Abstract
Somatic cell nuclear transfer (SCNT) offers great potential for developing better animal models of human disease. The domestic ferret (Mustela putorius furo) is an ideal animal model for influenza infections and potentially other human respiratory diseases such as cystic fibrosis, where mouse models have failed to reproduce the human disease phenotype. Here, we report the successful production of live cloned, reproductively competent, ferrets using species-specific SCNT methodologies. Critical to developing a successful SCNT protocol for the ferret was the finding that hormonal treatment, normally used for superovulation, adversely affected the developmental potential of recipient oocytes. The onset of Oct4 expression was delayed and incomplete in parthenogenetically activated oocytes collected from hormone-treated females relative to oocytes collected from females naturally mated with vasectomized males. Stimulation induced by mating and in vitro oocyte maturation produced the optimal oocyte recipient for SCNT. Although nuclear injection and cell fusion produced mid-term fetuses at equivalent rates (~3–4%), only cell fusion gave rise to healthy surviving clones. Single cell fusion rates and the efficiency of SCNT were also enhanced by placing two somatic cells into the perivitelline space. These species-specific modifications facilitated the birth of live, healthy, and fertile cloned ferrets. The development of microsatellite genotyping for domestic ferrets confirmed that ferret clones were genetically derived from their respective somatic cells and unrelated to their surrogate mother. With this technology, it is now feasible to begin generating genetically defined ferrets for studying transmissible and inherited human lung diseases. Cloning of the domestic ferret may also aid in recovery and conservation of the endangered black-footed ferret and European mink.
Keywords: Nuclear transfer, Somatic cell, Ferret
Introduction
Evolutionary conservation in organ physiology and cell biology between humans and various animal species has influenced the choice of alternative non-rodent models of human disease pathogenesis. Recent successes in creating cloned animals by SCNT (Baguisi et al., 1999; Chesne et al., 2002; Galli et al., 2003; Kato et al., 1998; Lee et al., 2005; Polejaeva et al., 2000; Shin et al., 2002; Wakayama et al., 1998; Wilmut et al., 1997; Woods et al., 2003; Zhou et al., 2003) have made animal modeling more feasible in alternative non-mouse species. The domestic ferret has emerged as an excellent model system for studies involving reproductive physiology, endocrinology, and virology (Marini et al., 2002). Indeed, the ferret is one of the most widely studied animal models of human influenza infection (Pearson and Gorham, 1998) and is also an ideal choice for modeling genetic lung diseases such as cystic fibrosis (CF) (Li and Engelhardt, 2003). In part, this is due to the remarkable similarity between ferret and human lung cell biology (Choi et al., 2000; Mercer et al., 1994; Wang et al., 2001). Moreover, the ferret has a 42-day gestation period and reaches sexual maturity in 4 to 5 months (Fox and Bell, 1998), making it one of the more rapidly reproducing species for animal modeling by SCNT.
We have previously developed technologies for superovulation in the ferret and nuclear transfer (NT) of G0/G1-phase ferret fetal fibroblasts into enucleated oocytes by direct cytoplasm injection (Li et al., 2003). More recently, these techniques were applied using electrofusion of somatic cells as the method of NT (Li et al., 2005b). However, maturation of late-stage NT ferret embryos using both these techniques remained an inefficient process and further optimization of SCNT procedures was needed to produce viable clones. In the present study, we compared the efficiency of SCNT using fetal fibroblasts and cumulus cell nuclear donors and two methods of NT (nuclear injection and cell fusion). By altering the oocyte harvesting procedures and improving the efficiency of cell fusion, we successfully developed methods of producing viable, reproductively competent, ferret clones. This success represents the first reported cloning in this species and has laid the foundation for genetic modeling with this species.
Materials and methods
Reagents and animal housing
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and Invitrogen Co. (Grand Island, NY, USA) unless otherwise noted. Ferrets were purchased from Marshall Farms (North Rose, NY, USA). Female sable coat-color ferrets (virgin, 6–7 months of age) and albino coat-color ferrets (primipara, 9–12 months of age) were in estrus when delivered. The vasectomized male ferrets (albino, 12 months of age) were used for mating to induce follicular oocyte maturation in oocyte donor ferrets and to induce pseudopregnancy in nuclear transfer (NT) embryo surrogate ferrets. All ferrets were housed in separate cages under controlled temperature (20–22°C) and long day light cycle (16 h light/8 h dark). Ferret chow was obtained from Marshall Farms, North Rose, NY, USA.
Oocyte collection and in vitro maturation (IVM)
Ferret immature oocytes were obtained from sable coat-color ferrets mated with vasectomized male ferrets 24 h prior to collection. To retrieve the oocytes, ferrets were euthanized by administration of pentobarbital sodium injection (50–100 mg/kg, i.p., Ovation Pharmaceuticals Inc., Deerfield, IL, USA). Ovaries were excised, and small vesicular follicles on the ovary surface were incised in mPBS [Dulbecco PBS supplemented with 0.1% (w/v) D-glucose, 36 mg/L of pyruvate, and 0.4% (w/v) BSA] to release the cumulus–oocyte complexes (COCs). COCs with uniform cytoplasm and several layers of cumulus cells were washed with mPBS and cultured in TCM-199 + 10% FBS + 10 IU/ml eCG (equine chorionic gonadotrophin; Sigma G-4527) + 5 IU/ml of hCG (human chorionic gonadotrophin; Sigma C-8554) at 38.5°C in 5% CO2, 95% air for 18, 20, and 22 h. Upon completion of in vitro culture, cumulus cells were removed by pipetting in mPBS containing 0.2% (w/v) hyaluronidase and oocytes were stained with 1% (w/v) orcein or 10 μg/ml of Hoechst 33342 (Sigma B-2261) as previously described (Li et al., 2002). Oocytes displaying a disassembled nuclear membrane, chromatin condensation, and a first polar body were considered to be mature. The number of COCs collected from each ferret ranged from 14 to 28 (19.5 ± 4.2, mean ± SE, n = 25).
Establishment of fetal fibroblast and cumulus cell lines
Ferret fetal fibroblasts were obtained from 28 dpc (day post copulation) fetuses derived from a Sable (female) × Cinnamon (male) mating (Marshall Farms, North Rose, NY, USA), and cell lines were established as previously reported (Li et al., 2003). Each fetus was treated individually. After karyotype analysis (Li et al., 2005a) on individual cell lines, fibroblasts from 3 male fetuses were used for NT (within 3 passages). Cumulus cells were collected from the COCs of sable coat-color ferrets, treated with 0.2% hyaluronidase/mPBS, and cultured in 10% FBS/DMEM medium for 3–7 days. The somatic cells were serum-starved for 24 h with DMEM containing 0.5% FBS before NT.
Nuclear transfer and activation
In vitro matured oocytes were transferred to mPBS medium containing 7.5 μg/ml of cytochalasin B (CB, Sigma C-6762) in the micromanipulation chamber. Using Nomarski optics, the first polar body and chromosome spindle were aspirated into the pipette with a minimal volume of oocyte cytoplasm with a 15 μm (inside diameter, ID) PeizoDrill glass pipette (Humagen™, Charlottesville, VA, USA). Nuclear transfers (NTs) were performed by two methods including microinjection of nuclei and electrofusion of intact cells as described below. In select experiments, premature chromosome condensation (PCC) and pronuclear (PN) formation was monitored at 2, 4, and 6 h post-NT or post-parthenogenetic activation by staining embryos with 10 μg/ml of Hoechst 33342 for 2–5 min. Fluorescent microscopy was then used to monitor the timing of PCC and PN formation.
Microinjection and activation
Serum-starved fetal fibroblasts or cumulus cells were transferred to a micromanipulation chamber in mPBS medium containing 7.5 μg/ml of CB. One fibroblast or cumulus cell (diameter ≥15 μm) was aspirated in and out of a 10 μm (ID) PeizoDrill glass pipette to break the cell membrane. One somatic cell nucleus was then microinjected into the oocyte cytoplasm. The NT embryos reconstructed by microinjection were transferred into electrical activation medium [0.3 M Mannitol, 0.1 mM MgCl2, 0.1 mM CaCl2, 0.5 mM HEPES, 0.01% (w/v) BSA], placed between two parallel electrodes, and subjected to an electrical pulse of 1 DC of 180 V/mm for 30 μs from an ECM 2001 (BTX, San Diego, CA). The NT embryos subjected to electrical pulse were kept in TCM-199 + 10% FBS medium for 1 h, and then incubated in TCM-199 medium containing 5 μg/ml of cycloheximide (Sigma C-7698) and 2 mM 6-dimethylaminopurine (6-DMAP, Sigma D-2629) for 1 h to facilitate chemical activation.
Electrofusion and activation
Serum-starved fetal fibroblasts or cumulus cells were transferred to a micromanipulation chamber in mPBS medium containing 7.5 μg/ml of CB. One or two fibroblasts or cumulus cells were aspirated and inserted into the perivitelline space (PVS) of enucleated oocytes using the same enucleation pipette (15 μm ID). Those cell–oocyte couplets were then transferred into electrical fusion medium (the same as the above activation medium), placed between two parallel electrodes, and manually aligned with a fine pipette so that the contact surface between the oocyte and the donor cell was parallel to the electrodes. Cell fusion was induced with an electrical pulse (as above). The electrofusion efficiency of reconstructed embryos was examined within 20–30 min after electrical pulse. The fused NT embryos were kept in TCM-199 + 10% FBS medium for 30 min (1 h from electrical pulse) then subjected 1 h of chemical activation, as above. Unfused cell–oocyte couplets were retreated with an electrical pulse, and fused NT embryos were then activated and cultured as above (Li et al., 2005b).
Immunocytochemical analysis of Oct4 protein expression in parthenogenetically activated oocytes
In vitro matured oocytes were subjected to a combination of electrical and chemical stimuli (as described for NT embryo activation) to induce parthenogenetic embryogenesis. The parthenogenetic embryos at <8-cell, 9- to 16-cell, morula, and blastocyst stages were washed twice in PBS containing 0.1% polyvinyl pyrrolidone (Sigma P-0930) (PBS–PVP) then fixed in 3.7% paraformaldehyde (Electron Microscopy Sciences, Front Washington, PA, USA) in PBS–PVP (pH 7.4) for 15 min at 4°C. After permeabilization with 0.1% Triton X-100 (Sigma T-9284) in blocking solution (3% goat serum in PBS–PVP) for 10 min at room temperature (RT), the specimens were washed three times and incubated for 1 h at RT in 3% goat serum PBS–PVP. Embryos were then incubated in 3% goat serum PBS–PVP containing anti-Oct4 (Santa Cruz Biotechnology, Santa Cruz, CA) rabbit polyclonal antibody (1:200 dilution) at 4°C for overnight. After extensive washing, the embryos were exposed to affinity-purified goat anti-rabbit secondary antibody conjugated with fluorescein isothiocyanate (FITC, 1:100 dilution, Santa Cruz Biotechnology, Santa Cruz, CA). The embryos were then co-stained with 40,60-diamidino-2-phenylindole (DAPI) in UltraCruz™ mounting medium (Santa Cruz Biotech-nology, Santa Cruz, CA), mounted onto slides, and examined with fluorescence microscopy. Controls were performed by omitting the primary antibody, and no staining was consistently observed.
Embryo transfer (ET)
After NT, electrofusion, and/or activation, the embryos were rinsed in mPBS and transferred into pseudopregnant ferrets immediately. A pseudopregnant state was achieved in surrogate albino primipara ferrets through mating with a vasectomized albino male 24 h prior to embryo transfer (ET). A single stock solution of saline containing 10 mg/ml each of ketamine HCl (Abbott Laboratories, N. Chicago, IL, USA) and xylazine (Phoenix Pharmaceutical INC., St. Joseph, MO, USA) was prepared. The surrogate ferrets were routinely anaesthetized by i.p. injection of this solution to a final concentration of 20 mg/kg of ketamine and 20 mg/kg xylazine. If the depth of anesthesia was insufficient, an additional dose of the stock solution was administered up to a total dose of 30 mg/kg. The abdomen was shaved and disinfected with 10% povidone-iodine and 70% ethanol. A 3–4 cm incision was made along the midline of the abdomen to expose the ovaries and oviducts. The 23-gauge needle was used to generate a hole on the surface of the left oviduct. Between 25 and 55 NT embryos were delivered into the left oviduct through the hole using a fine glass pipette with an inner diameter slightly larger than that of the embryo. After the surgical incision was sutured, the ferrets were returned to their cages and closely monitored until they were awake. Mid-term and full-term development of NT embryos was assessed at 3 weeks and 6 weeks post-ET, respectively.
Microsatellite genotyping of DNA
The microsatellite analysis was essentially performed as previously described (Wisely et al., 2002). Eleven primer sets were used based on microsatellite analysis in mink, black-footed ferret, and Canadian polar bear including: Mer022, Mvis072, Mer095, Mvis075 (Fleming et al., 1999); Mvi87, Mvi232, Mvis022, Mvi57 (O’Connell et al., 1996); G1A (Paetkau et al., 1995); Gg4 (Davis and Strobeck, 1998); Mer049 (Wisely et al., 2002). Briefly, the PCR products were amplified using ABI dye labeled primers (only the forward dye was labeled) and run in a thermocycler. The PCR products were run on an automated sequencer and analyzed by ABI GeneMapper version III software. The ABI dyes used were TAM, HEX, and FAM. ROX was used as the standard.
Statistical analysis
Statistical analysis was performed using SAS 8.0 statistical software (SAS Institute Inc., Cary, NC, USA). One-way ANOVA was used for statistical analysis. When ANOVA demonstrated a significant difference, the follow-up Tukey multiple comparison test was performed to determine P values for all possible two-group comparisons within the data set. For all statistical analyses, a difference was considered to be significant when the P value was < 0.05.
Results
In vitro maturation (IVM) of ferret oocytes
Immature ferret oocytes were obtained from Jills mated with vasectomized male ferrets 24 h prior to collection. This was a change from our previous strategy for SCNT in which we used oocytes collected following superovulation. As shown in Table 1, oocytes cultured for 20 and 22 h achieved in vitro maturation rates of 93%, significantly greater (P < 0.05) than that of oocytes cultured in vitro for 18 h (85%). This rate of in vitro oocyte maturation was significantly greater than that previously obtained using oocytes collected following hormonal superovulation (70%) (Li et al., 2002). Since no statistical differences were observed for IVM rates at 20 and 22 h of culture, we chose 20 h IVM oocytes with normal morphology, uniform cytoplasm, and containing a first polar body for enucleation and NT.
Table 1.
In vitro maturation of ferret oocytes
| Hours of IVM | No. of replicates | No. of oocytes examined | No. of matured oocytes | % of matured oocytes(mean ± SEM)* |
|---|---|---|---|---|
| 18 | 5 | 95 | 81 | 85.1 ± 1.9a |
| 20 | 5 | 96 | 89 | 93.0 ± 2.0b |
| 22 | 5 | 102 | 94 | 92.9 ± 2.6b |
Differences among percentages containing the different superscripted letters (a,b) are significant (P < 0.05).
Nuclear transfer by microinjection and in vivo development of cloned embryos
Using the direct injection protocol, nuclei from cumulus cells or fibroblasts were microinjected into enucleated oocytes using a Piezo drill pipette. Following electrical and chemical activation, NT embryos were transferred into pseudopregnant albino primipara ferrets. We carried out 30 such nuclear transfer procedures, which resulted in 851 cloned embryos (460 embryos from fibroblast cell nuclei and 391 embryos from cumulus cell nuclei) (Table 2). These cloned embryos were transplanted into 30 recipient Jills (15 with each cell type), and mid-term development (21 day) of cloned embryos was assessed in 26 surrogate females while the remaining 4 Jills were allowed to go to term. As shown in Table 2, embryos from fibroblast nuclei implanted into the uterus at a rate of 7.2% (25/349) and 3.4% (12/349) formed visible fetuses at 21 days (Fig. 1). Cloned embryos derived from injection of cumulus cell nuclei more efficiently implanted into the uterus (14.2%, 44/310), however, fetal formation rates were similar to that observed with fibroblasts (4.2%, 13/310) (Table 2). Of the remaining 4 surrogate Jills allowed to go to term, only one female pup was born from cumulus-cell-generated embryos (1.2%, 1/81) and this pup died shortly after birth. No live births occurred with the 111 fibroblast NT embryos transplanted into two Jills (Table 2).
Table 2.
Ferret cloning using different methods for nuclear transfer and somatic donor cell types
| Method of nuclear transfer | Donor cell (type) | Nuclear transfers (no. embryos transferred) | Embryo transfers (no. recipients) | 3-week implantation (%) | 3-week fetal development (%) | Birth rate (%) |
|---|---|---|---|---|---|---|
| Microinjection | Fibroblast | 460 | 15 | 7.2 (25/349) | 3.4 (12/349) | 0 (0/111) |
| Cumulus cell | 391 | 15 | 14.2 (44/310) | 4.2 (13/310) | 1.2 (1/81) a | |
| Cell Fusion | Fibroblast | 430 | 14 | 5.5 (18/326) | 3.1 (10/326) | 0.96 (1/104) b |
| Cumulus cell | 487 | 14 | 4.3 (16/375) | 3.2 (12/375) | 1.8 (2/112) c |
Female clone died hours after birth.
Male clone died 3 days after birth.
Healthy reproductively competent female clones.
Fig. 1.
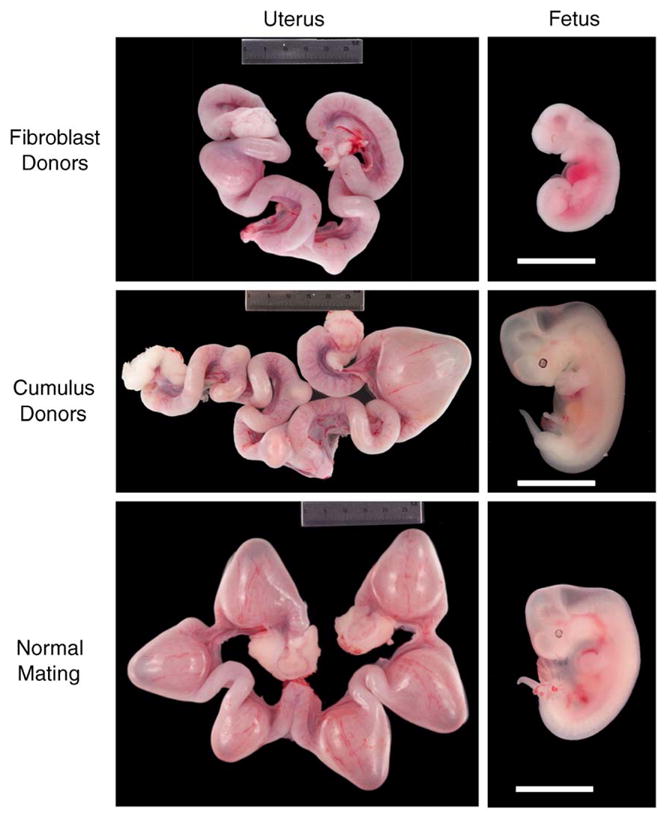
In vivo development of 21-day fetuses following SCNT. Gross anatomical examples of implantations and dissected fetuses at 21 days for fibroblast and cumulous clones as compared to natural mating with fertile males.
Nuclear transfer by cell fusion and in vivo development of cloned embryos
Utilizing the cell fusion technique, 1–2 intact fibroblasts, or cumulus cells was inserted into the perivitelline space (PVS) of enucleated oocytes and fused via electrical pulse. Following chemical activation, NT embryos were transferred into 28 surrogate females, 14 with each cell type. In total, 917 cloned embryos were produced, 430 embryos from fibroblast cells, and 487 embryos from cumulus cells (Table 2). The mid-term development of cloned embryos in 12 surrogate Jills for each donor cell type was evaluated at 3 weeks following ET. As shown in Table 2, 5.5% (18/326) of fibroblast NT embryos underwent implantation with 3.1% (10/326) showing visible fetal development. Similar results were observed with cumulus cell NT embryos with 4.3% (16/375) undergoing implantation and 3.2% (12/375) giving rise to a 21-day fetal formation (Table 2, Fig. 1). The remaining NT embryos in the four surrogate females were allowed to develop to term. One male pup was born that was derived from a fibroblast NT embryo (0.96%, 1/104) and died 3 days after birth. Two live-born female pups (1.8%, 2/112) were produced from cumulus cell NTs (Table 2) and named Libby and Lilly (Fig. 2A).
Table 5.
Microsatellite genotyping of ferret DNA
| Ferrets and cells | Microsatellite DNA markers
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mer022 | Mvi87 | G1A | Mvis072 | Mvi232 | Gg4 | Mvis022 | Mer095 | Mvi57 | Mvis075 | Mer049 | |
| Donor cumulus cells (Sable) | 245/245 | 78/78 | 160/162 | 269/269 | 155/155 | 90/90 | 282/284 | 148/150 b | 106/106 | 139/139 | 183/185 |
| Libby a | 245/245 | 78/78 | 160/162 | 269/269 | 155/155 | 90/90 | 282/284 | 148/150 b | 106/106 | 139/139 | 183/185 |
| Lillya | 245/245 | 78/78 | 160/162 | 269/269 | 155/155 | 90/90 | 282/284 | 148/150 b | 106/106 | 139/139 | 183/185 |
| Surrogate mother (albino) | 245/245 | 76/78 | 162/162 | 269/269 | 153/155 | 90/90 | 284/284 | 152/152 b | 106/106 | 139/139 | 185/185 |
| Donor fibroblast cells | 245/245 | 76/78 | 160/162 | 269/271 | 155/155 b | 90/90 | 284/284 | 150/152 | 106/108 | 139/139 | 183/183b |
| Clone c | 245/245 | 76/78 | 160/162 | 269/271 | 155/155 b | 90/90 | 284/284 | 150/152 | 106/108 | 139/139 | 183/183b |
| Surrogate mother | 245/245 | 76/78 | 160/162 | 269/271 | 153/159 b | 90/90 | 284/284 | 152/152 | 104/106 | 139/139 | 185/185b |
AL01–AL02: unrelated albino fibroblasts; SA01–SA10: unrelated sable fibroblasts; ND: not determined; FCC1: ferret cumulus cells unrelated to cloned animals.
Lilly and Libby–Sable coat-color ferrets cloned from cumulus cells (Fig. 1).
Informative markers distinguishing somatic-cell-derived clones from their surrogate mother. Analysis of 13 unrelated DNAs from Albino and Sable coat-color ferrets is shown in Table S1.
Clone–Live-born male ferret cloned from fibroblast cells and died 3 days after birth.
Fig. 2.

(A) Two cloned sable coat-color female ferret pups, Libby and Lilly (at 7 weeks of age), produced by SCNT and ET into an albino surrogate Jill. (B) Lilly with her 6 newborn pups. (C) Libby with her 10 newborn pups.
Since no viable offspring were obtained following SCNT using fibroblast cell fusion, we attempted to assess whether developmental abnormalities existed in fibroblast NT clones. To this end, we compared the histology of 21-day fetuses derived from in vivo fertilization with two 21-day fibroblast NT clones. These data are presented in Fig. S1. No obvious differences in the development of primordia/organs were observed between 21-day in vivo fertilized embryos and NT embryos including the mandibular component of the branchial arch, heart, lung bud, hepatic primordium, lumen of duodenum, centrum of axis, dorsal root ganglion, and third ventricle. However, as shown in whole mount embryo panels of Fig. 1 and Fig. S1 (NT-2), mid-gestation NT fibroblast clones were sometimes smaller, suggesting retarded growth. We could not easily obtain histologic data from full-term embryos that died after birth due to cannibalism of the newborn carcass by the Jill.
Nuclear reprogramming in vitro following SCNT and parthenogenetic activation
Since cell fusion was the most effective approach in developing live clones, we sought to determine whether in vitro development of cloned embryos was improved over previous attempted approaches. To this end, we evaluated the progression of fibroblast-cloned embryos to premature chromosome condensation (PCC) and pronuclei (PN) formation following fibroblast cell fusion and activation. As shown in Table 3 and Fig. 3, the majority of reconstructed embryos progressed to PCC by 2 h post-NT, while pronuclei formation was maximal by 6 h. Under these conditions, 80% of reconstructed embryos cleaved while 35% and 16% developed to morula and blastocyst stages, respectively (Table 3). These rates of morula and blastocyst development achieved by cell fusion were similar to that previously achieved following nuclear injection of fibroblast nuclei (morula 26.0%; blastocyst 17.6%), a method that failed to give rise to full-term embryo development (Li et al., 2003).
Table 3.
Timing of nuclear reprogramming events following SCNT
| Hours after activation | Interphase | PCC a | PN b |
|---|---|---|---|
| 2 | 23.8% (5/21) | 71.4% (15/21) | 4.8% (1/21) |
| 4 | 5.9% (2/34) | 41.2% (14/34) | 52.9% (18/34) |
| 6 | 3.7% (1/27) | 11.1% (3/27) | 85.2% (23/27) |
In these studies, we evaluated reconstruction of 174 embryos of which 82 were stained to evaluate PCC formation and 92 were cultured in vitro to evaluate embryogenesis. In these studies, 80% progressed to cleavage, 35% progressed to morula, and 16% progressed to blastocysts. The percentage in each category found at the given time points is shown with the fraction of reconstructed embryos analyzed in parentheses. A total of 16 Jills were used for these experiments, and oocytes were collected in the absence of hormonal stimulation by mating with vasectomized males.
PCC: premature chromosome condensation.
PN: pronuclei. Results are shown following fibroblast cell fusion and activation.
Fig. 3.
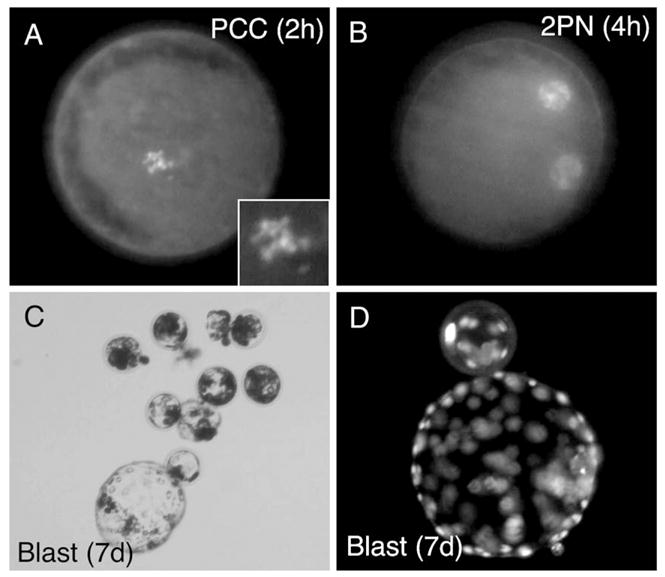
In vitro development of fibroblast NT embryos following cell fusion and activation. (A) DAPI staining of PCC formation at 2 h post-SCNT. (B) DAPI staining of pronuclei formation at 4 h post-SCNT. (C) Bright field photomicrograph of 7-day blastocysts formed following SCNT. (D) DAPI stained 7-day blastocysts formed following SCNT.
The cytoplasmic environment in mature oocytes promotes nuclear envelope breakdown (NEBD) and PCC following SCNT. The exposure of somatic chromatin to the oocyte cytoplasm is thought to promote more efficient nuclear reprogramming and in vitro development of the bovine NT embryo (Tani et al., 2001). Moreover, the time interval between SCNT and embryo activation appears to be an important determinant for successful in vitro development in these NT embryos (Choi et al., 2004). Therefore, we sought to confirm NEBD and PCC in the ferret oocyte following cell fusion as the basis for determining the optimal time for activation following SCNT. As shown in Table 4, approximately 19% of NT embryos progressed to PCC by 4 h following cell fusion. This percentage was strikingly similar to the extent of blastocyst formation (16%) following NT and activation (Table 3).
Table 4.
Progression of PCC following cell fusion
| Hours after cell fusion | Interphase | PCC a | PN % b |
|---|---|---|---|
| 2 | 100% (16/16) | 0% | 0 |
| 4 | 81.2% (13/16) | 18.8% (3/16) | 0 |
| 6 | 88.9% (8/9) | 11.1% (1/9) | 0 |
Progression to PCC was evaluated following fibroblast somatic cell fusion in the absence of activation. In total, 41 oocytes collected from four Jills in the absence of hormonal stimulation by mating with vasectomized males were analyzed. The percentage in each category found at the given time points is shown with the fraction of oocytes analyzed in parentheses.
PCC: premature chromosome condensation.
PN: pronuclei.
Oct4 expression patterns in parthenogenetically activated ferret oocytes derived from hormonally treated and naturally mated donors
To begin to determine why oocytes derived from non-hormonally stimulated donors appeared to have a greater in vivo developmental capacity following SCNT, we assessed the relative activation of the Oct4 embryonic gene product. Incomplete reactivation of Oct4 and Oct4-related genes has been suggested to hinder in vivo developmental capacity following SCNT leading to embryonic lethality of somatic cell clones in mice (Bortvin et al., 2003). To this end, we compared the protein expression of Oct4 in early and late stage parthenogenetically activated oocytes harvested from hormonally treated Jills or Jills mated with vasectomized males. Interestingly, parthenogenetically activated oocytes harvested from hormonally treated Jills expressed Oct4 in 47 ± 14% of nuclei in early stage embryos of ≤21 cells (Fig. 4). This level of Oct4 expression was significantly lower (P < 0.028) than that seen following parthenogenetic activation of oocytes harvested from Jills mated with vasectomized males in side-by-side experiments (83 ± 5%) (Fig. 4). However, no differences in the nuclear frequency of Oct4 staining were seen at morula and blastocyst stages in either of the two groups. Hence, hormonal stimulation of oocytes during the collection period appears to predispose ferret embryos to altered activation of Oct4.
Fig. 4.
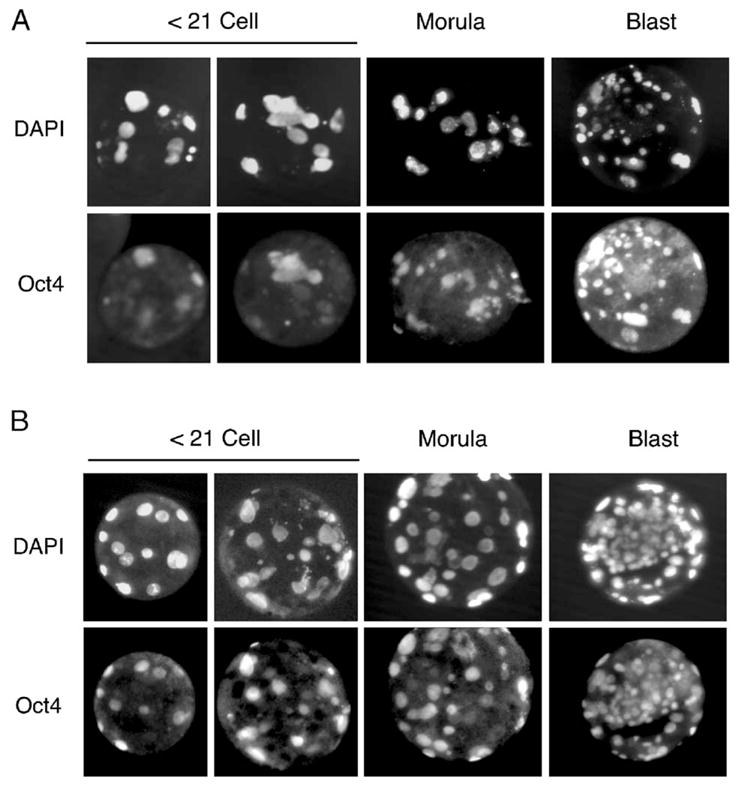
Oct4 staining of parthenogenetically activated oocytes. Oocytes were collected (A) following eCG treatment of donor Jills or (B) following natural mating with vasectomized males. Oocytes were activated as described in Materials and methods and stained with anti-Oct4 antibodies and DAPI at various stages as indicated. A total of four Jills were used to generate at least 6 embryos in each developmental group. Oct4 expression was seen in 47 ± 14% of nuclei from ≤21 cells embryos of the hormonally treated group and 83 ± 5% of nuclei from ≤21 cells embryos in the untreated group.
Genetic analysis of clones
Microsatellite marker analysis of live-born ferret clones was developed based on previous microsatellite primers used for related species (Davis and Strobeck, 1998; Fleming et al., 1999; O’Connell et al., 1996; Paetkau et al., 1995; Wisely et al., 2002). Approximately 90% of the primers tested had sufficient conservation in domestic ferrets to amplify the correct size microsatellite fragments. This microsatellite marker analysis confirmed that all three live-born clones were genetically indistinguishable from the respective somatic cells from which they were derived (Table 5). In contrast, analysis of DNA from 13 unrelated ferrets demonstrated multiple differences in the 11 microsatellite sequences analyzed, with none giving rise to the identical genotypes (Table S1). The values shown in Table 5 and Table S1 indicate the fragment size of both alleles in base pairs. Libby and Lilly are heterozygous at locus Mer095, identical to the genotype of the donor cells at that locus, but different from that of their surrogate mother. Similarly, the cloned male ferret has multi-locus genotypes identical to the donor cells (e.g., Mvi232 and Mer049), but different from his surrogate mother (e.g., Mvi232 and Mer049).
Fertility of cloned ferrets
Progression to estrus in ferrets is regulated by the duration of light to which the animals are exposed (Donovan, 1967; Fox and Bell, 1998). Ferrets normally reach sexual maturity within 4–5 months of age (Fox et al., 2002), and, typically, sexually mature female ferrets transferred to a long-light cycle (16 h light/8 h dark) enter estrus within 21 days. Libby and Lilly were reared in a continuous long-light cycle but failed to enter estrus after 6 months. As such, these clones were subsequently exposed to a short light cycle (10 h light/14 h dark) for approximately 100–120 days to reset their estrus “clock”. Upon reintroduction to a long-light cycle (16 h light/8 h dark), the two clones successfully entered estrus within 3–4 weeks. Each was then mated naturally with a fertile male. Lilly and Libby gave birth to 6 and 10 healthy pups (Figs. 2B, C), respectively. Lilly and Libby were subsequently bred a second time and gave rise to litters of 6 and 7 healthy pups, respectively. At 7 weeks postpartum, Lilly developed mastitis and died from sepsis within 3 days of diagnosis, despite antibiotic treatment. Escherichia coli was detected from nodules in the lung, liver, and mammary glands. Her pups were successfully nursed by hand and remain healthy.
Discussion
The successful production of viable ferret progeny following somatic cell nuclear transfer provides exciting new opportunities for basic research, investigating early embryogenesis and the propagation of endangered black-footed ferrets and European minks. To our knowledge, this study represents the first successful cloning of the domestic ferret by SCNT using in vitro matured oocytes as cytoplasm donors and in vitro cultured fetal fibroblast cells and cumulus cells as nuclear donors. Given the relatively short total reproductive cycle of the ferret, with a gestation period of 42 days and 4–5 months to sexual maturity (Fox et al., 2002), the ferret represents an attractive animal model for genetic manipulation in conjunction with SCNT.
Successful SCNT is highly dependent upon oocyte quality. To prepare donor oocytes for nuclear transfer (NT), we initially attempted to obtain in vivo matured oocytes by superovulating ferrets with hormone treatment (Li et al., 2001). This is a standard approach used in rabbit NT (Chesne et al., 2002). Although superovulated ferret oocytes are efficiently fertilized in vivo and developed to term (Li et al., 2001), oocytes harvested following superovulation were fragile and rapidly fragmented during NT procedures. To our knowledge, such rapid deterioration of superovulated oocytes has not been reported for other cloned species. To circumvent this limitation, we developed methods for in vitro maturation (IVM) of oocytes collected from ovaries of hormone-treated ferrets (Li et al., 2002). Although matured oocytes derived in this manner were stable throughout the NT process, they failed to give rise to cloned ferrets due to a significant embryogenic block during the last half term of gestation (Li et al., 2003).
Recognizing that natural ovulation in ferrets is induced by copulation (Chang and Yanagimachi, 1963), we modified the procedure of IVM by harvesting immature oocytes from ferrets mated with vasectomized male ferrets 24 h prior to collection in the absence of hormone treatment. IVM rates of oocytes collected in this manner (93%) were significantly higher than that previously reported for oocytes collected following hormonal superovulation (70%) (Li et al., 2002). This difference in the quality of oocytes for IVM may be one reason full-term development was achieved in the current studies as compared to previous methods (Li et al., 2003). Two studies demonstrating that eCG treatment protocols used for superovulation reduce developmental competence of in vivo fertilized embryos cultured in vitro and then transplanted into recipient mice and hamster support the notion that hormonal treatment may also affect in vivo development of cloned embryos in ferret (Ertzeid and Storeng, 2001; McKiernan and Bavister, 1998). Furthermore, our studies demonstrating that oocytes harvested from hormonally treated Jills have delayed onset of Oct4 protein expression during early stages of parthenogenetic embryogenesis, as compared to those harvested following mating with vasectomized males, support the hypothesis that oocyte exposure to eCG/hCG during superovulation adversely affects developmental reprogramming.
We observed no correlation between the rate of implantation of NT embryos and full-term birth of clones. For example, the highest rates of implantation were achieved following nuclear transfer of cumulus nuclei (14.2%), but only one live birth was obtained and the clone died within hours. Despite the lower implantation rate of NT embryos produced by cell fusion of cumulus cells (4.3%), two viable clones were produced. The increased success of cell fusion, using two or more somatic cells, may have also contributed to this approach producing the most viable cloned offspring.
Many somatic cell types, including mammary epithelial cells, ovarian cumulus cells, fibroblasts, sertoli cells, macrophages, and blood leukocytes, have been successfully utilized for nuclear transfer (Kato et al., 2000; Shi et al., 2003; Tian et al., 2003; Yanagimachi, 2002). In accordance with previous work with mice (Rideout et al., 2001; Wakayama et al., 1998), cattle (Kato et al., 1998, 2000; Tian et al., 2003), rabbits (Chesne et al., 2002), and cat (Shin et al., 2002), our results also suggest that donor cell type can significantly affect NT embryo development in the ferret, with cumulus cells proving to be more effective than fibroblasts for SCNT. It is reported that abnormal phenotypes have been seen in animals derived from NT. These include respiratory distress (Carter et al., 2002; Garry et al., 1996; Walker et al., 1996), contracted tendons and cardiovascular problems (Carter et al., 2002; Hill et al., 1999; Lai et al., 2002; Schnieke et al., 1997), and large birth weights (Boquest et al., 2002; Walker et al., 1996). It has been reported that approximately 60–70% of the cloned calves born survive normally to the adult stage and present an apparently normal physiology (Heyman, 2005). In ferret clones that died shortly after birth, no obvious gross developmental abnormalities were seen in the remainder of the carcass that was not cannibalized by the Jill. However, detailed histopathology was not possible due to the post-mortem state in which the pups were found. Hence, we cannot rule out that post-natally failed clones had developmental abnormalities. Although 21-day NT clones derived from fibroblasts often appeared to have retarded growth compared to those derived from NT cumulus cells or natural mating (Fig. 1 and Fig. S1), no obvious histologic differences in primordia/organ development were observed in 21-day NT fibroblast clones (Fig. S1).
Our reproductive data on Libby and Lilly are consistent with several studies demonstrating normal reproductive behavior and fertility in numerous cloned species including mice (Tamashiro et al., 2002; Wakayama and Yanagimachi, 1999), cattle (Tian et al., 2003; Wells et al., 2004), and pigs (Boquest et al., 2002; Mir et al., 2005). It is currently unclear if the death of Lilly, due to mastitis-induced sepsis, was an event caused by her cloning. Mastitis caused by Hemolytic E. coli is frequently seen in ferrets and, if untreated, leads to sepsis and death (Liberson et al., 1983).
Taken together, two procedural modifications of current cloning protocols in the ferret led to the production of these live healthy clones. These included optimizing recipient oocyte quality by omitting hormonal treatments typically used to increase oocyte yield and the implementation of cell fusion as the means of nuclear transfer with two or more somatic cells inserted into the perivitelline space. With further improvements, these studies will likely enhance the utility of the ferret as a new species to study developmental biology and model human diseases.
Supplementary Material
Acknowledgments
The authors want to thank Dr. R. Scipioni Ball and her staff at Marshall Farms for their kind assistance with ferret care and reproduction. The authors also thank Dr. M. Parker and his staff in the University of Iowa Animal Facility for their efforts in maintenance of the ferret colony. The Cystic Fibrosis Foundation, HL61234 (M.J.W) and DK47967 (J.F.E.), funded this research. J.F.E. holds the Roy J. Carver Chair in Molecular Medicine.
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ydbio.2006.02.016.
References
- Baguisi A, Behboodi E, Melican DT, Pollock JS, Destrempes MM, Cammuso C, Williams JL, Nims SD, Porter CA, Midura P, Palacios MJ, Ayres SL, Denniston RS, Hayes ML, Ziomek CA, Meade HM, Godke RA, Gavin WG, Overstrom EW, Echelard Y. Production of goats by somatic cell nuclear transfer. Nat Biotechnol. 1999;17:456–461. doi: 10.1038/8632. [DOI] [PubMed] [Google Scholar]
- Boquest AC, Grupen CG, Harrison SJ, McIlfatrick SM, Ashman RJ, d’Apice AJ, Nottle MB. Production of cloned pigs from cultured fetal fibroblast cells. Biol Reprod. 2002;66:1283–1287. doi: 10.1095/biolreprod66.5.1283. [DOI] [PubMed] [Google Scholar]
- Bortvin A, Eggan K, Skaletsky H, Akutsu H, Berry DL, Yanagimachi R, Page DC, Jaenisch R. Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development. 2003;130:1673–1680. doi: 10.1242/dev.00366. [DOI] [PubMed] [Google Scholar]
- Carter DB, Lai L, Park KW, Samuel M, Lattimer JC, Jordan KR, Estes DM, Besch-Williford C, Prather RS. Phenotyping of transgenic cloned piglets. Cloning Stem Cells. 2002;4:131–145. doi: 10.1089/153623002320253319. [DOI] [PubMed] [Google Scholar]
- Chang MC, Yanagimachi R. Fertilization of ferret ova by deposition of epididymal sperm into the ovarian capsule with special reference to the fertilizable lie of ova and the capacitation of sperm. J Exp Zool. 1963;154:175–187. doi: 10.1002/jez.1401540205. [DOI] [PubMed] [Google Scholar]
- Chesne P, Adenot PG, Viglietta C, Baratte M, Boulanger L, Renard JP. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat Biotechnol. 2002;20:366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat. 2000;197 (Pt 3):361–372. doi: 10.1046/j.1469-7580.2000.19730361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Kim CI, Park CK, Yang BK, Cheong HT. Effect of activation time on the nuclear remodeling and in vitro development of nuclear transfer embryos derived from bovine somatic cells. Mol Reprod Dev. 2004;69:289–295. doi: 10.1002/mrd.20131. [DOI] [PubMed] [Google Scholar]
- Davis CS, Strobeck C. Isolation, variability, and cross-species amplification of polymorphic microsatellite loci in the family Mustelidae. Mol Ecol. 1998;7:1776–1778. doi: 10.1046/j.1365-294x.1998.00515.x. [DOI] [PubMed] [Google Scholar]
- Donovan BT. Light and the control of the oestrous cycle in the ferret. J Endocrinol. 1967;39:105–113. doi: 10.1677/joe.0.0390105. [DOI] [PubMed] [Google Scholar]
- Ertzeid G, Storeng R. The impact of ovarian stimulation on implantation and fetal development in mice. Hum Reprod. 2001;16:221–225. doi: 10.1093/humrep/16.2.221. [DOI] [PubMed] [Google Scholar]
- Fleming MA, Ostrander EA, Cook JA. Microsatellite markers for American mink (Mustela vison) and ermine (Mustela erminea) Mol Ecol. 1999;8:1352–1354. doi: 10.1046/j.1365-294x.1999.00701_2.x. [DOI] [PubMed] [Google Scholar]
- Fox JG, Bell JA. Grouth, reproduction, and breeding. In: Fox JG, editor. Biology and Diseases of the Ferret. Williams and Wilkins; Baltimore: 1998. pp. 211–227. [Google Scholar]
- Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory Animal Medicine. Academic Press; San Diego, CA, USA: 2002. [Google Scholar]
- Galli C, Lagutina I, Crotti G, Colleoni S, Turini P, Ponderato N, Duchi R, Lazzari G. Pregnancy: a cloned horse born to its dam twin. Nature. 2003;424:635. doi: 10.1038/424635a. [DOI] [PubMed] [Google Scholar]
- Garry FB, Adams R, McCann JP, Odde KG. Postnatal characteristics of calves produced by nuclear transfer cloning. Theriogenology. 1996;45:141–152. [Google Scholar]
- Heyman Y. Nuclear transfer: a new tool for reproductive biotechnology in cattle. Reprod Nutr Dev. 2005;45:353–361. doi: 10.1051/rnd:2005026. [DOI] [PubMed] [Google Scholar]
- Hill JR, Roussel AJ, Cibelli JB, Edwards JF, Hooper NL, Miller MW, Thompson JA, Looney CR, Westhusin ME, Robl JM, Stice SL. Clinical and pathologic features of cloned transgenic calves and fetuses (13 case studies) Theriogenology. 1999;51:1451–1465. doi: 10.1016/s0093-691x(99)00089-8. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil. 2000;120:231–237. [PubMed] [Google Scholar]
- Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Shamim MH, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005;436:641. doi: 10.1038/436641a. [DOI] [PubMed] [Google Scholar]
- Li Z, Engelhardt JF. Progress toward generating a ferret model of cystic fibrosis by somatic cell nuclear transfer. Reprod Biol Endocrinol. 2003;1:83. doi: 10.1186/1477-7827-1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Jiang QS, Zhang YL, Liu XM, Engelhardt JF. Successful production of offspring after superovulation and in vitro culture of embryos from domestic ferrets (Mustela putorius furos) Reproduction. 2001;122:611–618. [PubMed] [Google Scholar]
- Li Z, Jiang Q, Rezaei Sabet M, Zhang Y, Ritchie TC, Engelhardt JF. Conditions for in vitro maturation and artificial activation of ferret oocytes. Biol Reprod. 2002;66:1380–1386. doi: 10.1095/biolreprod66.5.1380. [DOI] [PubMed] [Google Scholar]
- Li Z, Rezaei Sabet M, Zhou Q, Liu X, Ding W, Zhang Y, Renard JP, Engelhardt JF. Developmental capacity of ferret embryos by nuclear transfer using G0/G1-phase fetal fibroblasts. Biol Reprod. 2003;68:2297–2303. doi: 10.1095/biolreprod.102.012369. [DOI] [PubMed] [Google Scholar]
- Li Z, Chen X, Sun X, Zhou Q, Chen J, Leno GH, Engelhardt JF. Nuclear transfer of M-phase ferret fibroblasts synchronized with the microtubule inhibitor demecolcine. J Exp Zool, Part A. 2005a;303:1126. doi: 10.1002/jez.a.234. [DOI] [PubMed] [Google Scholar]
- Li Z, Sun X, Chen J, Leno GH, Engelhardt JF. Factors affecting the electrofusion of mouse and ferret oocytes with ferret somatic cells. Mol Reprod Dev. 2005b;72:40–47. doi: 10.1002/mrd.20321. [DOI] [PubMed] [Google Scholar]
- Liberson AJ, Newcomer CE, Ackerman JI, Murphy JC, Fox JG. Mastitis caused by hemolytic Escherichia coli in the ferret. J Am Vet Med Assoc. 1983;183:1179–1181. [PubMed] [Google Scholar]
- Marini RP, Otto G, Erdman S, Palley L, Fox JG. Biology and diseases of ferrets. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory Animal Medicine. Academic Press; San Diego, CA: 2002. pp. 483–517. [Google Scholar]
- McKiernan SH, Bavister BD. Gonadotrophin stimulation of donor females decreases post-implantation viability of cultured one-cell hamster embryos. Hum Reprod. 1998;13:724–729. doi: 10.1093/humrep/13.3.724. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- Mir B, Zaunbrecher G, Archer GS, Friend TH, Piedrahita JA. Progeny of somatic cell nuclear transfer (SCNT) pig clones are phenotypically similar to non-cloned pigs. Cloning Stem Cells. 2005;7:119–125. doi: 10.1089/clo.2005.7.119. [DOI] [PubMed] [Google Scholar]
- O’Connell M, Wright JM, Farid A. Development of PCR primers for nine polymorphic American mink Mustela vison microsatellite loci. Mol Ecol. 1996;5:311–312. doi: 10.1111/j.1365-294x.1996.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Paetkau D, Calvert W, Stirling I, Strobeck C. Microsatellite analysis of population structure in Canadian polar bears. Mol Ecol. 1995;4:347–354. doi: 10.1111/j.1365-294x.1995.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Pearson RC, Gorham JR. Viral disease models. In: Fox JD, editor. Biology and Disease of Ferret. Lippincott Williams and Wilkins; Baltimore, MD: 1998. pp. 487–497. [Google Scholar]
- Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KH. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407:86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- Rideout WM, III, Eggan K, Jaenisch R. Nuclear cloning and epigenetic reprogramming of the genome. Science. 2001;293:1093–1098. doi: 10.1126/science.1063206. [DOI] [PubMed] [Google Scholar]
- Schnieke AE, Kind AJ, Ritchie WA, Mycock K, Scott AR, Ritchie M, Wilmut I, Colman A, Campbell KH. Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science. 1997;278:2130–2133. doi: 10.1126/science.278.5346.2130. [DOI] [PubMed] [Google Scholar]
- Shi W, Zakhartchenko V, Wolf E. Epigenetic reprogramming in mammalian nuclear transfer. Differentiation. 2003;71:91–113. doi: 10.1046/j.1432-0436.2003.710201.x. [DOI] [PubMed] [Google Scholar]
- Shin T, Kraemer D, Pryor J, Liu L, Rugila J, Howe L, Buck S, Murphy K, Lyons L, Westhusin M. A cat cloned by nuclear transplantation. Nature. 2002;415:859. doi: 10.1038/nature723. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Wakayama T, Akutsu H, Yamazaki Y, Lachey JL, Wortman MD, Seeley RJ, D’Alessio DA, Woods SC, Yanagimachi R, Sakai RR. Cloned mice have an obese phenotype not transmitted to their offspring. Nat Med. 2002;8:262–267. doi: 10.1038/nm0302-262. [DOI] [PubMed] [Google Scholar]
- Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol Reprod. 2001;64:324–330. doi: 10.1095/biolreprod64.1.324. [DOI] [PubMed] [Google Scholar]
- Tian XC, Kubota C, Enright B, Yang X. Cloning animals by somatic cell nuclear transfer—Biological factors. Reprod Biol Endocrinol. 2003;1:98. doi: 10.1186/1477-7827-1-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R. Cloning of male mice from adult tail-tip cells. Nat Genet. 1999;22:127–128. doi: 10.1038/9632. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Walker SK, Hartwich KM, Seamark RF. The production of unusually large offspring following embryo manipulation: concepts and challenges. Theriogenology. 1996;45:111–120. [Google Scholar]
- Wang X, Zhang Y, Amberson A, Engelhardt JF. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol. 2001;24:195–202. doi: 10.1165/ajrcmb.24.2.3918. [DOI] [PubMed] [Google Scholar]
- Wells DN, Forsyth JT, McMillan V, Oback B. The health of somatic cell cloned cattle and their offspring. Cloning Stem Cells. 2004;6:101–110. doi: 10.1089/1536230041372300. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Wisely SM, Buskirk SW, Fleming MA, McDonald DB, Ostrander EA. Genetic diversity and fitness in black-footed ferrets before and during a bottleneck. J Hered. 2002;93:231–237. doi: 10.1093/jhered/93.4.231. [DOI] [PubMed] [Google Scholar]
- Woods GL, White KL, Vanderwall DK, Li GP, Aston KI, Bunch TD, Meerdo LN, Pate BJ. A mule cloned from fetal cells by nuclear transfer. Science. 2003;301:1063. doi: 10.1126/science.1086743. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. Cloning: experience from the mouse and other animals. Mol Cell Endocrinol. 2002;187:241–248. doi: 10.1016/s0303-7207(01)00697-9. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Renard JP, Le Friec G, Brochard V, Beaujean N, Cherifi Y, Fraichard A, Cozzi J. Generation of fertile cloned rats by regulating oocyte activation. Science. 2003;302:1179. doi: 10.1126/science.1088313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


