Abstract
Objective
Targeted regulation of β-like globin genes was studied using designer zinc finger transcription factors containing the DNA binding domain of the red cell specific transcription factor EKLF fused to repression domains.
Methods
Globin gene expression was analyzed after introduction of the modified transcription factors into cell lines, embryonic stem cells and transgenic mice.
Results
As would be predicted, when introduced transiently into cells these transcription factors were effective in repressing the adult β-globin promoter CACCC element which is the natural target for EKLF. In murine erythroleukemia cells repression of the adult β-globin gene was accompanied by a reactivation of the endogenous embryonic βH1-globin gene. Studies in differentiated embryonic stem cells and transgenic mice confirmed the reactivation of embryonic gene expression during development.
Conclusion
Our studies support a competition model for β-globin gene expression and underscore the importance of EKLF in the embryonic/fetal to adult globin switch. They also demonstrate the feasibility of designer zinc finger transcription factors in the study of transcriptional control mechanisms at the β-globin locus and as potential gene therapy agents for sickle cell disease and related hemoglobinopathies.
Introduction
The ability to willfully regulate the expression of endogenous genes using designed transcription factors is an emerging field. There is tremendous appeal in utilizing the understanding of transcriptional control pathways to design tools that will elucidate molecular mechanisms and provide potential therapeutic tools. Expression of the β-like globin genes is regulated in a specific temporal sequence during mammalian development [1]. This well characterized “switch” of globin genes provides a target for such an approach. In humans, the first blood cells formed within the yolk sac express the embryonic (ε) globin variant. After the site of hematopoiesis switches to the fetal liver, the fetal (γ) globin genes are expressed. This is followed by yet another switch in expression to the adult (β) globin genes within the bone marrow. In mice, there is a single switch from embryonic (βH1, βH0, εy) globin genes in the yolk sac to the adult (β) globin gene in the fetal liver, adult bone marrow and spleen. These β-like globin genes, both in humans and mice, are arranged in a cluster and expressed in a specific temporal sequence during ontogeny [2,3]. Regulation of these genes is a result of a complex interplay between cis and trans acting regulatory elements. Important cis acting elements include the promoters of each of these genes and the upstream locus control region (LCR) [4] Trans acting elements are the tissue specific transcription factors [1].
Two mechanisms of molecular control of switching have been proposed –gene silencing and gene competition [1]. The model of gene silencing posits that individual globin genes are turned off autonomously [5,6] whereas the latter model proposes that competition between the individual β-like genes for interaction with the LCR determines which gene is expressed. The competition model for globin switching was first demonstrated in the chicken globin system by Choi and Engel [7] and recapitulated in transgenic mice [2,8]. Various studies exploring the mechanism of competition favor a looping model in which the LCR forms direct chromatin interactions with local regulatory elements to activate transcription of a single β-like globin gene [9,10]. Evidence for this phenomenon is provided by 2 ingenious techniques, RNA TRAP (tagging and recovery of associated proteins) [11] and 3C (“capturing chromosome conformation”), and this 3D clustering of hypersensitive sites has been named the active chromatin hub or ACH [12–14]. Palstra et al demonstrated that globin genes switched their interaction with the ACH during development correlating with the switch in their transcriptional activity. In agreement with the notion that there is competition between genes for inclusion within the ACH, Patrinos et al showed that deletion of the β-globin promoter resulted in higher frequency of association of the γ-globin gene with the ACH and higher level of expression.
Erythroid Kruppel like factor (EKLF) is an erythroid specific transcription factor critical for the activation of the β-globin promoter [15–17]. EKLF null mice die of anemia at the time of switch to adult globin [18,19]. Relevant to the studies described in this manuscript is its important but somewhat less well characterized role in consolidating the switch from γ- to β-globin gene expression [20–22]. To examine this important biologic role of EKLF, Perkins et al interbred EKLF heterozygous (+/−) mice with those harboring a human β-globin yeast artificial chromosome (YAC) transgene. In the absence of EKLF not only was human β-globin expression dramatically reduced, γ-globin expression was increased 5-fold and persisted into a later stage in gestation [21]. In a similar animal model, Wijgerde et al and Drissen et al demonstrated an altered chromatin structure at the respective promoters coincident with the reduction of β and increased γ globin gene expression [22,23]. Specifically, EKLF is necessary for hypersensitivity at and participation of the LCR and the β-globin promoter in the ACH [23]. The importance of EKLF in switching is further underscored by individuals that harbor mutations at the CACCC motif within the adult β-globin promoter (−87 C to G, −87C to T and −88 C to T), all of which lead to β+ thalassemia [24–27]. This is the site at which EKLF binds, and each of these mutations significantly decreases EKLF affinity for this sequence. Of particular relevance to the present studies, these patients have a mild thalassemic phenotype resulting from Hb F levels that are markedly elevated to 55 and 70% of total hemoglobin.
These observations suggest that EKLF plays an important role in stabilizing the interaction of the β-globin gene with the LCR and in its absence, the balance is shifted in favor of the γ-globin gene – LCR interaction. This led us to consider whether manipulating EKLF’s molecular properties so that it acts as a transcriptional repressor might, with greater potency than its mere absence, augment the expression of murine embryonic globins. In order to do this EKLF was redesigned in 2 ways. First, the repression domain from the Drosophila engrailed protein (ENG), one that has been widely used as a potent repressor of transcription [28,29], was fused to the zinc finger domain (ZnF) of EKLF. Second, a fusion product of the Sin 3a/Histone deacetylase interaction domain (HID) and the EKLF ZnF was constructed. Sin 3A is a protein that establishes a link between DNA bound proteins and histone deacetylases (HDAC) [30,31]. Chromatin immunoprecipitation (ChIP) analyses in GATA-1-null cells (G1E), with or without a conditionally active estrogen receptor ligand binding domain fusion to GATA-1, have measured EKLF occupancy at various sites within the B globin locus. Occupancy was detected at HS2, HS3, and the β major promoter and weakly at HS1. No occupancy was detected at Ey and βH1 sites [32]. Further, Feng et al have directly tested if point mutations in the CACCC site affect DNA binding of EKLF and in vitro analyses have demonstrated a 40–100 fold decrease in binding affinity, emphasizing the specificity of the binding of the zinc finger domain [33]. Since the repressor constructs have a preserved EKLF DNA binding domain, both these constructs should specifically bind the CACCC site in the β-major gene promoter. We postulated that this will produce transcriptional repression of β-major instead of activation, and that this will alter the expression levels of genes within the β-globin cluster in complementary ways consistent with the competition model.
Materials and Methods
Plasmid Constructions
A fragment containing aa 1–298 from Drosophila Engrailed was fused in frame to an EKLF ZnF (aa 293–376) to generate ENG/ZnF. The HID (aa 524–899)/ZnF construct, HID/ZnF, was generated in the same manner. For the MEL studies, ENG/ZnF was subcloned downstream of the 3kb BamH1/Stu1 EKLF promoter [34] in a vector containing a puromycin selection marker. Plasmid constructs pSG5/ZnF, pSG5 EKLF, pCACCCtk CAT, pαLCR βglob-CAT have been previously described [17].
Cell Culture and Transfections
CV-1, Cos7 and MEL (745A) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM). 32D Epo 1 cells were maintained in IMDM supplemented with IL-3. CV-1 and 32D epo1 cells were transfected, harvested, normalized and assayed for CAT activity as described [15]. The Engrailed antibody was a kind gift from Pat O’Farrell. The anti myc antibody was purchased from the Mount Sinai Hybridoma core and used to detect the myc tagged HID/ZnF protein.
Establishment and analyses of Stable MEL Clones
Transfections into 745A MEL cells [35] was performed with the cationic lipid reagent Tfx 50 (Promega) and selected with puromycin. For differentiation, cells were exposed to 5mM hexamethylene bisacetamide (HMBA) and aliquots were removed for benzidine staining and RNA extraction. Total RNA was isolated in TRI Reagent (Sigma) and analyzed by RT/PCR as described [16]. Cycles for each primer pair were within the early exponential phase of synthesis. Quantitation was performed on a Phosphoimager using ImageQuant software. PCR primers for HPRT, have been previously described [16,36]. Globin gene levels were normalized to HPRT and expressed as fold increase from the levels prior to addition of HMBA.
Embryonic Stem cell/Embryoid Body studies
ENG/ZnF was inserted into a modifed plox vector [37] to generate ploxENG/IRES GFP. Ainv18 ES cells were targeted with ploxENG/IRES GFP by coelectroporation of ploxENG/IRES GFP and a Cre recombinase expression plasmid followed by selection in G418 to generate the inducible cell line, iENG/IRES GFP. Culture of iENG/IRES GFP after removal from feeder cells and EB differentiation precisely followed established protocols [38–40]. 1 μg/ml of Doxycycline was added on day 4 and EBs were harvested 48hrs later for FACS and RNA analysis. βmaj, and βh1 globin gene expression were normalized to α-globin and expressed as a ratio of the induced sample to the uninduced.
Studies in Transgenic Mice
ENG/ZnF was cloned downstream of the minimal ankyrin promoter (−291 to −20)[41] to generate 573Eng transgenic mice as described [42]. Founder animals were identified by PCR analysis of genomic DNA and were crossed for developmental studies. Total RNA was extracted from D13.5 fetal livers. Transgene expression was verified by RT-PCR. βmaj and βH1 message normalized to α-globin were determined by RT-PCR, and compared to wild type littermates.
Quantitative RT-PCR Assay
cDNA was prepared from 1 μg of purified total RNA. RT-PCRs (20 μl) contained 2 μl of cDNA solution with the appropriate primers designed using primer express. Product was measured by SYBR green fluorescence. Absolute expression levels were determined from a standard curve of serial dilutions of cDNA samples for ENG/ZNF and EKLF. Relative expression levels of β and βH1 globin RNA (normalized to α) were also measured.
Results
Design and Characterization of Repressor EKLF constructs
EKLF protein contains a DNA binding domain and an activation domain. The activation domain is specifically required for adult β-globin expression [17]. The 3 zinc finger DNA binding domain binds to the CACCC box motif within the β-globin promoter with high specificity. We redesigned EKLF as a repressor by replacing the activation domain with the 2 different repressor domains, ENG and HID (Fig 1A). We verified expression of both proteins by Western blot analysis after transfection into COS cells (data not shown). Both repressor proteins bind a synthetic oligonucleotide containing the mammalian β-globin CAC site (5′-AGCTAGCCACACCCTGAAGCT-3′) in gel shift analyses (data not shown). As a preliminary test of the ability of these constructs to repress the β-globin CACCC site, transient transfection assays were performed in CV1 cells with the reporter plasmid pCAC –tkCAT [16]. Cells cotransfected with the pSG5 vector alone give a basal level of CAT activity, normalized to 1, and an expected increase in CAT activity with pSG5/EKLF. However, both repressor constructs result in a reduction of CAT activity from baseline. A 3 – 4 fold repression is seen with the ENG/ZnF construct and a 2- fold repression with HID/ZnF (Fig 1B). As a more stringent test of their functional ability, the repressor constructs were transiently transfected into 32D Epo 1 cells [43] chosen for their ability to express EKLF and adult β-globins. The α-LCR-β-CAT reporter contains a human β-globin promoter driving the CAT gene with an α LCR linked upstream as an enhancer (Fig 2) [17]. Cells transfected with pSG5 vector alone have a basal level of CAT activity (normalized to 1) derived from the endogenous EKLF. Cells transfected with the zinc fingers alone show only a modest reduction in CAT activity. ENG/ZnF and HID/ZnF result in a robust reduction in CAT activity, about 2–3 fold with HID/ZnF and 5 fold with ENG/ZnF. Since ENG/ZnF is the more potent repressor, this construct was used for all further experiments.
Figure 1.
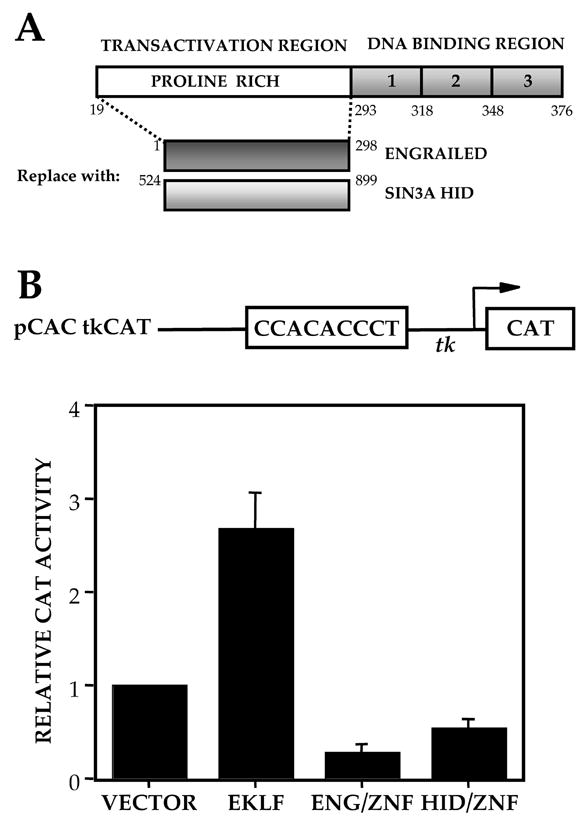
(A) Design of Modified EKLF Constructs. Transactivation domain of EKLF is replaced by repressor domains, Engrailed and Sin 3/HID. (B) Transcriptional activities of EKLF repressor proteins on pCAC-tkCAT. The pCAC-tkCAT reporter was cotransfected with vector alone, full length EKLF or the repressor constructs into CV1 cells. CAT activity in extracts was normalized to cotransfected growth hormone. The average of 3 experiments is shown.
Figure 2.
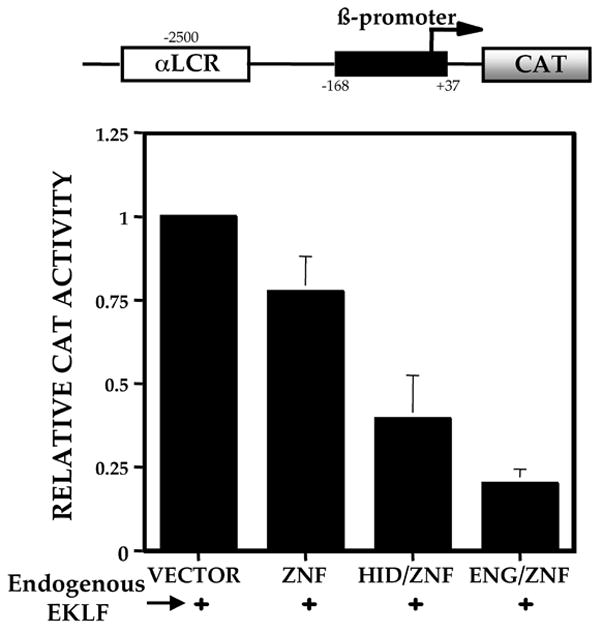
Transcriptional activities of EKLF repressor proteins on αLCR-βCAT. The αLCR-βCAT reporter was cotransfected with vector alone, the zinc finger domain alone or the repressor constructs into 32D Epo cells. These cells express endogenous EKLF. CAT activity in extracts was normalized to cotransfected growth hormone. The average of 3 experiments is shown.
Altered Transcriptional Regulation of the Endogenous Beta like Globin Genes in MEL cells
Discrepancies between the ability of artificial transcription factors to activate naked reporter DNA as opposed to an endogenous chromosome locus has previously been reported [44], leading us to study the effects of modified EKLF on an endogenous β-globin locus during erythroid differentiation in Murine erythroleukemia (MEL) cells. Cells that stably express ENG/ZnF were induced to differentiate with HMBA. As a control we used cells transfected with vector alone. Percentages of cells that stain blue with benzidine, an indicator of hemoglobinization, were determined on days 1 through 4 (Fig. 3A). In the control cells, hemoglobinized cells are first detected on day 1 and on day 4 nearly all cells are hemoglobinized. The ENG/ZnF transfected clones demonstrate a significant delay in hemoglobinization, and the peak number of hemoglobinized cells is approximately 2 fold lower. In order to determine the composition of hemoglobin in the stable MEL clones, globin RNA levels were studied. Expression of β major, the predominant murine adult hemoglobin, is reduced approximately 5 fold in clone 2–11 and 3 fold in clone 2–12 compared to the control clone (Fig 3B). In contrast, βH1 RNA, one of the murine embryonic globins, was reactivated with peak levels being 2 fold higher in clone 2–11 and 5 fold higher in clone 2–12. In studies performed by Lim et al [45] a 10 -fold increase in βH1 message was observed in definitive erythroid colonies derived from EKLF −/− ES cells compared to heterozygous ES cells. Since their studies showed a selective enrichment of βH1 message without an effect on εy message, we focused our attention on βH1 in these and all subsequent studies. The increase in βHI is consistent with the hypothesis of a competitive activation of embryonic globin by modified EKLF.
Figure 3.
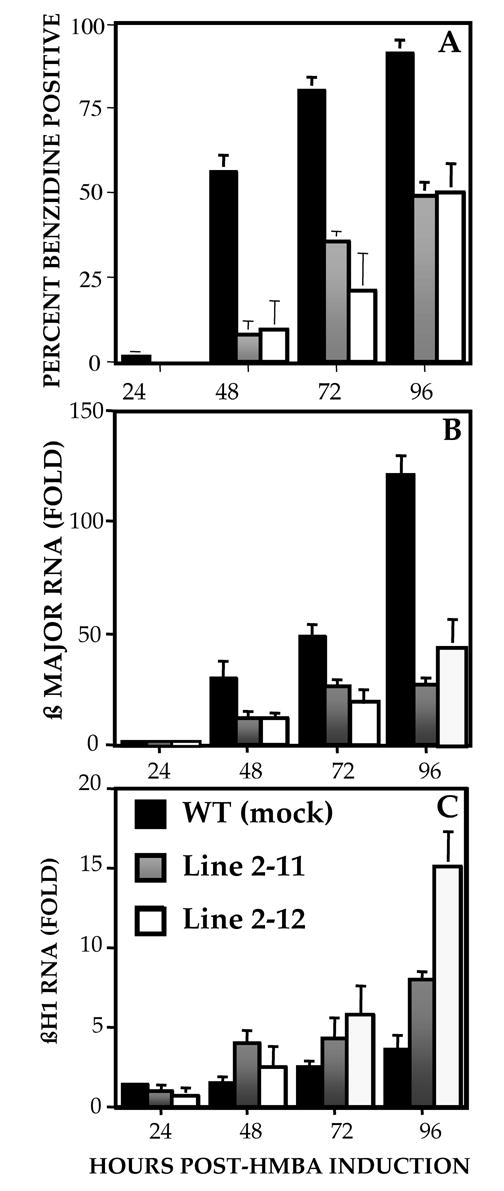
Erythroid differentiation of MEL clones stably expressing ENG/ZNF (2–11 and 2–12) with HMBA. Control cells are transfected with empty vector. Hemoglobinization was monitored by benzidine staining of whole cells at 24 hour intervals. The average of 3 experiments is shown (A). RNA was extracted at indicated time points and subject to semiquantitative PCR in the presence of primers for βmajor (B) and βH1 (C). Globin RNA levels are normalized to HPRT and are an average of 2 experiments.
Redesigned EKLF Constructs Augment Embryonic Globins During Primitive Erythropoiesis
We next turned to mouse embryonic stem cells (ES) to explore the effect of modified EKLF during primitive erythropoiesis as these cells, unlike MEL cells, have the benefit of a developmental program and plasticity. To achieve a consistent and homogenous induction of ENG/ZnF during differentiation in ES cells we utilized Ainv18 cells [37]. These cells contain a targeting site upstream of the HPRT locus such that integration of the tetracycline inducible transgene generates a functional antibiotic resistance gene thereby facilitating selection. The transgene contains an IRES-GFP downstream of ENG/ZnF, which serves as an internal control. FACS analysis showed robust levels of GFP expression in 70 to 80% of cells after doxycycline induction (Fig. 4A). The GFP signal specificity was confirmed by RT-PCR for ENG/ZnF with expression seen only after induction (Fig 4B). We wanted to measure the levels of ENG/ZnF relative to endogenous EKLF. Using Q-PCR to measure the absolute number of molecules of ENG/ZnF and EKLF in each sample we determined the ratio of the transgene to endogenous EKLF. This ratio is 7.8X, 10X and 20X respectively in the 3 clones. Levels of βH1 RNA by RT – PCR on Day 6 of EB differentiation are increased 2 fold in clones E11 and E19 and 3.5 fold in clone E9 upon doxycycline induction, with the greatest augmentation in the clone that had the highest amounts of ENG/ZnF relative to endogenous EKLF (Fig. 4C). At day 6, primitive erythroid progenitors are 100 fold more frequent than definitive erythroid progenitors and expressive primitive globins predominantly. Due to the relative paucity of β major at this stage of development [40], a two-fold decrease in β major was documented utilizing real time PCR (data not shown). It is striking that even at a developmental stage when β major is not being highly expressed and the embryonic genes are already active, embryonic globins could still be induced to even higher levels by expression of the ENG/ZnF repressor construct and in a dose dependent manner.
Figure 4.
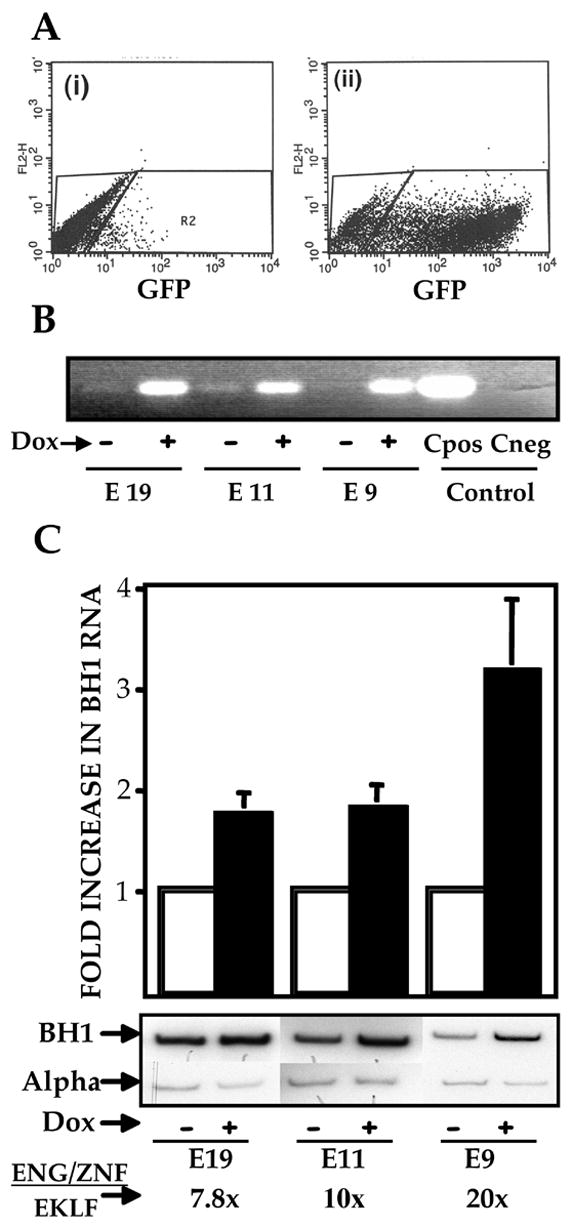
(A) FACS profile of an ES cell line modified for inducible ENG/ZNF expression. i) Uninduced ES cells ii) ES cells differentiated as EBs and grown in medium supplemented with 1 mcg/ml of doxycycline. (B) ENG/ZNF RNA expression in clones E11, E19, E9. RNA was extracted from EBs grown in the absence and presence of doxycycline and subjected to RT-PCR with primers specific for engrailed cDNA. (C)Augmented βH1 expression in EBs expressing ENG/ZNF. Clones E11, E19, E9 were differentiated to EBs in the presence and absence of doxycycline. βH1 RNA levels were determined using semiquantitative RT-PCR and are depicted as fold increase from the uninduced state. Results are normalized to α globin transcripts and are an average of 3 experiments. Absolute number of molecules of ENG/ZNF and EKLF were determined by Q-PCR. The ratio of ENG/ZNF to EKLF in respective clones was thus obtained.
Fetal liver Erythroid Cells from ENG/ZnF Transgenic Mice Demonstrate a Greater Probability of Executing a βH1 Expression Program
To address the effects of ENG/ZnF expression in a more enriched population of cells that have already switched their β-like globin expression pattern to the adult program, we established transgenic lines that harbored ENG/ZnF under the control of a 271 bp 5′ flanking region of the ANK-1 gene. This promoter was chosen for its ability to provide erythroid specific expression with copy number dependence and minimal position dependence [41]. The transgenic mice are viable, fertile and appear phenotypically normal. D13.5 fetal livers were subject to PCR analysis to establish the absolute amounts of ENG/ZNF and endogenous EKLF and to quantitate the ratios of the β-globins to α-globin transcripts (Fig. 5). The 9 transgenic embryos (from 3 separate founders) express βH1 mRNa in a range of values that is statistically higher than in 9 control littermates despite some overlap in the groups (Mann Whitney U test utilizing EXCEL with Stat Plus gives a p value of 0.02). At the same time, β Major expression demonstrates a trend towards significant reduction. Since the promoter used for the transgenic studies had previously been extensively validated in transgenic assays as being copy number independent and largely position independent [41,46], we proceeded directly to examine efficacy of transgene expression by measuring ratios of the transgene to endogenous EKLF.
Figure 5.
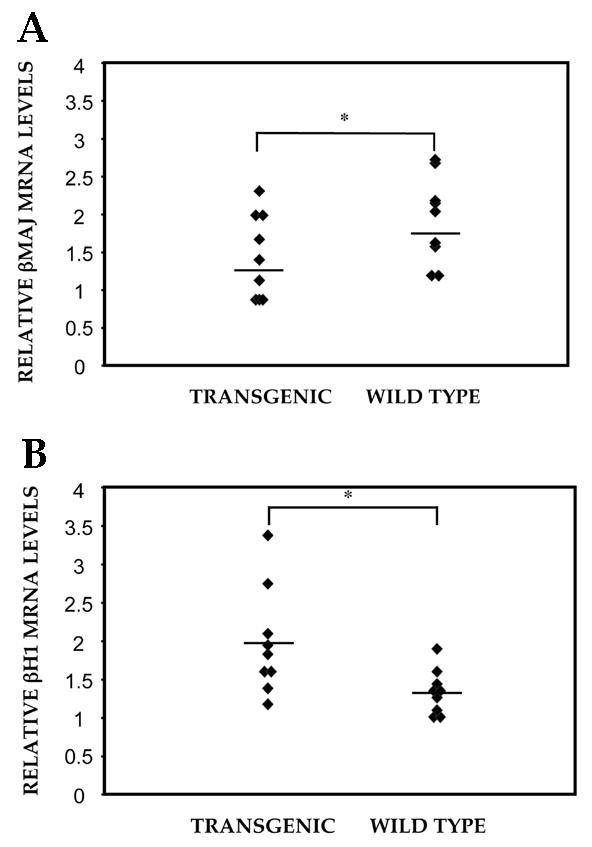
Altered Globin expression patterns in ENG/ZNF transgenic mice. βH1 (A) and β-major (B) transcripts in D13.5 fetal livers from transgenic embryos and wild type littermates were determined by semi-quantitative RT-PCR. Mann Whitney U/Rank sum test gave a p value of 0.02 and 0.05 for βH1 and β-major transcripts respectively.
The ratio of ENG/ZNF to endogenous EKLF ranges between 0.1X and 10X and there is no obvious correlation in the alteration of globin RNAs. We conclude that expression of ENG/ZNF alters the normal levels of theβ-like globins, more significantly by increasing embryonic globin expression. The lack of dose dependence and a modest effect could be attributed to complex regulatory processes in the whole animal, a closed chromatin context of the βH1 promoter in day 13.5 fetal livers or due to lower levels of transgene expression than in the ES/EB system. It is likely that stress erythropoiesis or alteration of the embryonic promoter context with a demethylating agent might augment the effect and uncover a clear dose dependence.
Discussion
Feasibility Of The Use Of Designer EKLF For Altered Transcription At The β-Globin Locus
Genomes are regulated at the level of transcription primarily through the effect of transcription factors that bind DNA in a sequence specific fashion. Artificially designed or synthetic transcription factors have been increasingly used to study aspects of transcriptional regulation [47,48]. Zinc fingers of the C2H2 type for this type of design are DNA binding motifs of choice due to their adaptability and specificity of binding [49,50]. Since they are functionally modular and each finger targets a 3 to 4 base pair sequence with few direct contacts between fingers, a DNA binding domain to any area in the genome can be designed. They have been used alone or linked to activation or repression domains. The feasibility of zinc finger transcription factors to provide precise regulation of a specific target gene has validated in a report in which the target gene was the only gene repressed when 16000 genes were analyzed by micro array analysis [51]. Selective activation and repression of genes has been successfully accomplished by such strategies demonstrating the feasibility of gene regulation on demand in vivo [52–55]. Our studies show that modified EKLF constructs effectively target the endogenous β-globin locus.
The Role of EKLF in Globin Gene Regulation
Our findings have the following important implications in the understanding of the role of EKLF at the β-globin locus. First, repression at the adult β-globin CACCC site in our studies once again establishes this sequence as a selective target for EKLF, even in its modified form. Despite the presence of a consensus EKLF-type CACCC motif in the embryonic globin βH1 promoter, its expression is increased rather than decreased. This is consistent with the observation that EKLF has a 4 fold greater binding efficiency to the murine βmaj versus βH1 CACCC site [33]. Second, the role of EKLF in consolidating the switch from γ-globin to β-globin during development was first demonstrated in EKLF −/− mice harboring the human β-globin transgene. Since the β-globin cluster in mice does not have a fetal stage-specific gene it has been a matter of some debate whether the embryonic genes are regulated in a similar fashion during development. In studies reported by Perkins et al, βH1 levels in heterozygous as well as EKLF knockout animals were not greater than wild type, however animals bearing the human β globin YAC showed increased levels of γ message[19,21]. In direct contrast to this data, Constantini et al [45] demonstrated a 10 fold increase in βH1 message in definitive erythroid colonies derived from EKLF −/− ES cells compared to heterozygous ES cells. The activation of βH1 globin in this report supports a developmental regulation that is analogous to the γ globin gene. It is likely that the repressor constructs create a closed chromatin context at the adult β-globin promoter, excluding it from the ACH (Active Chromatin Hub). Consistent with the competition model, this would favor the participation of the βH1 promoter in the ACH and augmented expression.
Designer EKLF in Transgenic Studies – Lessons to be Learned
Functional assays of designed ZFP have predominantly been performed in cell culture. There are reports of expression in the whole animal using adenoviral vectors [56]; however, ours is the first report describing the use of designed ZFP in transgenic animals. In these animals the heterogeneity in the level of βH1 and β major expression is consistent with stochastic models in which it is envisioned that the LCR may interact in an alternate fashion with downstream βH1 or β major globin genes in individual cells [13,14]. In transgenic animals, the probability of expression of a more embryonic program is statistically increased over wild type littermates, in keeping with our hypothesis. It is intriguing how these observations contrast with the effects of naturally occurring point mutations in the CACCC site of the adult β-globin gene in humans. Since the mutated CACCC sites disrupt transactivation of the adult β globin promoter by EKLF, we had originally predicted that humans carrying these mutations provide a biologic model of what one might expect when ENG/ZNF is expressed in transgenic assays. The −87 C to G substitution [25,26], −87 C to T mutation [27] and the −88 C to T [24] confer the mild phenotype of β-thalassemia intermedia in individuals homozygous for these mutations [57] and also in combination with β0-thalassemia [25]. The mild clinical presentation in these individuals is a combination of residual adult β-globin expression as well as a dramatic activation of γ globin genes with Hb F levels of 60–65%. In our transgenic mice we do not see a dramatic effect on embryonic globin reactivation. This could certainly be a function of the experimental design, especially since the β-major reduction is modest. Future experiments in mice harboring the human β-globin transgene will elucidate differential effects on the human versus mouse locus.
Potential Tools for Gene Therapy
Availability of designer transcriptional factors provides a greater likelihood of finding therapeutic agents that will provide optimal benefits with minimal side effects. Lack of a pan-genomic effect and a mutagenesis-free genetic modulation makes the use of zinc finger transcription factors for the gene therapy of sickle cell anemia an attractive approach.
Acknowledgments
We thank Robert Eisenman for plasmid clones and Pat O’Farrell for the Engrailed antibody. We are grateful to Gordon Keller and Marion Kennedy for advice on ES/EB techniques, Michael Kyba and George Daley for the Ainv18 cells, and Xue Li for guidance with the transgenic studies. Jonathan Licht, George Atweh, and Gordon Keller provided helpful comments throughout the project. The transgenic mice were generated in the Mouse Genetics Shared Research Facility which is partially funded by NCI grant R24 CA88302. This work was supported by National Institutes of Health Grants K08 DK002871 to DM and P60 HL28381 to JJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamatoyannopoulos GGF. Hemoglobin Switching. In: Majerus RMPPW, Varmus H, editors. The Molecular Basis of Blood Diseases. Philadelphia: W.B Saunders; 2001. [Google Scholar]
- 2.Behringer RR, Ryan TM, Palmiter RD, Brinster RL, Townes TM. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990;4:380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- 3.Trimborn T, Gribnau J, Grosveld F, Fraser P. Mechanisms of developmental control of transcription in the murine alpha- and beta-globin loci. Genes Dev. 1999;13:112–124. doi: 10.1101/gad.13.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosveld F, van Assendelft GB, Greaves DR, Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 5.Dillon N, Grosveld F. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature. 1991;350:252–254. doi: 10.1038/350252a0. [DOI] [PubMed] [Google Scholar]
- 6.Guy LG, Mei Q, Perkins AC, Orkin SH, Wall L. Erythroid Kruppel-like factor is essential for beta-globin gene expression even in absence of gene competition, but is not sufficient to induce the switch from gamma-globin to beta-globin gene expression. Blood. 1998;91:2259–2263. [PubMed] [Google Scholar]
- 7.Choi OR, Engel JD. Developmental regulation of beta-globin gene switching. Cell. 1988;55:17–26. doi: 10.1016/0092-8674(88)90005-0. [DOI] [PubMed] [Google Scholar]
- 8.Enver T, Raich N, Ebens AJ, Papayannopoulou T, Costantini F, Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990;344:309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- 9.Hanscombe O, Whyatt D, Fraser P, et al. Importance of globin gene order for correct developmental expression. Genes Dev. 1991;5:1387–1394. doi: 10.1101/gad.5.8.1387. [DOI] [PubMed] [Google Scholar]
- 10.Peterson KR, Stamatoyannopoulos G. Role of gene order in developmental control of human gamma- and beta-globin gene expression. Mol Cell Biol. 1993;13:4836–4843. doi: 10.1128/mcb.13.8.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter D, Chakalova L, Osborne CS, Dai YF, Fraser P. Long-range chromatin regulatory interactions in vivo. Nat Genet. 2002;32:623–626. doi: 10.1038/ng1051. [DOI] [PubMed] [Google Scholar]
- 12.Patrinos GP, de Krom M, de Boer E, et al. Multiple interactions between regulatory regions are required to stabilize an active chromatin hub. Genes Dev. 2004;18:1495–1509. doi: 10.1101/gad.289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palstra RJ, Tolhuis B, Splinter E, Nijmeijer R, Grosveld F, de Laat W. The beta-globin nuclear compartment in development and erythroid differentiation. Nat Genet. 2003;35:190–194. doi: 10.1038/ng1244. [DOI] [PubMed] [Google Scholar]
- 14.Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 15.Miller IJ, Bieker JJ. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Kruppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Southwood CM, Downs KM, Bieker JJ. Erythroid Kruppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev Dyn. 1996;206:248–259. doi: 10.1002/(SICI)1097-0177(199607)206:3<248::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Bieker JJ, Southwood CM. The erythroid Kruppel-like factor transactivation domain is a critical component for cell-specific inducibility of a beta-globin promoter. Mol Cell Biol. 1995;15:852–860. doi: 10.1128/mcb.15.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375:316–318. doi: 10.1038/375316a0. [DOI] [PubMed] [Google Scholar]
- 19.Perkins AC, Sharpe AH, Orkin SH. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 20.Donze D, Townes TM, Bieker JJ. Role of erythroid Kruppel-like factor in human gamma- to beta-globin gene switching. J Biol Chem. 1995;270:1955–1959. doi: 10.1074/jbc.270.4.1955. [DOI] [PubMed] [Google Scholar]
- 21.Perkins AC, Gaensler KM, Orkin SH. Silencing of human fetal globin expression is impaired in the absence of the adult beta-globin gene activator protein EKLF. Proc Natl Acad Sci U S A. 1996;93:12267–12271. doi: 10.1073/pnas.93.22.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wijgerde M, Gribnau J, Trimborn T, et al. The role of EKLF in human beta-globin gene competition. Genes Dev. 1996;10:2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 23.Drissen R, Palstra RJ, Gillemans N, et al. The active spatial organization of the beta-globin locus requires the transcription factor EKLF. Genes Dev. 2004;18:2485–2490. doi: 10.1101/gad.317004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Redondo JM, Stoming TA, Lanclos KD, et al. Clinical and genetic heterogeneity in black patients with homozygous beta-thalassemia from the southeastern United States. Blood. 1988;72:1007–1014. [PubMed] [Google Scholar]
- 25.Rosatelli MC, Oggiano L, Battista Leoni G, et al. Thalassemia intermedia resulting from a mild beta-thalassemia mutation. Blood. 1989;73:601–605. [PubMed] [Google Scholar]
- 26.Gilman JG, Manca L, Frogheri L, et al. Mild beta+(−87)-thalassemia CACCC box mutation is associated with elevated fetal hemoglobin expression in cis. Am J Hematol. 1994;45:265–267. doi: 10.1002/ajh.2830450316. [DOI] [PubMed] [Google Scholar]
- 27.Kulozik AE, Bellan-Koch A, Bail S, Kohne E, Kleihauer E. Thalassemia intermedia: moderate reduction of beta globin gene transcriptional activity by a novel mutation of the proximal CACCC promoter element. Blood. 1991;77:2054–2058. [PubMed] [Google Scholar]
- 28.Jaynes JB, O’Farrell PH. Active repression of transcription by the engrailed homeodomain protein. Embo J. 1991;10:1427–1433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han K, Manley JL. Functional domains of the Drosophila Engrailed protein. Embo J. 1993;12:2723–2733. doi: 10.1002/j.1460-2075.1993.tb05934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadosh D, Struhl K. Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol Cell Biol. 1998;18:5121–5127. doi: 10.1128/mcb.18.9.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 32.Im H, Grass JA, Johnson KD, et al. Chromatin domain activation via GATA-1 utilization of a small subset of dispersed GATA motifs within a broad chromosomal region. Proc Natl Acad Sci U S A. 2005;102:17065–17070. doi: 10.1073/pnas.0506164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng WC, Southwood CM, Bieker JJ. Analyses of beta-thalassemia mutant DNA interactions with erythroid Kruppel-like factor (EKLF), an erythroid cell-specific transcription factor. J Biol Chem. 1994;269:1493–1500. [PubMed] [Google Scholar]
- 34.Xue L, Chen X, Chang Y, Bieker JJ. Regulatory elements of the EKLF gene that direct erythroid cell-specific expression during mammalian development. Blood. 2004;103:4078–4083. doi: 10.1182/blood-2003-09-3231. [DOI] [PubMed] [Google Scholar]
- 35.Elnitski L, Hardison R. Efficient and reliable transfection of mouse erythroleukemia cells using cationic lipids. Blood Cells Mol Dis. 1999;25:299–304. doi: 10.1006/bcmd.1999.0257. [DOI] [PubMed] [Google Scholar]
- 36.Weiss MJ, Keller G, Orkin SH. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 37.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy M, Keller GM. Hematopoietic commitment of ES cells in culture. Methods Enzymol. 2003;365:39–59. doi: 10.1016/s0076-6879(03)65003-2. [DOI] [PubMed] [Google Scholar]
- 39.Keller G, Webb S, Kennedy M. Hematopoietic Development of ES cells in culture. In: Jordan CAKaCT., editor. Methods in Molecular Medicine. Totowa: Humana Press; 2003. p. 209. [DOI] [PubMed] [Google Scholar]
- 40.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabatino DE, Wong C, Cline AP, et al. A minimal ankyrin promoter linked to a human gamma-globin gene demonstrates erythroid specific copy number dependent expression with minimal position or enhancer dependence in transgenic mice. J Biol Chem. 2000;275:28549–28554. doi: 10.1074/jbc.M004043200. [DOI] [PubMed] [Google Scholar]
- 42.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo. 1994. [Google Scholar]
- 43.Migliaccio G, Migliaccio AR, Kreider BL, Rovera G, Adamson JW. Selection of lineage-restricted cell lines immortalized at different stages of hematopoietic differentiation from the murine cell line 32D. J Cell Biol. 1989;109:833–841. doi: 10.1083/jcb.109.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu PQ, Rebar EJ, Zhang L, et al. Regulation of an endogenous locus using a panel of designed zinc finger proteins targeted to accessible chromatin regions. Activation of vascular endothelial growth factor A. J Biol Chem. 2001;276:11323–11334. doi: 10.1074/jbc.M011172200. [DOI] [PubMed] [Google Scholar]
- 45.Lim SK, Bieker JJ, Lin CS, Costantini F. A shortened life span of EKLF−/− adult erythrocytes, due to a deficiency of beta-globin chains, is ameliorated by human gamma-globin chains. Blood. 1997;90:1291–1299. [PubMed] [Google Scholar]
- 46.Sabatino DE, Seidel NE, Aviles-Mendoza GJ, et al. Long-term expression of gamma-globin mRNA in mouse erythrocytes from retrovirus vectors containing the human gamma-globin gene fused to the ankyrin-1 promoter. Proc Natl Acad Sci U S A. 2000;97:13294–13299. doi: 10.1073/pnas.230453097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blancafort P, Segal DJ, Barbas CF., 3rd Designing transcription factor architectures for drug discovery. Mol Pharmacol. 2004;66:1361–1371. doi: 10.1124/mol.104.002758. [DOI] [PubMed] [Google Scholar]
- 48.Urnov FD, Rebar EJ, Reik A, Pandolfi PP. Designed transcription factors as structural, functional and therapeutic probes of chromatin in vivo. Fourth in review series on chromatin dynamics. EMBO Rep. 2002;3:610–615. doi: 10.1093/embo-reports/kvf140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhanasekaran M, Negi S, Sugiura Y. Designer zinc finger proteins: tools for creating artificial DNA-binding functional proteins. Acc Chem Res. 2006;39:45–52. doi: 10.1021/ar050158u. [DOI] [PubMed] [Google Scholar]
- 50.Papworth M, Kolasinska P, Minczuk M. Designer zinc-finger proteins and their applications. Gene. 2006;366:27–38. doi: 10.1016/j.gene.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Tan S, Guschin D, Davalos A, et al. Zinc-finger protein-targeted gene regulation: genomewide single-gene specificity. Proc Natl Acad Sci U S A. 2003;100:11997–12002. doi: 10.1073/pnas.2035056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartsevich VV, Miller JC, Case CC, Pabo CO. Engineered zinc finger proteins for controlling stem cell fate. Stem Cells. 2003;21:632–637. doi: 10.1634/stemcells.21-6-632. [DOI] [PubMed] [Google Scholar]
- 53.Jouvenot Y, Ginjala V, Zhang L, et al. Targeted regulation of imprinted genes by synthetic zinc-finger transcription factors. Gene Ther. 2003;10:513–522. doi: 10.1038/sj.gt.3301930. [DOI] [PubMed] [Google Scholar]
- 54.Urnov FD, Rebar EJ. Designed transcription factors as tools for therapeutics and functional genomics. Biochem Pharmacol. 2002;64:919–923. doi: 10.1016/s0006-2952(02)01150-4. [DOI] [PubMed] [Google Scholar]
- 55.Graslund T, Li X, Magnenat L, Popkov M, Barbas CF., 3rd Exploring strategies for the design of artificial transcription factors: targeting sites proximal to known regulatory regions for the induction of gamma-globin expression and the treatment of sickle cell disease. J Biol Chem. 2005;280:3707–3714. doi: 10.1074/jbc.M406809200. [DOI] [PubMed] [Google Scholar]
- 56.Rebar EJ, Huang Y, Hickey R, et al. Induction of angiogenesis in a mouse model using engineered transcription factors. Nat Med. 2002;8:1427–1432. doi: 10.1038/nm1202-795. [DOI] [PubMed] [Google Scholar]
- 57.Camaschella C, Alfarano A, Gottardi E, Serra A, Revello D, Saglio G. The homozygous state for the −87 C----G beta + thalassaemia. Br J Haematol. 1990;75:132–133. doi: 10.1111/j.1365-2141.1990.tb02629.x. [DOI] [PubMed] [Google Scholar]


