 40.8 ± 1.7 | 41.9 ± 6.9 | 42.7 ± 3.5 |
| Stimulated HSVECs | ||||
| MMLV | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.3 |
| MMLV + polybrene | 10.8 ± 0.3 | 2.8 ± 3.9 | 10.7 ± 3.4 | 10.6 ± 4.9 |
| Virosomes | 22.2 ± 8.1 | 22.7 ± 3.2 | 22.3 ± 4.5 | 24.1 ± 3.8 |
| Anti-CD62E/P immunovirosomes | 35.2 ± 1.8 | 36.9 ± 3.3 | 37.1 ± 5.2 | 38.1 ± 7.1 |
HSVECs (either nonactivated or activated) were treated with amphotropic, ecotropic, GALV- and VSV-G-pseudotyped MMLV. These pseudotyped MMLV were then used to generated virosomes [Tfx-50, lipofectin (data not shown) or LipofectAMINE (data not shown)] and anti-CD71 containing immunovirosomes for nonactivated ECs (upper panel) or anti-CD62E/P containing immunovirosomes for cytokine-activated ECs (lower panel). All results are expressed as mean ± SD of transfection efficiency of triplicate wells and all experiments were carried out at least three times.
Effect of Virosomes and Immunovirosomes on Viral Particle Entry to Cells
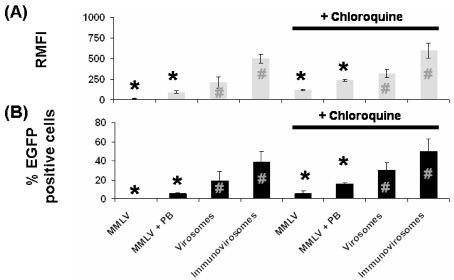
We investigated the internalisation of viral particles in target cells by labelling the viruses with fluorescent dye and then following of internalisation of the virus by flow cytometry (using washing with NaOH to remove surface bound material). The data show that when immunovirosomes are targeted at endocytic receptors, such as CD71, there is more rapid uptake than virus alone or incorporated into a virosome (Figure 3a). Chloroquine has been shown to increase gene expression in a number of vector systems, by inhibiting acidification of the endolysosomal pathways and thus increasing the amount of cDNA reaching the nucleus (35). We showed that treatment with this endosomolytic agent can increase cellular entry of MMLV particles in the presence and absence of polybrene. However, less impressive improvement in cellular entry was seen when the particles were packaged by liposomes and immunoliposomes (Figure 3a).
Figure 3.
Viral particle entry and transduction efficiency after endosomolytic agent treatment. (A) To address entry of viral particles following transduction, we labeled the particles with YOYO-1 before transduction. In some cases, cells were treated with chloroquine before transduction. After removal of uninternalized and surface-bound particles, fluorescence intensity was assessed using flow cytometry at 4 h after transduction. The results are expressed as mean relative fluorescence intensity ± SD. (B) HSVECs were transduced with MMLV, MMLV plus polybrene (PB), virosomes and immunovirosomes (carrying anti-CD71 antibodies). Percentage of transduced cells was analyzed using flow cytometry. Results comparing treatment with or without chloroquine were compared using the paired t test (shown with + chloroquine data) with * indicates P < 0.001 and # indicates P < 0.05. These are representative of three independent experiments. Results were analyzed with paired t test. *P < 0.001; #P < 0.05.
Transduction of Virosomes and Immunovirosomes After an Endosomolytic Treatment
As observed in Figure 2, transduction of HSVECs with MMLV alone and MMLV in the presence of polybrene resulted in poor transduction efficiency. When comparing MMLV transduction of HSVECs in the absence of polybrene with virosomes and immunovirosomes, we saw enhancement in transduction efficiency of ~100-fold and ~250-fold, respectively (Figure 3b). On the other hand, when MMLV transduction in the presence of polybrene was compared to virosomes and immunovirosomes, only ~two-fold and ~five-fold increases, respectively, was observed (Figure 3b).
Cellular treatment with chloroquine can improve transduction efficiency of MMLV alone or MMLV in the presence of polybrene by ~25-fold and ~two-fold, respectively (Figure 3b). However, in the case of virosome and immunovirosome transduction, the addition of chloroquine resulted in only marginal increase in transduction efficiencies (Figure 3b) (albeit statistically significant but probably with limited any biological significance).
Effect of Virosomes and Immunovirosomes on Kinetics of Gene Expression
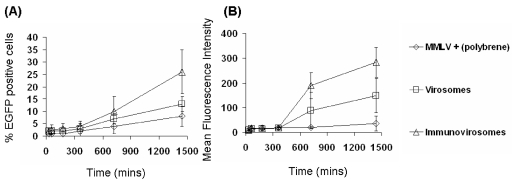
The time-course of expression after gene transfer with virus, virosomes, or immunovirosomes was compared. Following amphotropic MMLV, virosome, or immunovirosome transduction/transfection, the early expression of EGFP (less than 24 h) indicated that there was a slow increase in EGFP-positive cells and mean fluorescence intensity of cells (Figure 4) in all methods of transduction/transfection.
Figure 4.
Early kinetics of gene expression. HSVECs that had been stimulated with 80 ng/mL TNF- α , 80 ng/mL IFN-γ , and 80 ng/mL IL-1β ) for 8 h were treated with amphotropic MMLV-EGFP in combination with polybrene or amphotropic MMLV-EGFP containing virosomes or immunovirosomes (made with anti-CD71 mAb). After transduction/transfection, the cells were cultured for 24 h. Flow cytometric analysis was performed at 30, 60, 180, 360, 720, and 1440 min to determine the percentage of EGFP-positive cells (transfection efficiency) (A) and the mean fluorescence intensity of cell (B). All results are expressed as the mean (% transfection efficiency) ± SD of triplicates (A) and the mean fluorescence intensity (B).
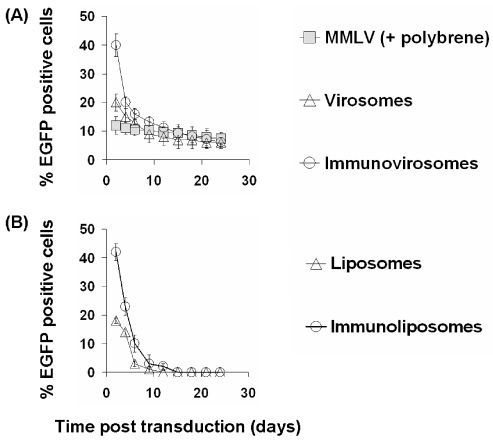
After 24 h following amphotropic MMLV gene transfer, the expression of EGFP was stable with only a slight decrease over time in the proportion of cells expressing EGFP (Figure 5). However, in cells treated with either virosomes or immunovirosomes made with anti-CD71 (Figure 5) or anti-CD62E/P (data not shown), there was a decrease over two weeks in the proportion of cells expressing EGFP, at which time expression was equivalent to that seen following retroviral transduction. This reduction in the proportion of EGFP-expressing cells showed a similar kinetics to the reduction in expression seen following transfection with plasmid DNA and liposomes or immunoliposomes alone (with the exception that with these transient nonviral vectors, expression fell to background levels over two weeks) (Figure 5a, lower panel).
Figure 5.
Kinetics of gene expression after gene transfer. HSVECs that had been stimulated with 80 ng/mL TNF- α , 80 ng/mL IFN-γ , and 80 ng/mL IL-1β ) for 8 h were treated with amphotropic MMLV-EGFP in combination with polybrene or amphotropic MMLV-EGFP containing virosomes or immunovirosomes (made with anti-CD71 mAb) (upper panel). Alternatively, the cells were transfected with pCMV-EGFP plasmid DNA using liposomes or immunoliposomes containing anti-CD71 mAb (lower panel). After gene transfer, the cells were cultured for 25 days. Flow cytometric analysis was performed every 3 days to determine the percentage of EGFP positive cells. All results are expressed as the mean (% of EGFP positive cells) ± SD of triplicates.
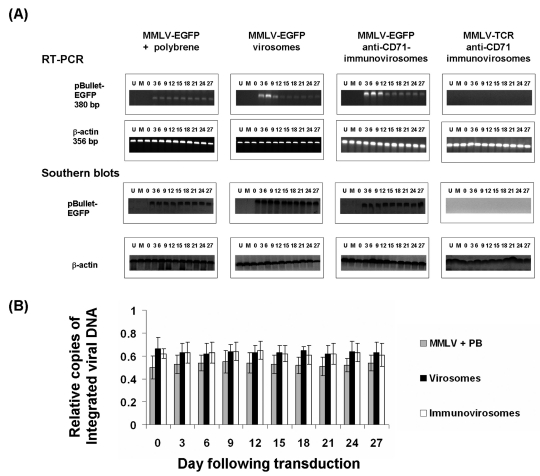
We compared the kinetics of mRNA expression following treatment with amphotropic MMLV (Figure 6, pBullet-EGFP) (LZRS-EGFP, data not shown), virosomes, or immunovirosomes. As can be seen in Figure 6, upper panels, expression of EGFP mRNA was similar at all times following retroviral gene transfer. However, following treatment with either virosomes or immunovirosomes, EGFP mRNA expression was higher at early time-points but decreased over time to reach levels comparable to retroviral-transduced levels [shown for anti-CD71 monoclonal antibodies for activated EC (Figure 6, upper panels), but similar data (not shown) were seen with anti-CD71 for nonactivated EC and anti-CD62E/P for activated EC].
Figure 6.
Lack of enhancement in integration after gene transfer with virosomes and immunovirosomes. HSVECs were transduced with amphotropic MMLV-EGFP either in the presence of polybrene or as virosomes or immunovirosomes (made with anti-CD71 mAb). As a control, immunovirosomes were used containing anti-CD71 and the control amphotropic MMLV-TCR (23). (A) After gene transfer, the cells were cultured for 27 days and on different days (indicated by numbers above the gel) mRNA and genomic DNA was prepared for RT-PCR (top) and Southern blotting (bottom) for detection of EGFP mRNA or DNA, respectively. Also shown are RT-PCR or Southern blots from untransfected cells (U), mock-transduced cells (M), or immediately after exposure to the virus (0). (B) Copies of integrated viral DNA were analyzed. The results are expressed as mean relative copies of integrated DNA ± SD of three independent experiments.
To assess the persistence of EGFP gene integrated into the EC genome, we carried out Southern blot analysis of genomic DNA derived from ECs following gene transfer. Interestingly, the data showed that the number of copies of EGFP integrated into EC genomic DNA remained stable at all times following treatment with viruses, virosomes, or immunovirosomes (Figure 6, lower panels).
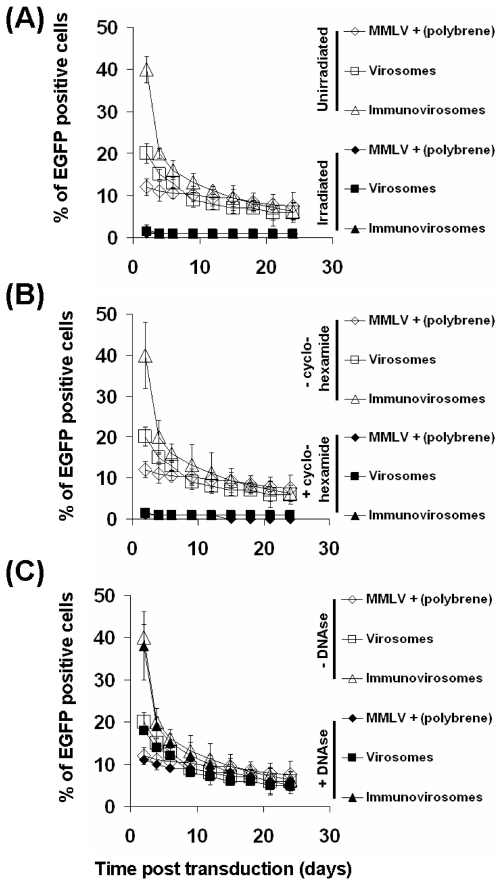
To exclude the possibility that the augmentative effects of liposomes and immunoliposomes were due merely to the transfer of fluorescent protein (produced in the packaging cell) by the viral particles, the particles were irradiated (90 kGy) before use. As can be seen (Figure 7a), no expression of EGFP seen after irradiation, indicating that passive uptake of fluorescent viral particles was not responsible. This was further confirmed by treating HSVECs with cycloheximide. Again, no EGFP expression was seen, indicating that EGFP expression is due to the de novo synthesis rather than transfer of EGFP protein (Figure 7b).
Figure 7.
Expression of EGFP is dependent on de novo synthesis. To exclude the possibility that the increase in fluorescence after virosome or immunovirosome (containing anti-CD71 mAb) gene transfer was due to transfer of fluorescent protein by viruses, irradiated viruses were used (A). Alternatively, ECs were treated with cycloheximide to prevent de novo synthesis of protein (B). Furthermore, to exclude that the increase in fluorescence was due to the carryover transfection of EGFP plasmids, the supernatants were treated with DNAse (C). Unstimulated HSVECs were treated with MMLV-EGFP + polybrene, virosomes, or immunovirosomes. The numbers of EGFP-expressing cells were analyzed by flow cytometry on day 3. All results are expressed as mean ± SD of triplicate wells and all experiments were carried out at least three times.
To exclude the likelihood that the EGFP-containing plasmids used to make the retrovirus particles, which might still be present in the viral supernatants, were responsible, we treated the supernatant with DNAse before use. As can be seen Figure 7c, treatment of DNAse had minimal effect on gene expression, indicating that carryover of EGFP plasmids were unlikely to be the cause.
DISCUSSION
We have demonstrated a quick and easy method to alter viral tropism without the need of generating genetic modification of viral capsid proteins. This allows a rapid assessment of the consequences of changing viral tropism in terms of transfection efficiency and cell-type specificity. In addition, we also highlighted that altering viral tropism to allow viral entry through highly endocytic receptors can increase transfection efficiency transiently but has no effect on proviral integration.
Since the initial description of lipofection as a mean of DNA transfection (39), cationic liposomes have been widely used for gene delivery both in vitro and in vivo (40,41). We, like others, have used liposomes to package viral particles to increase the infectivity (17,18,42). Using immunoliposomes targeted to endothelial receptors, such as CD71 (nonactivated ECs) or activated endothelial receptors, such as CD62E/P, we can enhance gene transfer of MMLV. We showed selectivity of viral tropism when viral particles were complexed with immunoliposomes containing anti-CD71 antibodies. Although expression of CD71 is high on ECs, its ubiquitous expression restricts its use as a specific target for gene delivery. We have, therefore, developed this approach with anti-CD62E/P. This allows a target-specific delivery of viral particles to activated ECs. On the other hand, other adhesion molecules, such as CD54 (data not shown) and CD106 expressed by activated ECs (21), did not show enhancement of transduction efficiency. Our observations suggest that the cellular receptors chosen to be targeted are important in determining the success of the receptor-mediated approach. Whereas some antigens may be attractive targets in terms of patterns of expression, they are poor at delivering the viral particles to the appropriate cellular compartment. For example, even though CD106 has been shown to internalize through clathrin-coated vesicles (43), when the molecule is bound by antibody, it is shed rather than internalized (44). CD54, on the other hand, has a 10-fold lower endocytosis rate than E-selectin (19,45).
As MMLV on its own has poor transduction efficiency on HSVECs, we have therefore chosen this group of viruses as a model. On the basis of their host range, MMLVs have been classified into six subgroups (ecotropic, amphotropic, polytropic, xenotropic, MDEV, and 10A1) (9). The envelope motifs that bind to the various viral receptors and determine the infection specificity have been mapped within the N-terminal one-third of the surface protein SU (gp70), which bears the most variable regions of MMLV envelope (46–48). Thus, ecotropic MMLV enters cells after binding to a cationic amino acid transporter (CAT-1 or REC1) (49), and amphotropic MMLV enters cells after binding to a sodium phosphate symporter (PIT-2 or GLVR1) (50,51). However, the fine mechanism by which retro-viruses enter the cell is one of the most poorly understood aspects of the viral life cycle. We have shown that MMLV pseudotyped with various viral envelopes [amphotropic, ecotropic, gibbon ape leukemia virus (GALV), vesicular stomatitis virus G (VSV-G) (Table 1)] can be targeted using immunoliposomes, indicating that the coat protein has little effect on the interaction of the virus with liposomes or their subsequent delivery to cells. By generating immunovirosomes, there are three independent pathways for cell entry which likely will compete with each other. Our data suggest the most efficient pathway of entry would be the predominant entry delivery mechanism in our mixed system. It is therefore not surprising that viral entry pathway mediated by its pseudotyped envelope is negligible, as it represents the slowest entry pathway (Figure 3).
Using virosomes or immunovirosomes targeting at highly endocytic receptors, we could improve transduction efficiency of MMLV alone by ~100- and ~250-fold, respectively. Compared with MMLV transduction in the presence of polybrene, however, these increases are less impressive. These findings are not surprising, as the use of polybrene is a well-optimized method to improve transduction efficiency of MMLV in vitro. Whereas the use of polybrene to assist transduction of MMLV is effective in vitro, its application for in vivo transduction is limited. On the other hand, virosomes or, in our case, immunovirosomes may allow in vivo application (18).
Parameters that control the kinetics of transduction of viral vectors are poorly understood. The first step is binding of the virus to its receptor on the cell surface. This is thought to be the rate-limiting step of the infection process (52). It has been argued that the Brownian motion of viral particles in the medium imposes a significant limitation for infection of adherent cells (53). However, the binding kinetics of MMLV vectors to cells in suspension fits a bimolecular, noncooperative model, which rapidly reaches equilibrium at 37°C when virus is in excess (54). The association rate constant is significantly lower than the calculated limitation imposed by viral diffusion, suggesting that binding, rather than encounter, is the rate-limiting step (55). In our experiments, the cells were exposed to viral particles with or without liposomes or immunoliposomes for 2 h. Longer time exposure to viral particles often results in higher cellular toxicity (data not shown). The use of immunoliposomes or liposomes may increase the rate of binding of MMLV.
Further studies have shown that MMLV infection follows internalization of intact virions by endocytosis, followed by a membrane fusion event releasing virion cores to the cytoplasm (56,57). To determine if MMLV transduction is endocytosis dependent, we showed that transduction efficiency could be augmented by treating the cells with the endosomolytic agent chloroquine, as previously reported (58). This suggests that once the complex is internalized, it is directed into the endolysosomal pathway. By inhibiting the lysosomal enzymes with the endosomolytic agents and thereby increasing endolysosomal escape, gene expression was increased. However, in the case of virosomes and immunovirosomes, treatment with the endosomolytic agents resulted in only a marginal improvement in the number of viral particles retained intracellularly and productive transduction. This may be because the efficient delivery of viral particles overwhelms the degradation capacity of endolysosomal pathways. However, with a less efficient delivery system such as MMLV alone or in the presence of polybrene, these pathways have more influence on the total number of particles remained intracellularly (Figure 3a) at the 4-h time-point and the productive transduction (Figure 3b).
The enhancement of expression with immunovirosomes or virosomes was transient. Previous groups have also shown short-term expression after transduction with either lentivirus or MMLV. This could be due to transfer of protein by the viral particles (59,60) or, in the case of viruses that have been disabled in such as a way as to prevent either reverse transcription of RNA or integration of virally derived DNA into the host genome, as a result of direct translation from virally delivered RNA (61) or transcription of episomal viral DNA (60,62). Such “pseudo-transduction” has been suggested both as a way of transient expression of toxic proteins (61) and as a safe, nonintegrating, viral vector for gene therapy (62).
The reason viral particles, when delivered into a cell using immunovirosomes or virosomes, are unable to enhance the integration of the virally derived DNA into the genome is not clear. We have previously reported that HSVECs are actively dividing cells (21), and stable integration was seen with MMLV alone, indicating that lack of enhancement in integration with virosomes or immunovirosomes is not due to cell division or cell senescence. It is interesting to speculate that the mechanism of transduction occurring with virus alone versus virosomes or immunovirosomes might be different. Our data suggest that pseudo-transduction due to delivery of fluorescent proteins is unlikely to be the cause for lack of integration seen with virosomes or immunovirosomes. Early EGFP expression data indicate that de novo synthesis of EGFP protein is required. Owing to increased delivery of retroviral RNA with virosomes and immunovirosomes, direct translation from virally delivered RNA (60) or transcription of episomal viral DNA (59,61) may be possible. It may also be possible that sites for retroviral integration in the human genome are a rate-limiting step for viral transduction. We cannot exclude that the use of liposomes may interfere with the integration process.
Modifications of the structure of the virus capsid proteins and expression cassette to generate vectors that are highly selective and efficient for target cell binding and entry have been described (6). For example, fusing a gene for a single chain antibody (sFv) to the region of the envelope gene encoding its N-terminal amino acids has been carried out (63). This technique has been attempted using different sFvs and other proteins capable of specifically attaching to cellular receptors (62). Most have resulted in very low infectivity, less than 3%, and poor virion stability (63). It is therefore difficult to compare the approach with our approach.
It may be possible to exploit the different pathways utilized by virus alone or virosomes/immunovirosomes to alter the behavior of retroviral vectors. Retroviral vectors are likely to be of greatest use in the context of requiring long-term expression of transgene. However, nonviral vectors or adenovirus that resulted in transient expression of transgene may be useful for clinical settings requiring transient expression. In our system, we saw biphasic expression profile with higher and transient expression followed by low and sustained expression of transgene. This might be in particular useful for transplantation, as the initial expression of an immunomodulatory transgene would be required at higher levels to prevent acute rejection (3). Later and sustained expression of this transgene might be required to prevent chronic rejection or maintain tolerance (3).
The successful utilization of a new generation of viral vectors in the clinic may well pave the way for superior gene delivery systems in the future that specifically deliver the therapeutic gene to a particular target cell. Immunovirosomes may offer an opportunity to address some of the current deficiency of viral vectors, in particular for transplantation (3), and to test different targets for gene transfer. They can be easily and quickly assembled, avoiding complicated molecular engineering of the viral envelope. However, it is important to understand how these vectors alter long-term expression of genes in target cells. Our findings have significant implication for viral vector design and improved tropism.
ACKNOWLEDGMENTS
This research is supported by a Research Training Fellowship from the MRC and the Royal College of Surgeons Edinburgh 2001–2004 (PHT) and project and program grants from the BBSRC and the MRC (AJTG). AJTG was a BBSRC Research Development Fellow. The authors thank Prof. K Taylor and his team for supplying human vascular tissues for the work and the theatre nursing staff at Hammersmith and St. Mary’s Hospitals for collecting and preserving human vessels.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Tan PH, Chan CL, Chan C, George AJT. The evolving role of gene-based treatment in surgery. Br J Surg. 2005;92:1466–80. doi: 10.1002/bjs.5181. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Deisseroth A. Tumor vascular targeting therapy with viral vectors. Blood. 2006;107:3027–3033. doi: 10.1182/blood-2005-10-4114. [DOI] [PubMed] [Google Scholar]
- 3.Tan PH, Tan PL, George AJ, Chan CL. Gene therapy for transplantation with viral vectors: how much of the promise has been realized? Expert Opin Biol Ther. 2006;6:759–72. doi: 10.1517/14712598.6.8.759. [DOI] [PubMed] [Google Scholar]
- 4.Beck C, Uramoto H, Boren J, Akyurek LM. Tissue-specific targeting for cardiovascular gene transfer: potential vectors and future challenges. Curr Gene Ther. 2004;4:457–67. doi: 10.2174/1566523043346138. [DOI] [PubMed] [Google Scholar]
- 5.Turunen MP, et al. Peptide-retargeted adenovirus encoding a tissue inhibitor of metalloproteinase-1 decreases restenosis after intravascular gene transfer. Mol Ther. 2002;6:306–12. doi: 10.1006/mthe.2002.0668. [DOI] [PubMed] [Google Scholar]
- 6.Kasahara N, Dozy AM, Kan YW. Tissue-specific targeting of retroviral vectors through ligand-receptor interactions. Science. 1994;266:1373–6. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 7.Cosset FL, Morling FJ, Takeuchi Y, Weiss RA, Collins MK, Russell SJ. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J Virol. 1995;69:6314–22. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valsesia-Wittmann S, Morling FJ, Hatziioannou T, Russell SJ, Cosset FL. Receptor co-operation in retrovirus entry: recruitment of an auxiliary entry mechanism after retargeted binding. EMBO J. 1997;16:1214–23. doi: 10.1093/emboj/16.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battini JL, Heard JM, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–75. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnierle BS, et al. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc Natl Acad Sci U S A. 1997;94:8640–5. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatziioannou T, Delahaye E, Martin F, Russell SJ, Cosset FL. Retroviral display of functional binding domains fused to the amino terminus of influenza hemagglutinin. Hum Gene Ther. 1999;10:1533–44. doi: 10.1089/10430349950017860. [DOI] [PubMed] [Google Scholar]
- 12.Morizono K, Bristol G, Xie YM, Kung SK, Chen IS. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001;75:8016–20. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandrin V, et al. Lentiviral vectors pseudo-typed with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and non-human primates. Blood. 2002;100:823–32. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- 14.Roux P, Jeanteur P, Piechaczyk M. A versatile and potentially general approach to the targeting of specific cell types by retroviruses: application to the infection of human cells by means of major histocompatibility complex class I and class II antigens by mouse ecotropic murine leukemia virus-derived viruses. Proc Natl Acad Sci U S A. 1989;86:9079–83. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snitkovsky S, Young JA. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc Natl Acad Sci U S A. 1998;95:7063–8. doi: 10.1073/pnas.95.12.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boerger AL, Snitkovsky S, Young JA. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc Natl Acad Sci U S A. 1999;96:9867–72. doi: 10.1073/pnas.96.17.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodgson CP, Solaiman F. Virosomes: cationic liposomes enhance retroviral transduction. Nat Biotechnol. 1996;14:339–42. doi: 10.1038/nbt0396-339. [DOI] [PubMed] [Google Scholar]
- 18.Porter CD, Lukacs KV, Box G, Takeuchi Y, Collins MK. Cationic liposomes enhance the rate of transduction by a recombinant retroviral vector in vitro and in vivo. J Virol. 1998;72:4832–40. doi: 10.1128/jvi.72.6.4832-4840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan PH, et al. Antibody targeted gene transfer to endothelium. J Gene Med. 2003;5:311–23. doi: 10.1002/jgm.358. [DOI] [PubMed] [Google Scholar]
- 20.Tan PH, et al. Immunolipoplexes: an efficient, nonviral alternative for transfection of human dendritic cells with potential for clinical vaccination. Mol Ther. 2005;11:790–800. doi: 10.1016/j.ymthe.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Tan PH, et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis. 2004;173:171–83. doi: 10.1016/j.atherosclerosis.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Tan PH, et al. Effect of vectors on human endothelial cell signal transduction: implications for cardiovascular gene therapy. Arterioscler Thromb Vasc Biol. 2006;26:462–7. doi: 10.1161/01.ATV.0000200083.95349.9e. [DOI] [PubMed] [Google Scholar]
- 23.Xue SA, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106:3062–7. doi: 10.1182/blood-2005-01-0146. [DOI] [PubMed] [Google Scholar]
- 24.Morris EC, Tsallios A, Bendle GM, Xue SA, Stauss HJ. A critical role of T cell antigen receptor-transduced MHC class I-restricted helper T cells in tumor protection. Proc Natl Acad Sci U S A. 2005;102:7934–9. doi: 10.1073/pnas.0500357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller AD, Garcia JV, von Suhr N, Lynch CM, Wilson C, Eiden MV. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–4. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan PH, et al. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood. 2005;105:3824–32. doi: 10.1182/blood-2004-10-3880. [DOI] [PubMed] [Google Scholar]
- 27.Weijtens ME, Willemsen RA, Hart EH, Bolhuis RL. A retroviral vector system ‘STITCH’ in combination with an optimized single chain antibody chimeric receptor gene structure allows efficient gene transduction and expression in human T lymphocytes. Gene Ther. 1998;5:1195–203. doi: 10.1038/sj.gt.3300696. [DOI] [PubMed] [Google Scholar]
- 28.Dardalhon V, et al. Green fluorescent protein as a selectable marker of fibronectin-facilitated retroviral gene transfer in primary human T lymphocytes. Hum Gene Ther. 1999;10:5–14. doi: 10.1089/10430349950019147. [DOI] [PubMed] [Google Scholar]
- 29.Dardalhon V, et al. Highly efficient gene transfer in naive human T cells with a murine leukemia virus-based vector. Blood. 2000;96:885–93. [PubMed] [Google Scholar]
- 30.Chandrashekran A, Gordon MY, Casimir C. Targeted retroviral transduction of c-kit+ hematopoietic cells using novel ligand display technology. Blood. 2004;104:2697–703. doi: 10.1182/blood-2003-10-3717. [DOI] [PubMed] [Google Scholar]
- 31.Chandrashekran A, Gordon MY, Darling D, Farzaneh F, Casimir C. Growth factor displayed on the surface of retroviral particles without manipulation of envelope proteins is biologically active and can enhance transduction. J Gene Med. 2004;6:1189–96. doi: 10.1002/jgm.625. [DOI] [PubMed] [Google Scholar]
- 32.Manunta M, Tan PH, Sagoo P, Kashefi K, George AJ. Gene delivery by dendrimers operates via a cholesterol dependent pathway. Nucleic Acids Res. 2004;32:2730–9. doi: 10.1093/nar/gkh595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guindulain T, Comas J, Vives-Rego J. Use of nucleic acid dyes SYTO-13, TOTO-1, and YOYO-1 in the study of Escherichia coli and marine prokaryotic populations by flow cytometry. Appl Environ Microbiol. 1997;63:4608–4611. doi: 10.1128/aem.63.11.4608-4611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nermut MV, Fassati A. Structural analyses of purified human immunodeficiency virus type 1 intracellular reverse transcription complexes. J Virol. 2003;77:8196–8206. doi: 10.1128/JVI.77.15.8196-8206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue SA, Lu QL, Poulsom R, Karran L, Jones MD, Griffin BE. Expression of two related viral early genes in Epstein-Barr virus-associated tumors. J Virol. 2000;74:2793–803. doi: 10.1128/jvi.74.6.2793-2803.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue SA, Jones MD, Lu QL, Middeldorp JM, Griffin BE. Genetic diversity: frameshift mechanisms alter coding of a gene (Epstein-Barr virus LF3 gene) that contains multiple 102-base-pair direct sequence repeats. Mol Cell Biol. 2003;23:2192–201. doi: 10.1128/MCB.23.6.2192-2201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan PH, et al. Inhibition of NF-κ B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol. 2005;174:7633–44. doi: 10.4049/jimmunol.174.12.7633. [DOI] [PubMed] [Google Scholar]
- 38.Felgner PL, Ringold GM. Cationic liposome-mediated transfection. Nature. 1989;337:387–8. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 39.Caplen NJ, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 40.Tan PH, Chan CL, George AJ. Strategies to improve non-viral vectors: potential applications in clinical transplantation. Expert Opin Biol Ther. 2006;6:619–30. doi: 10.1517/14712598.6.6.619. [DOI] [PubMed] [Google Scholar]
- 41.Porter CD. Rescue of retroviral envelope fusion deficiencies by cationic liposomes. J Gene Med. 2002;4:622–33. doi: 10.1002/jgm.310. [DOI] [PubMed] [Google Scholar]
- 42.Ricard I, Payet MD, Dupuis G. VCAM-1 is internalized by a clathrin-related pathway in human endothelial cells but its alpha 4 beta 1 integrin counter-receptor remains associated with the plasma membrane in human T lymphocytes. Eur J Immunol. 1998;28:1708–18. doi: 10.1002/(SICI)1521-4141(199805)28:05<1708::AID-IMMU1708>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 43.Harrison AA, et al. Expression of vascular cell adhesion molecule-1 by vascular endothelial cells in immune and nonimmune inflammatory reactions in the skin. J Immunol. 1997;159:4546–54. [PubMed] [Google Scholar]
- 44.von Asmuth EJ, Smeets EF, Ginsel LA, Onderwater JJ, Leeuwenberg JF, Buurman WA. Evidence for endocytosis of E-selectin in human endothelial cells. Eur J Immunol. 1992;22:2519–26. doi: 10.1002/eji.1830221009. [DOI] [PubMed] [Google Scholar]
- 45.Battini JL, Danos O, Heard JM. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J Virol. 1995;69:713–9. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992;66:4632–8. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan RA, Nussbaum O, Muenchau DD, Shu L, Couture L, Anderson WF. Analysis of the functional and host range-determining regions of the murine ecotropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:4712–21. doi: 10.1128/jvi.67.8.4712-4721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kavanaugh MP, et al. Control of cationic amino acid transport and retroviral receptor functions in a membrane protein family. J Biol Chem. 1994;269:15445–50. [PubMed] [Google Scholar]
- 49.Miller DG, Edwards RH, Miller AD. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci U S A. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller DG, Miller AD. A family of retro-viruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–6. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss RA, Tailor CS. Retrovirus receptors. Cell. 1995;82:531–533. doi: 10.1016/0092-8674(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 52.Chuck AS, Clarke MF, Palsson BO. Retroviral infection is limited by Brownian motion. Hum Gene Ther. 1996;7:1527–34. doi: 10.1089/hum.1996.7.13-1527. [DOI] [PubMed] [Google Scholar]
- 53.Yu H, Soong N, Anderson WF. Binding kinetics of ecotropic (Moloney) murine leukemia retrovirus with NIH 3T3 cells. J Virol. 1995;69:6557–62. doi: 10.1128/jvi.69.10.6557-6562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andersen KB, Nexo BA. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983;125:85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- 55.Aboud M, Shoor R, Salzberg S. Adsorption, penetration, and uncoating of murine leukemia virus studied by using its reverse transcriptase. J Virol. 1979;30:32–7. doi: 10.1128/jvi.30.1.32-37.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Risco C, Menendez-Arias L, Copeland TD, Pinto da Silva P, Oroszlan S. Intracellular transport of the murine leukemia virus during acute infection of NIH 3T3 cells: nuclear import of nucleocapsid protein and integrase. J Cell Sci. 1995;108:3039–50. doi: 10.1242/jcs.108.9.3039. [DOI] [PubMed] [Google Scholar]
- 57.Zhong Q, Kolls JK, Schwarzenberger P. Retrovirus molecular conjugates: a novel, high transduction efficiency, potentially safety-improved, gene transfer system. J Biol Chem. 2001;276:24601–7. doi: 10.1074/jbc.M010318200. [DOI] [PubMed] [Google Scholar]
- 58.Nash KL, Lever AM. Green fluorescent protein: green cells do not always indicate gene expression. Gene Ther. 2004;11:882–3. doi: 10.1038/sj.gt.3302246. [DOI] [PubMed] [Google Scholar]
- 59.Haas DL, Case SS, Crooks GM, Kohn DB. Critical factors influencing stable transduction of human CD34+ cells with HIV-1-derived lentiviral vectors. Mol Ther. 2000;2:71–80. doi: 10.1006/mthe.2000.0094. [DOI] [PubMed] [Google Scholar]
- 60.Galla M, Will E, Kraunus J, Chen L, Baum C. Retroviral pseudotransduction for targeted cell manipulation. Mol Cell. 2004;16:309–15. doi: 10.1016/j.molcel.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 61.Vargas J, Jr, Gusella GL, Najfeld V, Klotman ME, Cara A. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum Gene Ther. 2004;15:361–72. doi: 10.1089/104303404322959515. [DOI] [PubMed] [Google Scholar]
- 62.Jiang A, Chu TH, Nocken F, Cichutek K, Dornburg R. Cell-type-specific gene transfer into human cells with retroviral vectors that display single-chain antibodies. J Virol. 1998;72:10148–56. doi: 10.1128/jvi.72.12.10148-10156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]