Abstract
Gene therapy for patients with hemoglobin disorders has been hampered by the inability of retrovirus vectors to transfer globin genes and their cis-acting regulatory sequences into hematopoietic stem cells without rearrangement. In addition, the expression from intact globin gene vectors has been variable in red blood cells due to position effects and retrovirus silencing. We hypothesized that by substituting the globin gene promoter for the promoter of another gene expressed in red blood cells, we could generate stable retrovirus vectors that would express globin at sufficient levels to treat hemoglobinopathies. Recently, we have shown that the human ankyrin (Ank) gene promoter directs position-independent, copy number-dependent expression of a linked γ-globin gene in transgenic mice. We inserted the Ank/Aγ-globin gene into retrovirus vectors that could transfer one or two copies of the Ank/Aγ-globin gene to target cells. Both vectors were stable, transferring only intact proviral sequences into primary mouse hematopoietic stem cells. Expression of Ank/Aγ-globin mRNA in mature red blood cells was 3% (single copy) and 8% (double copy) of the level of mouse α-globin mRNA. We conclude that these novel retrovirus vectors may be valuable for treating a variety of red cell disorders by gene replacement therapy including severe β-thalassemia if the level of expression can be further increased.
The hemoglobinopathies comprise a large group of disorders characterized by absent synthesis of either the α- or β-like globin chains, or synthesis of a mutant globin protein (1). Taken as a group, the hemoglobinopathies are the most common inherited disease of the hematopoietic system worldwide. Absence of β-globin chains [β-thalassemia (β-thal)] causes precipitation of α-globin chains, resulting in red cell destruction during erythropoiesis or in the circulation (2). One approach to the treatment of the hemoglobinopathies has been allogeneic bone marrow transplantation, which has been limited by donor availability and the complications of graft versus host disease (1).
In severe β-thal patients, the predominant hemoglobin tetramer formed is hemoglobin F, composed of two α-globin and two γ-globin chains (2). The γ-globin chain is the most abundant β-like globin synthesized before birth. After birth, the β-globin gene becomes active, and γ-globin synthesis is decreased (3). Several experimental strategies have been attempted to increase the level of γ-globin gene expression in patients with severe β-thal. These include treatment with 5-azacytidine or hydroxyurea, both of which can raise the level of hemoglobin F (1, 4, 5). Although these treatments have been effective for some patients, these therapies do not result in a complete correction of β-thal.
The hemoglobinopathies were originally considered excellent candidates for treatment by gene replacement therapy. The globin genes were available, and many of the critical aspects of their regulation were known. Ideally, a β-like globin gene would be transferred via a retrovirus vector into the hematopoietic stem cells from a patient with a β-chain hemoglobinopathy. After integration of the retrovirus into the stem cell genome, the globin gene would be passed along to all of the progeny of that stem cell and eventually expressed in mature red blood cells (6, 7). Early work demonstrated that retrovirus vectors could transfer human β-like globin genes into mouse hematopoietic stem cells. However, the level of globin mRNA was far below the level needed to treat a β-thal patient (8–10). The locus control region (LCR) of the human β-globin cluster contains powerful cis-acting regulatory elements that are critical for high level, position-independent expression of the globin genes in transgenic mice (11–14). The incorporation of LCR elements into the original retrovirus vectors causes the retrovirus to rearrange during the life cycle.
The modification of cryptic RNA processing sites in the LCR sequences and deletion of sequences in IVS2 of the globin gene produced a “second generation” of globin vectors with higher titer and more stable integration (7, 15–22). Others have found that the HS-40 regulatory region from the α-globin locus could be substituted for β-globin LCR sequences with increased transmission of intact retrovirus into murine erythroleukemia cells and higher levels of expression (22, 23). Despite these advances, globin gene expression has been shown to be highly position dependent and prone to silencing over time in mouse models (7, 19, 20). Recently May et al. (24) have used an HIV-based vector to transfer and express a human β-globin gene in mouse erythroid cells. The HIV rev protein, which is expressed during packaging, suppresses processing of the vector RNA, and the larger capacity of the HIV vector compared with retrovirus vectors allowed for larger LCR sequences to be incorporated. Human β-globin mRNA levels were approximately 13% of the endogenous β-globin, and sufficient β-globin protein was present to improve the red cell indices in mice heterozygous for the β-thalassemia, inducing knockout of the mouse β-globin genes (24).
We have taken an alternative approach that takes full advantage of the relative safety of the murine retrovirus system compared with HIV vectors. We have previously shown that the promoter of the red cell membrane protein, ankyrin (Ank), directed erythroid-specific, position-independent, copy number-dependent expression of a γ-globin gene in transgenic mice (25). We hypothesized that by replacing the γ-globin gene promoter with the Ank promoter in a retrovirus vector, we would eliminate the need for LCR sequences in the vector and promote both higher titer and more stable gene transfer. In this report, we demonstrate that retrovirus vectors containing one or two Ank/Aγ-globin transgenes could be generated at high titers and were transferred intact into primary mouse progenitor cells (colony-forming units of the spleen, CFU-S) and hematopoietic stem cells. The expression of single-copy and double-copy Ank/Aγ-globin mRNA in CFU-S and long-term mice was approximately 3% and 8% the level of mouse α-globin mRNA per vector copy, respectively. We conclude that the level of γ-globin gene expression from the double-copy Ank/Aγ-globin vector may be sufficient to treat many erythroid disorders, including severe β-thal if expression can be further increased.
Materials and Methods
Retrovirus Constructs.
The plasmid pHS40–5 (22) was digested with BamHI/HindIII to generate a 766-bp fragment containing sequences from exon 2 and the 3′ end of the Aγ gene minus a 717-bp deletion (XhoI/HincII) in IVS2. This fragment replaced the corresponding sequences in pSP72 Ank/Aγ (25) to generate pSP72 Ank/Aγdel. A 1,494-bp Ank/Aγdel fragment was excised with HindIII/XbaI and cloned into BspEI/HindIII-digested LITMUS 38 (38 Ank/Aγdel, New England Biolabs). A 1,563-bp Ank/Aγdel fragment was excised from 38 Ank/Aγdel with SnaBI/SalI and cloned into the retrovirus vector pGCsam (26) digested with SnaBI/XhoI to generate the GC Ank/Aγdel retrovirus vector.
To generate the Ank double-copy retrovirus, a 1,496-bp fragment containing Ank/Aγdel was excised from pSP72 Ank/Aγdel with SmaI/HindIII and inserted into pBluescript II KS+ digested with SmaI/HindIII to generate BS Ank/Aγdel. A 1,538-bp NotI/SalI Ank/Aγdel fragment was excised from BS Ank/Aγdel and inserted into LITMUS 38 digested with EagI/EcoRV to generate 38 Ank/Aγdel*. A 1,532-bp MluI/ClaI Ank/Aγdel fragment from 38 Ank/Aγdel* was cloned into the MluI/BstBI sites of a polylinker located in the 3′ long terminal repeat (LTR) of the retrovirus vector pG3 (a gift from Genetic Therapy) to generate G3 Ank/Aγdel. Finally, the 3′ LTR in pGCsam (26) was excised with NdeI/NotI and replaced with the NdeI/NotI fragment of G3 Ank/Aγdel containing the 3′ LTR to generate DC Ank/Aγdel.
Producer Cell Lines.
The single-copy retrovirus producer cell line was generated by cotransfection of GC Ank/Aγdel and the neomycin resistance gene into GP+E86 (27) packaging cells by calcium phosphate precipitation (Cellphect Kit, Amersham Pharmacia). Individual clones were selected with G418 and analyzed for virus production by slot blot analysis of supernatant RNA. The double-copy retrovirus producer cell line was generated by transfecting BING cells (28) with DC Ank/Aγdel. Supernatant from transfected BING cells was used to transduce GP+E86 cells, and individual colonies were isolated and characterized as above.
Transduction of Hematopoietic Stem Cells.
Donor mice (C57BL/6J) were treated with 5-fluorouracil (150 mg/kg) 48 h before bone marrow harvest. Bone marrow cells were collected from donor mice and cultures for 48 h in IL-3 (10 ng/ml), IL-6 (100 ng/ml, Amgen Biologicals), and stem cell factor (100 ng/ml, Amgen Biologicals). The donor cells were then cocultured with producer cells for 48 h in the presence of polybrene (6 mg/ml), IL-3, IL-6, and stem cell factor. The nonadherent cells were collected, washed, and injected into WBB6F1-W/Wv recipients (9, 29).
CFU-S Analysis.
CFU-S were dissected from the spleen 14 days after transplant of 1 × 104 to 1 × 105 cells, which were transduced with one of the Ank retrovirus vectors into irradiated (900R) WBB6F1+/+ recipients. RNA and DNA were isolated from each CFU-S and confluent spleen with TRIZOL (GIBCO/BRL).
Long-Term Repopulation Studies.
W/Wv mice were transplanted with 2 × 106 cells transduced with either Ank retrovirus and were analyzed 16 weeks posttransplant. The animals were phlebotomized for 3 consecutive days into 1 × red cell lysis buffer prepared from a 10× stock solution (82.9 g NH3Cl/10 g KHCO3/4 ml of 250 mM EDTA/dH2O to 1000 ml). DNA was isolated from the peripheral blood by digesting the cells in 1 M Tris/0.1 M NaCl/0.1 M EDTA/1%SDS/0.1 mg/ml proteinase K (GIBCO/BRL) at 55°C for 2 h followed by phenol and chloroform extractions and ethanol precipitation. On day 5, peripheral blood was collected in PBS with sodium heparin, and RNA was isolated from the reticulocytes with TRIZOL (GIBCO/BRL). RNA and DNA were isolated from bone marrow, spleen, and thymus with TRIZOL (GIBCO/BRL).
RNase Protection Assays.
Linear DNA templates for the RNase protection assay were prepared by EcoRI (Ank/Aγ) and HindIII (mouse α) digestion of cesium chloride-purified plasmid preparations. The templates were purified by agarose gel electrophoresis and purified using a Geneclean II Kit (Bio 101). 32P-labeled RNA probes were transcribed by using the MAXIscript in vitro transcription kit (Ambion). The hybridization of the probe and RNA (1 μg) was carried out overnight according to the standard procedure of the RPA II of the RNase protection assay kit (Ambion). RNase digestion was performed by using a RNase A/RNase T1 mixture in RNase digestion buffer (Ambion), and the protected fragments were separated on an 8% nondenaturing polyacrylamide gel.
Quantification of mRNA Levels.
The level of Ank/Aγ mRNA was estimated by using a Molecular Dynamics phosphorimager (Amersham Pharmacia). The relative amounts of the bands of human Aγ-globin exon 2 (175 bp) and mouse α-globin exon 2 (186 bp) were calculated by the following formula: (Aγ-globin RNA/transgene copy number)/(mouse α-globin RNA) × 100.
Immunofluoresence Analysis.
Peripheral blood cells were prepared as described (30). The cells fixed in ice-cold (4°C) 4% formaldehyde solution. The cells were washed with 1:1 acetone/water (−20oC), acetone (−20oC), and 1:1 acetone/water (−20oC) and resuspended in PBS/2% FBS (4oC). Hemoglobin tetramers containing human γ-globin were identified with an FITC-conjugated human hemoglobin F antibody (Perkin–Elmer). All incubations and washes were performed at 4oC.
Results
Ank/Aγ-Globin Retrovirus Vectors.
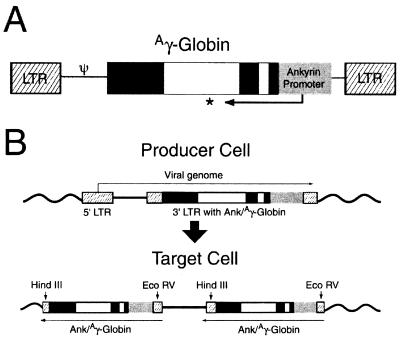
Two retrovirus vectors containing the Ank/Aγ-globin gene were constructed. The single copy retrovirus contains the Ank/Aγ-globin gene between the LTR sequences (Fig. 1A). The double-copy retrovirus contains the Ank/Aγ-globin gene in the 3′ LTR. During the retrovirus life cycle, the 3′ LTR is duplicated, generating Ank/Aγ-globin genes in both LTRs of the integrated provirus (Fig. 1B). The Aγ-globin gene in both vectors contains the IVS2 deletion shown to promote more stable gene transfer (22). Slot blot analysis of RNA isolated from medium conditioned by isolated clones of GP+E86 ecotropic producer cells demonstrated titers estimated to be between 1 × 105 (double copy) and 1 × 106 (single copy) cfu/ml. The titer was confirmed by transduction of NIH 3T3 cells followed by Southern blot analysis of DNA. Bands of the predicted size for both proviral genomes could be visualized (data not shown).
Figure 1.
Ank/Aγ-globin retrovirus constructs. (A) The integrated single-copy provirus. The LTR sequences are shown as hatched boxes, the ankyrin promoter as a gray box, exon sequences in the γ-globin gene as dark boxes, and intron sequences as open boxes. The asterisk indicates the presence of a 717-bp deletion in IVS2 of the γ-globin gene. (B) The double-copy retrovirus vector in the producer cell genome (Upper) and the integrated provirus (Lower). The elements are identified as in A. Note that during the conversion of the RNA genome to DNA, the 3′ LTR, which includes the Ank/Aγ-globin gene, is duplicated into the 5′ LTR. The positions of the restriction enzyme sites for HindIII and EcoRV are shown. HindIII digestion detects intact 5′ LTR sequences and generates a specific 3′ junction fragment for each insertion. EcoRV digestion detects intact 3′ LTR sequences and a specific 5′ junction fragment for each insertion.
Gene Transfer and Expression of the Ank/Aγ-Globin Gene in Primary Mouse Erythroid Progenitor Cells.
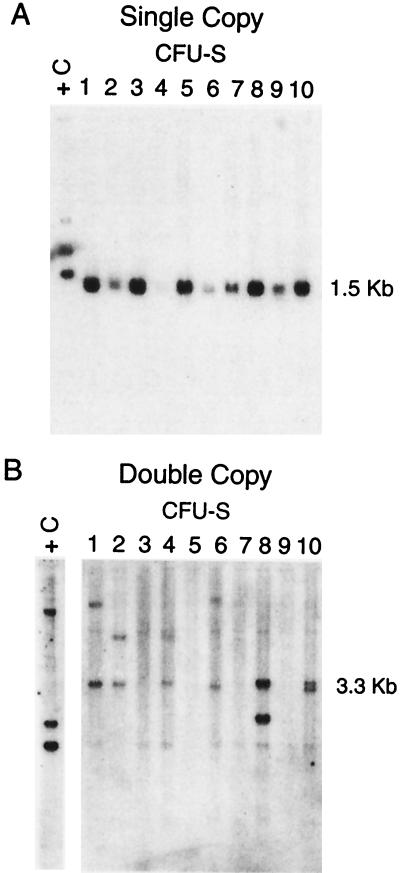
Both the single- and double-copy Ank/Aγ-globin vectors were used to transduce primary bone marrow cells from 5-fluorouracil treated mice. Limited numbers of cells were injected into irradiated mice for analysis of gene transfer and expression of the Ank/Aγ-globin retroviruses in CFU-S at 14 days posttransplant. Southern blot analysis of DNA from individual CFU-S colonies probed with a 600-bp probe containing the Ank promoter and Aγ-globin exon 1 and intron 1 revealed only the predicted 1.5-kb band for the single-copy vector (Fig. 2A). HindIII (Fig. 2B) and EcoRV digests (not shown) of DNA extracted from CFU-S transduced with the double-copy vector were performed to confirm the integration of the Ank/Aγ-globin gene in the 5′ and 3′ LTR, respectively. The 3.3-kb band in Fig. 2B represents an intact 5′ Ank/Aγ-globin gene, whereas the second band in each lane represents the 3′ junction fragment, which varies in size from colony to colony depending on the insertion site. No bands other than the predicted bands were observed in an analysis of 28 positive (single copy) and 19 positive (double copy) CFU-S (Table 1).
Figure 2.
Southern Blot analysis of single-copy (A) and double-copy (B) Ank/Aγ-globin provirus in CFU-S colonies. Each lane contains 10 μg of DNA isolated from a single dissected CFU-S digested with HindIII. The sizes of the bands indicated at the right indicate the predicted size of the intact provirus. +C, EcoRI-digested DNA from a transgenic mouse with 1 intact copy of the Ank/Aγ-globin gene.
Table 1.
Analysis of Ank/Aγ-globin gene transfer and expression in CFU-S
| Ankyrin retrovirus | No. CFU-S analyzed (Southern) | No. CFU-S with provirus | No. CFU-S with intact provirus | No. CFU-S analyzed (RNA) | Human γ-globin mRNA Mouse α-globin mRNA (mean %) |
|---|---|---|---|---|---|
| Single copy | 51 | 28 | 28 | 9 | 2.9 ± 1.2 |
| Double copy | 38 | 19 | 19 | 9 | 7.9 ± 3.1* |
*, P = 0.003 two tailed t-test.
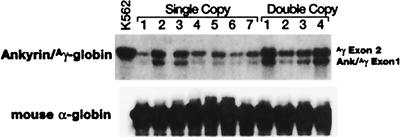
RNase protection analysis of RNA extracted from the same CFU-S colonies revealed expression of properly initiated and spliced Ank/Aγ-globin mRNA from both vectors (Fig. 3). Comparison of the intensities of the signals from the mouse α-globin and Ank/Aγ-globin indicated that the level of Ank/Aγ-globin mRNA was 2.9 ± 1.2% of the level of mouse α-globin mRNA in colonies containing the single-copy vector (Table 1). Concurrent analysis of CFU-S containing the double-copy vector indicated that the level of Ank/Aγ-globin mRNA was 7.9 ± 3.1 the level of α-globin mRNA (P = 0.003; Table 1).
Figure 3.
RNase protection of Ank/Aγ-globin mRNA from CFU-S colonies containing the single- or double-copy Ank/Aγ-globin vector. One microgram of total RNA was analyzed in each lane except the K562 positive control lane, which contained 10 μg. (Upper) Bands protected by the Ank/Aγ-globin probe are shown (exon 1, 139 bp; exon 2, 175 bp). (Lower) The band protected by the mouse α-globin probe is shown (exon 2, 186 bp).
Gene Transfer and Expression of the Ank/Aγ-Globin Gene in Long-Term Repopulated Mice.
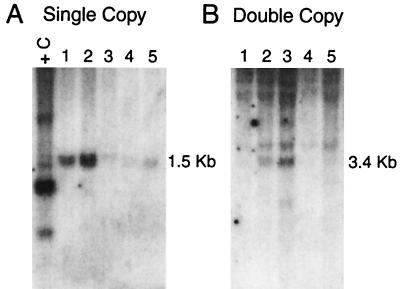
Approximately 2 × 106 cells transduced with either the single- or double-copy Ank/Aγ-globin vectors were injected into genetically anemic W/Wv mice for long-term analysis of gene transfer and expression. Conversion of the hemoglobin pattern from the single diffuse pattern of the recipient to the single pattern of the donor confirmed complete repopulation of the erythroid lineage in transplant recipients. Southern blot analysis of DNA isolated from the peripheral blood, bone marrow, and spleen of repopulated mice revealed only the bands of the predicted size for the single-copy vectors (Fig. 4A). Using the endogenous bands as loading controls, we compared the intensities of the Ank/Aγ-globin bands in DNA from the transplanted animals with the intensity of the band in DNA from a control transgenic mouse with one intact copy of the Ank/Aγ-globin gene. We estimated that the provirus copy number in the nucleated cells of the animals depicted in Fig. 4A was between 0.1 and 2 copies per animal. Analysis of HindIII- (not shown) and EcoRV-digested peripheral blood DNA extracted from animals transduced with the double-copy vector confirmed intact integration of the Ank/Aγ-globin gene in both the 5′ and 3′ LTR (Fig. 4B, showing the 3.4-kb 3′ Ank/Aγ-globin fragment). Additional fainter bands were rarely observed, consistent with integration at multiple locations. The estimated proviral copy number of the animals in Fig. 4B ranged between 0.1 and 0.5 copies per animal.
Figure 4.
Southern blot analysis of single-copy (A) and double-copy (B) Ank/Aγ-globin retrovirus vectors in long-term repopulated mice. Each lane contains 10 μg of DNA isolated from the peripheral blood of mice. (A) HindIII digest. (B) EcoRV digest. The sizes of the bands indicated at the right indicate the predicted size of the intact provirus. +C: EcoRI-digested DNA from a transgenic mouse with one intact copy of the Ank/Aγ-globin gene.
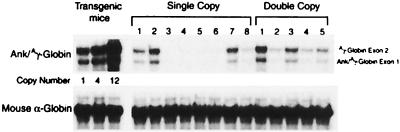
RNase protection analysis of RNA extracted from the same animals revealed expression of properly initiated and spliced Ank/Aγ-globin mRNA from both vectors (Fig. 5). In animals where no RNA was detected (e.g., lanes 3–6 in Fig. 5), no provirus was detected by Southern blot analysis of peripheral blood, bone marrow, spleen, or thymus DNA. The level of Ank/Aγ-globin mRNA in reticulocytes of transplanted animals was compared with the level of Ank/Aγ-globin mRNA in a panel of transgenic mice. The transgenic mice express Ank/Aγ-globin mRNA expressing 4, 15, and 45% of the level of α-globin mRNA (25) (Fig. 5), indicating relatively high levels of expression in many of the transplanted animals.
Figure 5.
RNase protection of Ank/Aγ-globin mRNA from reticulocytes of long-term repopulated mice transplanted with bone marrow cells exposed to the single- or double-copy Ank/Aγ-globin vectors. The transgenic mouse controls at the left have 1, 4, and 12 copies of the Ank/Aγ-globin transgene and express Ank/Aγ-globin mRNA at levels of approximately 3.7, 15, and 45% of mouse α-globin. (Upper) Bands protected by the Ank/Aγ-globin probe are shown (exon 1, 139 bp; exon 2, 175 bp). (Lower) The band protected by the mouse α-globin probe is shown (exon 2, 186 bp).
FACS Analysis of Ank/Aγ-Globin in Mouse Erythrocytes.
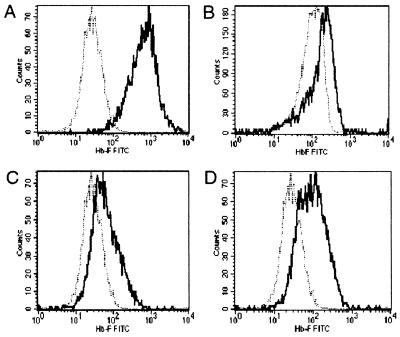
To estimate the relative number of red blood cells expressing γ-globin, we analyzed mouse α2/human γ2 tetramer formation in red blood cells from several animals with proviral copy numbers of 1 or higher using a monoclonal antibody against human γ-globin. As a positive control, we used red blood cells from a transgenic mouse expressing Aγ-globin mRNA at a level that is approximately 35% the level of α-globin mRNA (31). The control cells stained strongly with the anti-γ-globin antibody compared with a negative control mouse (Fig. 6A). The animal depicted in Fig. 6B was repopulated with bone marrow cells exposed to the single-copy Ank/Aγ-globin retrovirus, whereas the animals depicted in Fig. 6 C and D were repopulated with bone marrow cells exposed to the double-copy Ank/Aγ-globin retrovirus. In these animals, virtually all red blood cells expressed γ-globin. Two populations of γ-globin positive red blood cells are present in the animal depicted in Fig. 6D, consistent with differing levels of Ank/Aγ-globin gene expression in the two populations.
Figure 6.
FACS analysis of g-globin chains of red blood cells from long-term repopulated mice transplanted with bone marrow cells exposed to the single- or double-copy retrovirus vectors. Red blood cells were fixed and stained with a monoclonal antibody against human γ-globin. The lighter line in each panel shows untreated mouse red blood cells stained with the anti-γ-globin antibody. (A) Analysis of a transgenic mouse expressing γ-globin. (B) Analysis of a mouse repopulated with cells exposed to the single-copy vector. (C and D) Analysis of mice repopulated with bone marrow exposed to the double-copy vector.
We estimated the relative number of transduced cells based on a combination of provirus copy number in nucleated cells (Fig. 4), FACS analysis, and direct immunofluorescent staining of red blood cells (data not shown; courtesy of Thalia Papayannopoulou and Betty Mastropaolo). We calculate that in animals repopulated with cells exposed to the single-copy and double-copy retrovirus vectors, the level of Ank/Aγ-globin mRNA in the positive cells is approximately 3% and 7% of the level of mouse α-globin, respectively. These values are consistent with the Ank/Aγ-globin mRNA levels in CFU-S (Table 1).
Discussion
To be useful for gene therapy of hemoglobin disorders, a globin gene transfer vector should not rearrange during transduction and should express the transferred globin gene in a position-independent manner, without being silenced over time. Our double-copy Ank/Aγ-globin retrovirus vector is transduced unrearranged and expresses about 50% of the level of globin mRNA needed to treat severe β-thal. Silencing of retrovirus vectors over time has been a serious problem in the development of hematopoietic stem cells-based gene therapy (7, 19, 20, 32). We have seen no evidence of vector silencing in our experiments. We observed a 1:1 correspondence between the presence of the provirus in DNA from nucleated hematopoietic cells and expression of Ank/Aγ-globin mRNA in reticulocytes. We also observed a direct correlation between the estimated number of provirus copies and the level of Ank/Aγ-globin mRNA. We hypothesize that the relatively position-independent expression from the double-copy vector is due to the fact that the human Ank promoter contains all of the sequences that are necessary and sufficient for position-independent, copy number-dependent transgene expression in transgenic mice (25).
The sequence of the ANK-1 promoter is very GC rich and resembles a housekeeping promoter despite the fact that it directs erythroid-specific expression of transgenes (33). In previous studies, we demonstrated that the GC-rich β-spectrin (βsp) promoter also directs erythroid-specific expression (31). However, the βsp promoter differs from the human Ank promoter in that the βsp promoter requires an enhancer element (5′ HS2 from the β-globin LCR) for position-independent expression in transgenic mice (31). Interestingly, retrovirus vectors containing HS2 βsp/Aγ-globin were prone to rearrangement similar to LCR/globin retrovirus vectors (data not shown). One hypothesis that has been advanced by others is that the context of LCR sequences and globin genes in retrovirus vectors promotes processing of the vector RNA at cryptic sites after transcription, leading to reduced transfer of intact provirus. This hypothesis is strengthened by the recent observation that modification to LCR/globin vectors to eliminate cryptic splice sites has improved the ability of these vectors to be transferred intact to target cells (16, 17). We conclude that elimination of the need for LCR sequences allows the Ank/Aγ-globin retrovirus RNA to be packaged without significant processing after transcription.
The incorporation of the Ank/Aγ-globin gene into single-copy or double-copy retrovirus vectors led to Ank/Aγ-globin mRNA levels that closely approximate or exceed the levels we observed in transgenic mice carrying a similar transgene without the retrovirus backbone (25). Even if problems with RNA processing can be solved, it is unlikely that adding LCR sequences to the Ank/Aγ-globin would increase expression. We have previously shown that unlike the promoters of the β-spectrin (31) or globin genes (11–14), the 5′HS2 enhancer element from the locus control region decreases expression of the Ank/Aγ-globin gene in adult transgenic mice (25). The compact size of the human Ank promoter lends itself to site-directed mutagenesis, which could be used to identify mutations that lead to higher levels of expression. Insulator elements have been shown to protect transgenes from the influences of heterochromatin, and these elements may be incorporated into Ank/Aγ-globin retrovirus vectors (34). Finally, the woodchuck hepatitis virus sequences that improve RNA stability and nuclear export of RNA could be added to our vectors. This approach could increase the level of translated protein from the same amount of transcribed mRNA, as has been demonstrated in related systems (35).
The level of γ-globin mRNA in mice repopulated with cells transduced with the double-copy Ank/Aγ-globin retrovirus is approximately 50% of the level of mRNA expressed from an LCR/β-globin gene in an HIV vector background reported by May et al. (24). However, we feel that developing retrovirus vectors that express relatively high levels of globin mRNA offers an important advantage over the HIV-based system. Whereas second- and third-generation lentivirus vectors have been generated that substantially reduce the possibility that wild-type HIV can be produced along with the recombinant vector (36), murine retroviruses have a long history of safety in human gene therapy trials. The human Ank promoter may also be used to drive the expression of genes other than β- or γ-globin genes in erythroid cells. For example, the Ank-1 promoter could be fused to the G6PD gene to treat the severe hemolytic anemia seen in some G6PD-deficient patients (37). Other applications could include red cell membrane protein genes as suggested by the in vitro experiments of Dooner et al. (38).
Acknowledgments
We thank Thalia Papayannopoulou and Betty Mastropouloo for the direct imunofluorescence analysis. This work was supported in part by grants from the National Institutes of Health and The March of Dimes Birth Defects Foundation (to P.G.G.).
Abbreviations
- Ank
ankyrin
- β-thal
β-thalassemia
- LCR
locus control region
- LTR
long terminal repeat
- CFU-S
colony-forming unit-spleen
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230453097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230453097
References
- 1.Weatherall D J. In: The Molecular Basis of Blood Diseases. Stamatoyannopoulos G, Nienhuis A W, Majerous P W, Varmus H, editors. Philadelphia: Saunders; 1994. pp. 157–205. [Google Scholar]
- 2.Bunn H F, Forget B G. Hemoglobin: Molecular, Genetic and Clinical Aspects. Philadelphia: Saunders; 1986. [Google Scholar]
- 3.Stamatoyannopoulos G, Nienhuis A W. In: Molecular Basis of Blood Diseases. Stamatoyannopoulos G, Nienhuis A W, Varmus H, editors. Philadelphia: Saunders; 1993. [Google Scholar]
- 4.Ley T J, DeSimone J, Anagnou N P, Keller G H, Humphries R K, Turner P H, Young N S, Keller P, Nienhuis A W. N Engl J Med. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 5.Ley T J. Blood. 1991;77:1146–1147. [PubMed] [Google Scholar]
- 6.Anderson W F. Science. 1984;226:401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- 7.Sadelain M. Br J Haematol. 1997;98:247–253. doi: 10.1046/j.1365-2141.1997.2313048.x. [DOI] [PubMed] [Google Scholar]
- 8.Dzierzak E A, Papayannopoulou T, Mulligan R C. Nature (London) 1988;331:35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- 9.Bodine D M, Karlsson S, Nienhuis A W. Proc Natl Acad Sci USA. 1989;86:8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender M A, Gelinas R E, Miller A D. Mol Cell Biol. 1989;9:1426–1434. doi: 10.1128/mcb.9.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosveld F, van Assendelft G B, Greaves D R, Kollias G. Cell. 1987;51:975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- 12.Fraser P, Hurst J, Collis P, Grosveld F. Nucleic Acids Res. 1990;18:3503–3508. doi: 10.1093/nar/18.12.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrester W C, Takegawa S, Papayannopoulou T, Stamatoyannopoulos G, Groudine M. Nucleic Acids Res. 1987;15:10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan T M, Behringer R R, Martin N C, Townes T M, Palmiter R D, Brinster R L. Genes Dev. 1989;3:314–323. doi: 10.1101/gad.3.3.314. [DOI] [PubMed] [Google Scholar]
- 15.Plavec I, Papayannopoulou T, Maury C, Meyer F. Blood. 1993;81:1384–1392. [PubMed] [Google Scholar]
- 16.Leboulch P, Huang G M, Humphries R K, Oh Y H, Eaves C J, Tuan D Y, London I M. EMBO J. 1994;13:3065–3076. doi: 10.1002/j.1460-2075.1994.tb06605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadelain M, Wang C H, Antoniou M, Grosveld F, Mulligan R C. Proc Natl Acad Sci USA. 1995;92:6728–6732. doi: 10.1073/pnas.92.15.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takekoshi K J, Oh Y H, Westerman K W, London I M, Leboulch P. Proc Natl Acad Sci USA. 1995;92:3014–3018. doi: 10.1073/pnas.92.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivella S, Sadelain M. Semin Hematol. 1998;35:112–125. [PubMed] [Google Scholar]
- 20.Raftopoulos H, Ward M, Leboulch P, Bank A. Blood. 1997;90:3414–3422. [PubMed] [Google Scholar]
- 21.Li Q, Emery D W, Fernandez M, Han H, Stamatoyannopoulos G. Blood. 1999;93:2208–2216. [PubMed] [Google Scholar]
- 22.Emery D W, Morrish F, Li Q, Stamatoyannopoulos G. Hum Gene Ther. 1999;10:877–888. doi: 10.1089/10430349950018283. [DOI] [PubMed] [Google Scholar]
- 23.Ren S, Wong B Y, Li J, Luo X N, Wong P M, Atweh G F. Blood. 1996;87:2518–2524. [PubMed] [Google Scholar]
- 24.May C, Rivella S, Callegari J, Heller G, Gaensler K L M, Luzzatto L, Sadelain M. Nature (London) 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- 25.Sabatino D E, Wong C, Cline A P, Pyle L, Garrett L J, Gallagher P G, Bodine D M. J Biol Chem. 2000;275:28549–28554. doi: 10.1074/jbc.M004043200. [DOI] [PubMed] [Google Scholar]
- 26.Chuah M K, Vandendriessche T, Morgan R A. Hum Gene Ther. 1995;6:1363–1377. doi: 10.1089/hum.1995.6.11-1363. [DOI] [PubMed] [Google Scholar]
- 27.Markowitz D, Hesdorffer C, Ward M, Goff S, Bank A. Ann NY Acad Sci. 1990;612:407–414. doi: 10.1111/j.1749-6632.1990.tb24328.x. [DOI] [PubMed] [Google Scholar]
- 28.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bodine D M, McDonagh K T, Seidel N E, Nienhuis A W. Exp Hematol. 1991;19:206–212. [PubMed] [Google Scholar]
- 30.Thorpe S J, Thein S L, Sampietro M, Craig J E, Mahon B, Huehns E R. Br J Haematol. 1994;87:125–132. doi: 10.1111/j.1365-2141.1994.tb04881.x. [DOI] [PubMed] [Google Scholar]
- 31.Sabatino D E, Cline A P, Gallagher P G, Garrett L J, Stamatoyannopoulos G, Forget B G, Bodine D M. Mol Cell Biol. 1998;18:6634–6640. doi: 10.1128/mcb.18.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins P B, Skelton D C, Yu X J, Halene S, Leonard E H, Kohn D B. Proc Natl Acad Sci USA. 1998;95:10182–10187. doi: 10.1073/pnas.95.17.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher P G, Romana M, Tse W T, Lux S E, Forget B G. Blood. 2000;96:1136–1143. [PubMed] [Google Scholar]
- 34.Emery D W, Yannaki E, Tubb J, Stamatoyannopoulos G. Proc Natl Acad Sci USA. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zufferey R, Donello J, Trono D, Hope T. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zufferey R, Dull T, Mandel R J, Bukovsky A, Quiroz D, Naldini L, Trono D. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beutler E. In: The Molecular Basis of Blood Diseases. Stamatoyannopoulos G, Nienhuis A W, Majerus P W, Varmus H, editors. Philadelphia: Saunders; 1994. pp. 331–349. [Google Scholar]
- 38.Dooner G, Barker J, Gallagher P, Debatis M, Brown A, Forget B, Becker P. Exp Hematol. 2000;28:765–774. doi: 10.1016/s0301-472x(00)00185-5. [DOI] [PubMed] [Google Scholar]