Abstract
The mosquito Anopheles gambiae is a primary vector of Plasmodium parasites in Africa. The effect of aging on reproductive output in A. gambiae females from three strains that differ in their ability to melanize Plasmodium and in their systemic levels of hydrogen peroxide (H2O2), a reactive oxygen species (ROS), was analyzed. The number of eggs oviposited after the first blood meal decreases with age in all strains; however, this decline was much more pronounced in the G3 (unselected) and R (refractory to Plasmodium infection) strains than in the S (highly susceptible to Plasmodium) strain. Reduction of ROS levels in G3 and R females by administration of antioxidants reversed this age-related decline in fecundity. The S and G3 strains were fixed for two functionally different catalase alleles that differ at the second amino acid position (Ser2Trp). Biochemical analysis of recombinant proteins revealed that the Trp isoform has lower specific activity and higher Km than the Ser isoform, indicating that the former is a less efficient enzyme. The Trp-for-Ser substitution appears to destabilize the functional tetrameric form of the enzyme. Both alleles are present in the R strain, and Ser/Ser females had significantly higher fecundity than Trp/Trp females. Finally, a systemic reduction in catalase activity by dsRNA-mediated knockdown significantly reduced the reproductive output of mosquito females, indicating that catalase plays a central role in protecting the oocyte and early embryo from ROS damage.
Keywords: insect, malaria, Plasmodium, reproduction, single-nucleotide polymorphism
Anopheline mosquitoes are the natural vectors that transmit Plasmodium parasites, the causative agents of malaria, to humans worldwide. Previous studies have shown that higher levels of reactive oxygen species (ROS) in mosquito hemolymph limit Plasmodium development (1). ROS are continuously generated from the reduction of molecular oxygen to the superoxide anion (·O2•−) by single electrons that have escaped the mitochondrial respiratory chain. ROS accumulation can cause chronic cell damage and has been associated with aging (2); high levels of ROS can also be generated abruptly as part of the immune response to pathogens (3). Several enzymes such as superoxide dismutase (SOD), glutathione peroxidase, and catalase are involved in ROS detoxification. SOD converts O22•− into hydrogen peroxide (H2O2), a less toxic product. H2O2, however, also needs to be detoxified by peroxidases or catalase because, in the presence of reduced metal atoms, it can transform into a highly reactive hydroxyl radical (·OH). Catalase is a tetrameric enzyme that efficiently converts H2O2 to water and oxygen without production of other ROS.
ROS have received considerable attention for their putative role in aging processes. The free radical theory of aging proposes that, in biologic systems, ROS attack and degrade molecules, causing functional decline (2). This theory predicts that increasing antioxidant defenses would enhance longevity and protect functionality. Overexpression of SOD or catalase or treatment with mimetics of these enzymes in animal models such as mouse, Drosophila, and Caenorhabditis elegans has resulted in measurable increases in animal longevity in some studies (4–7), although not in all (8–10). ROS are also thought to negatively affect reproductive ability in both males and females. Reduced female fertility has been observed in mice and in Drosophila lacking Cu-Zn SOD (11, 12). In Drosophila, lack of Cu-Zn SOD also causes nearly complete sterility in males (12). A growing body of evidence associates ROS in humans with reduced sperm vitality (13), ovarian DNA damage (14), and preeclampsia and other pregnancy complications (15). Surprisingly, mice in which the catalase gene is disrupted grow normally and are as fertile as wild-type mice (16), indicating that, in vertebrates, this enzyme is not essential to protect spermatozoids or the ovaries from oxidative damage. In contrast, flies in which the catalase gene is disrupted and that lack catalase activity are extremely weak and die soon after eclosion, but flies with a single functional copy of the gene (17) or hypomorphic mutants with very low enzymatic activity (6–10% of wild-type) have normal viability (18). Fecundity studies have not been performed in these hypomorphic mutant flies.
Anautogenous mosquitoes such as anophelines must ingest a blood meal to obtain the nutrients required for oogenesis. The blood is digested by the midgut and the nutrients are transported to the fat body, where vitellogenin and other major proteins of the egg yolk are synthesized (19). In Anopheles gambiae, H2O2 levels in hemolymph increase dramatically after a blood meal, presumably because of increased metabolic activity during the processes of blood digestion and oogenesis; and hydrogen peroxide levels at 24 h post-blood meal (PBM) differ significantly among strains of A. gambiae (1). A strain genetically selected to be highly susceptible (S) to Plasmodium development has significantly lower levels of hemolymph H2O2 than the unselected, susceptible G3 strain 24 h after feeding. Furthermore, a strain that has been genetically selected to be refractory (R) has significantly higher levels (2 to 3 times) than the other two strains (1). We postulated that the higher H2O2 levels associated with enhanced immunity to Plasmodium infection may come with a cost, such as an adverse effect on fecundity. We show here that an age-related reduction in fecundity does occur in some strains, that it is likely caused by oxidative damage, and that it is mediated by catalase alleles of differing activities. Also, our results indicate that, unlike vertebrates, A. gambiae and possibly other insects require catalase to protect the ovary and/or embryo from oxidative damage.
Results
Fecundity Declines with Aging in Strains with Higher ROS Levels.
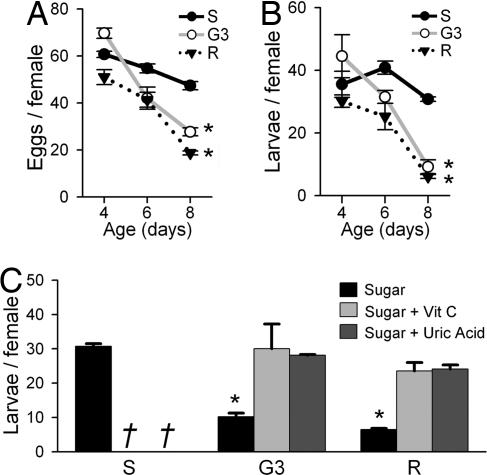
A. gambiae S, G3, and R females had similar reproductive outputs in terms of the number of eggs oviposited and the number of viable larvae hatched per female when fed at 4 days post-emergence (PE) (Fig. 1 A and B). However, when fed at older ages, the number of larvae per female declined in both G3 and R females, but not in S females (Fig. 1B). G3 and R females that were fed 8 days PE oviposited significantly lower numbers of eggs (Fig. 1A) and produced less viable larvae (Fig. 1B) than when they were fed 4 or 6 days PE (ANOVA, P < 0.002). G3 and R females that were fed at 8 days PE oviposited less eggs and produced 3.5- and 5-fold less viable larvae per female than S females, respectively (Fig. 1B; ANOVA, P < 0.002). The declines in the number of viable larvae (Fig. 1B) were the combined result of reductions in eggs laid (Fig. 1A) and in the percentage of larvae hatched (data not shown).
Fig. 1.
Age-associated loss of fecundity in the G3 and R strains. (A) Average number of eggs per female oviposited ± SE. (B) Average number of larvae produced per female ± SE. The first blood meal was given at 4, 6, or 8 days PE (n = three groups of 10 females each). (C) Effect of supplemental antioxidant feeding (vitamin C or uric acid) on fecundity when females were blood fed at 8 days PE. Both antioxidant treatments restored fecundity of G3 and R to levels not significantly different from S females. The same antioxidant treatment was deleterious to S females; all of them died within 48 h after blood feeding (indicated by †). Asterisks indicate values significantly lower than all others (P < 0.003, ANOVA).
Antioxidants Restore the Decline in Fecundity with Aging.
To confirm that differences in ROS levels in the three strains were responsible for their differences in fecundity, two different strong antioxidants, vitamin C and uric acid, were supplemented to all females in their sugar meal before and after blood feeding. Antioxidant supplementation to G3 and R females blood-fed 8 days PE restored reproductive output of these strains to levels similar to those of the untreated S strain (Fig. 1C). This dramatic result strongly supports the hypothesis that ROS are at least partially responsible for the decline in fecundity seen in G3 and R strains as they age. A curious observation was that when antioxidants were provided to S mosquitoes, which already have low basal levels of ROS, a deleterious effect was observed, because all females died within 48 h PBM. These data indicate some minimal level of ROS is required for normal physiology of blood-fed females, because the same dose of antioxidants did not cause mortality in females that were not blood-fed (data not shown). ROS may play an important role in maintaining immune competence to opportunistic endopathogens, such as bacteria, which are known to proliferate in the mosquito gut as the blood meal is digested (20), or ROS may be important as signaling molecules (21).
Catalase mRNA and Protein Accumulate in the Developing Oocyte.
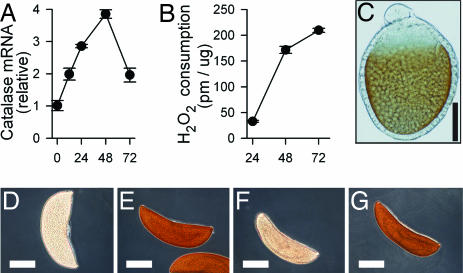
To investigate the potential role of catalase in protecting the oocyte from ROS damage, the levels of catalase mRNA and enzymatic activity were determined in ovaries at time points up to 72 h PBM. Both catalase mRNA and enzymatic activity increased significantly as the ovaries developed (Fig. 2 A and B). The cellular localization of the enzyme was determined by staining ovaries 24 h after feeding by using diaminobenzidine (DAB) as a substrate (22). Catalase activity was present only in the oocyte (Fig. 2C), and the intensity of the DAB staining increased as ova matured (Fig. 2E). The DAB assay can give a positive staining to both catalase and peroxidase activities (22). The specificity of the DAB staining for catalase was confirmed by the following criteria. (i) Peroxidase activity, determined with a peroxidase-specific substrate in ovary homogenates, was not induced as ova matured (data not shown). (ii) The eggs stained readily under pH conditions favorable to catalase (pH 10.0) but less favorable to peroxidase (22). (iii) The DAB staining was strongly inhibited by 3-amino-1,3,4-triazole (AT) (Fig. 2F), a catalase inhibitor that did not inhibit peroxidase activity in our ovarian extracts (data not shown). As expected, the inhibition was reversible (Fig. 2G).
Fig. 2.
Catalase mRNA and protein expression over time in developing oocytes. (A) Relative amounts of catalase mRNA in ovaries at times PBM as measured by quantitative PCR normalizing with ribosomal protein S7. (B) Enzymatic activity of catalase in ovary homogenates from G3 females normalized for total protein in the extract. (C–G) Catalase-mediated DAB staining of whole oocytes. (Scale bars: in C, 30 μm; in D–G, 150 μm.) (C) Developing oocytes (24 h PBM) are positive for DAB staining (brown), whereas unstained oocytes are clear (not shown). (D–G) Mature oocytes (72 h PBM) accumulate catalase protein. (D) Unstained oocyte. (E) Oocyte after DAB staining (dark brown). (F) DAB staining is minimal in oocytes incubated with AT, a catalase inhibitor. (G) AT is removed by extensive washing with PBS, and DAB staining is restored, indicating that AT inhibition is reversible.
S and G3 Strains Are Fixed for Different Catalase Alleles.
To explore the possibility that the differences in fecundity between strains could be due to differences in catalase activity, complete nucleotide sequences were obtained for catalase from 10 individuals of each strain. The catalase gene consists of exon 1 (54 nt) and exon 2 (1,461 nt). Four synonymous polymorphisms were found in exon 2 (codons 218, 243, 309, and 349) of some individuals, but were not restricted to any one strain. Three of these polymorphisms were also present among the EST and genomic information for A. gambiae. Two nonsynonymous polymorphisms were found in exon 1: A C/G substitution in the second codon resulted in a Ser2Trp amino acid change and a G/A substitution in the 14th codon resulted in a Arg14Lys change. The latter polymorphism was found in only two individuals, and the amino acids encoded by both alleles are similar in size and properties. However, the Ser/Trp polymorphism in the second amino acid position was of particular interest, because all 10 S individuals were homozygous for serine, and all 10 G3 individuals were homozygous for tryptophan. A TaqMan SNP assay (Applied Biosystems, Foster City, CA) was designed and used to verify these results as well as to genotype 90 more individuals from each strain. Remarkably, of 100 S individuals, all were C/C (Ser/Ser), and of 100 G3 individuals, all were G/G (Trp/Trp) (Table 1). Serine and tryptophan differ substantially: Serine is a small polar amino acid, whereas tryptophan is a large hydrophobic residue. Genotyping confirmed that both alleles are present in the R strain and are in Hardy–Weinberg equilibrium (Table 1).
Table 1.
Catalase genotype data for laboratory strains of A. gambiae
| Strain | n | C/C (Ser/Ser) | C/G (Ser/Trp) | G/G (Trp/Trp) |
|---|---|---|---|---|
| S | 100 | 100 | 0 | 0 |
| G3 | 100 | 0 | 0 | 100 |
| R | 100 | 36 | 52 | 12 |
The Ser Catalase Allele Is More Active and Stable than the Trp Allele.
Ovary homogenates of S (Ser allele) and G3 females (Trp allele) were prepared 48 h after feeding, and catalase activity per μg of total protein was measured across a broad pH range (from pH 5 to 12). At pH 5 and 5.5 there was no difference in activity between the two strains (Fig. 3A). However, in the pH range from 6 to 12, the homogenates from S females consumed significantly more H2O2 than those of G3, and their optimal pH was slightly different (9 and 8.5, respectively). To ensure catalase specificity in the reaction, only H2O2 consumption that could be inhibited by AT was measured. The experiments were done with whole-ovary extracts and, because the amount of catalase enzyme present could not be ascertained, H2O2 consumption was normalized on the basis of the total protein concentration in the sample.
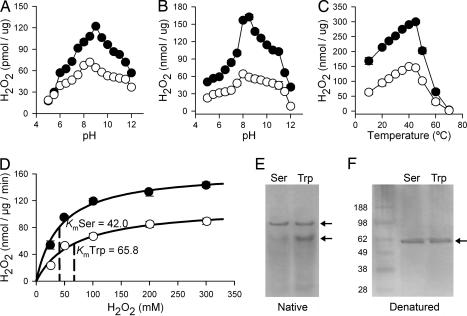
Fig. 3.
Enzymatic activities of Ser and Trp catalase alleles. (A) Effect of pH on catalase enzymatic activity in ovary homogenates from S (Ser allele) and G3 (Trp allele) females, normalized for total protein in the extract. Means are plotted ± SE. Ser allele, filled circles; Trp allele, open circles. (B) Effect of pH on enzymatic activity of purified recombinant Ser and Trp catalase isoforms. (C) Effect of temperature on enzymatic activity of purified recombinant Ser and Trp catalase isoforms. (D) Effect of substrate concentration on initial velocity of catalysis and determination of Km and Vmax of purified recombinant Ser and Trp catalase isoforms. (E) Native gel electrophoresis of recombinant Ser and Trp isoforms, showing that the Trp isoform has relatively less protein in tetramer form. (F) SDS/PAGE of Ser and Trp recombinant protein isoforms shows a single band.
Recombinant cDNA clones of the two catalase alleles, differing only by a single nucleotide that determined Ser or Trp in the second amino acid, were constructed by PCR from mRNA, cloned, and sequenced. Recombinant soluble enzymes were expressed in Escherichia coli, purified, and found to be catalytically active. Specific activity measurements of the recombinant enzymes indicated that the Ser allele is 2 to 3 times more active than the Trp allele over a broad pH range (pH 5–12) (Fig. 3B). Moreover, specific activity curves of the recombinant forms are similar to those of the ovarian homogenates (Fig. 3A), except for the Ser allele already having a higher activity at pH 5 and 5.5. The Ser allele also had a pH optimum slightly more basic than the Trp allele, 8.5 vs. 8.0, respectively. The specific activity of the Ser allele was also higher over a broad temperature range (10–60°C), but for both alleles the activity decreased abruptly at temperatures >45°C (Fig. 3C). Because catalase is known to follow Michaelis–Menten kinetics, the Km of both enzyme alleles was determined by measuring the increase in catalytic rate with increasing H2O2 concentrations (Fig. 3D). The Km of the Ser alleles (42.0 ± 5.6 mM) was significantly lower than that of the Trp alleles (65.8 ± 11.3 mM), indicating that the former is a more efficient enzyme (t test, P = 0.034).
The N terminus of catalases is regarded as important in the formation of catalase tetramers (23, 24). A silver-stained native gel showed that the Ser allele consisted almost entirely of the tetrameric form, whereas the equilibrium for the subunit association in the Trp allele was significantly shifted to a smaller form (Fig. 3E). A single band was observed when the same samples were analyzed under denaturing conditions (Fig. 3F).
Females Homozygous for the Trp Allele Have Lower Fecundity.
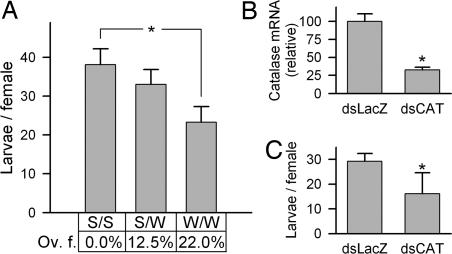
The R strain has both Ser and Trp alleles. To determine whether a female's catalase genotype was correlated with fecundity, 117 R females were blood-fed and allowed to oviposit eggs in individual tubes. The eggs and viable larvae were counted and the females were genotyped. Females which were homozygous for the Trp allele produced significantly less viable larvae than did those homozygous for the Ser allele (Fig. 4A), whereas heterozygous female output was intermediate. Furthermore, none of the fully engorged females with the Ser/Ser genotype had an oviposition failure (no eggs laid), whereas this increased to 12.5% for Ser/Trp and to 22.6% for Trp/Trp females (P = 0.005). Oviposition failures were not due to lack of insemination, because noninseminated females were removed from the dataset.
Fig. 4.
Effect of reduced catalase activity on fecundity. (A) Association between catalase allele genotypes and fecundity in R females. Asterisk indicates that Trp/Trp individuals produced significantly less offspring than those homozygous for the Ser allele. The table below the graph indicates the percentage of mosquitoes that exhibited oviposition failure (Ov. f.), which was significantly different among the groups (χ2 test, P = 0.005). (B and C) Effect of catalase knockdown on fecundity. (B) Relative amounts of catalase mRNA in G3 mosquitoes after injection with catalase dsRNA (dsCAT) compared with controls injected with LacZ dsRNA (dsLacZ). A 65% knockdown in abdomen catalase mRNA levels was achieved. (C) Mean number of larvae produced per female (± SE) decreased by 45% in dsCAT-knockdown vs. control G3 mosquitoes (t test, P = 0.046).
Knockdown of Catalase Reduces Fecundity.
To confirm the importance of catalase activity on A. gambiae fecundity, catalase expression was reduced by dsRNA-mediated gene knockdown in the G3 strain. Injection of catalase dsRNA decreased the basal whole body mRNA levels by >65%, relative to the dsLacZ control (Fig. 4B). Females with reduced catalase expression produced significantly less viable larvae (45% decrease) (Fig. 4C; t test, P = 0.046). These results support the working hypothesis that catalase has a central and nonredundant role in determining fecundity, most likely by protecting the egg and embryo from oxidative damage through H2O2 detoxification.
Discussion
Differences in ROS levels among the S, G3, and R strains of A. gambiae become acute after a blood meal (1), probably because of the increase in metabolic activity during blood digestion and oogenesis. We propose here that the dramatic decline in fecundity with age observed in G3 and R strains is primarily due to cumulative oxidative damage sustained from ROS on the basis of the compelling evidence that fecundity is restored in the G3 and R strains by supplemental feeding of antioxidants (Fig. 1C). It is unlikely that the reduction in fecundity is due to other factors. It is commonly reported that the number of eggs laid by anophelines (and other mosquitoes) decreases in successive gonotrophic cycles, which is caused by increasing ovarian follicle degeneration with each gonotrophic cycle (25, 26). However, in this study, the age and reproductive output was measured only after the first blood meal. Additionally, there is no reason to suspect nutritional differences between the different strains, because mosquitoes were reared under identical conditions, and, furthermore, antioxidants would not likely remedy a nutritional deficiency. Finally, we determined that antioxidant supplementation did not lead to larger blood meals; blood meal sizes were similar with or without antioxidants (data not shown).
The lower catalytic activity of the Trp catalase allele is expected to reduce the ability of G3 females to clear H2O2 and probably explains why this strain has higher levels of hemolymph H2O2 than S (1). If the three strains (S, G3, and R) had similar rates of ROS production, then the R strain, which has both catalase alleles, would be expected to have a fecundity intermediate between that of G3 and S. However, previous studies indicate that ROS are generated at a faster rate in R after a blood meal (1). The presence of the more active Ser catalase allele in some R females does not appear to be sufficient to compensate for the increased rate of ROS production.
It is apparent that A. gambiae females allocate resources to up-regulate catalase expression and accumulate this enzyme in the oocyte as it matures (Fig. 2). The strong effect of reduced catalase expression by dsRNA-mediated silencing on fecundity confirms the importance of this enzyme, as does the significantly lower fecundity associated with the Trp/Trp genotype in R females, when compared with those carrying the Ser/Ser allele. The thioredoxin system also has the ability to detoxify H2O2, but our data indicate that this system is unable to compensate for the deficiency in catalase. The abrupt increase in metabolic activity and the fast maturation of the ovaries that follows blood feeding may overwhelm other detoxification systems, whereas catalase has a remarkable catalytic rate that may be essential in physiologic conditions when ROS are generated rapidly.
Differences in enzymatic activity were first observed in whole ovary extracts from the S and G3 strains (Fig. 3A), which are homozygous for the high (Ser/Ser) and the low (Trp/Trp) catalase activity alleles, respectively. Detailed biochemical analysis of the recombinant isoforms that differ by a single amino acid revealed significant differences in specific activity and Km (Fig. 3 B–D). A common property of catalases is that the N-and C-terminal ends of each monomeric subunit overlap, or “interweave” with other catalase monomers in the formation of a catalytically active tetramer (23, 24). The N terminus affects tetramer formation; in Candida tropicans catalase (27) and E. coli catalase (28), small N terminus deletions (4- and 18-aa, respectively) reduce tetrameric accumulation. The Ser2Trp substitution appears to destabilize the tetrameric form and may account for the reduced efficiency of the Trp isoform (Fig. 3E). Similarly, an amino acid substitution at position 11 in mouse catalase also causes tetrameric instability and reduced activity (29).
Given the strong impact that decreased catalase activity has on A. gambiae fecundity, a reasonable prediction is that there would be strong selection for the Ser allele of catalase. In a preliminary analysis, a small sample of A. gambiae mosquitoes (n = 98) from various regions in Africa (Mali, Nigeria, Uganda, and Kenya) were genotyped, and all of them were homozygous for the Ser allele (data not shown), suggesting that the Trp allele is not common, at least not in the natural populations we sampled. In addition, natural populations may have functional polymorphisms in other parts of the catalase gene that are subject to local selective pressures.
However, higher ROS levels are associated with resistance to Plasmodium infection (1) and confer resistance to bacterial challenge (A.M.-C. and C.B.-M., unpublished work). Therefore, under some conditions, a decreased ability to degrade ROS may be favorable to mount a stronger, more effective immune response and/or maintain a normal physiology, so the Trp allele may be maintained in a population by balancing selection. Both the Ser and Trp alleles are found in the R strain (originally selected from G3) (30) and in G3 colonies from another laboratory (the Malaria Research and Reference Reagent Resource Center, Manassas, VA) (data not shown), suggesting that both alleles were present in the original G3 strain and that the Trp allele became fixed in our G3 laboratory colony as a result of a population bottleneck and/or an unintended selective process.
Whether catalase also plays such an important role in the reproductive success of other insect species remains to be determined. Female mosquitoes may present an extreme case of metabolic surge as they ingest and process a blood meal three times their body weight. Also of particular interest are insect species in which eggs undergo extensive hardening, because peroxidases involved in cross-linking of protective chorion proteins (31) may require high local levels of H2O2 that could potentially damage the embryo. In summary, genetic factors result in differences in basal levels of ROS between A. gambiae strains. ROS detoxification by catalase increases the reproductive output by protecting the ovary and early embryo from oxidative damage, but transient and local accumulation of ROS appears to be necessary for normal mosquito physiology.
Materials and Methods
Mosquito Strains.
A. gambiae strains used were as follows: 4A r/r, a strain genetically selected to be highly susceptible to Plasmodium (32) and referred to as the S strain; G3, a laboratory strain that has not been selected but is naturally susceptible to Plasmodium and melanization, is rarely observed; and L3-5, a strain genetically selected from G3 to be refractory, which melanizes many different Plasmodium species (30) and referred to as the R strain. Mosquitoes were maintained under standard conditions (1).
Fecundity Assays.
Female mosquitoes were allowed to mate in normal rearing conditions and then were fed on healthy BALB/c mice at 4, 6, or 8 days PE. A single blood meal is usually sufficient for A. gambiae females to produce an egg batch, particularly when larval nutrition and survival is high, resulting in large-size adults (33–35), as was the case for our colonies. Twenty-four hours PBM, females of each strain were separated into three groups of 10–12 mosquitoes each and were housed in pint-sized cages. A cup lined with moistened filter paper was provided to each cage 72 h PBM. The next day, filters were removed, and the eggs were counted and placed in hatching trays. New filter paper was provided for each of the next 2 days to allow for any late egg laying (minimal in our study). Larvae were counted 48 h after egg laying. Eggs or larvae produced per female was calculated using the number of females alive at the time of egg laying.
A fecundity assay involving only the R strain isolated individual females in 50-ml tubes, with a strip of filter paper around the inside of each tube. Water was added 72 h PBM to moisten the filter paper. Eggs and larvae were counted as above. Each individual R female was examined for insemination by crushing the spermatheca and looking for live sperm under a microscope. A low number of noninseminated individuals were removed from the analysis (3 females), leaving a total of 117 females. All females were stored in ethanol and genotyped for the Ser2Trp locus at a later time. Genotype results were later matched with fecundity measurements (n = 46 Ser/Ser, 40 Ser/Trp, and 31 Trp/Trp).
Antioxidant Feeding.
At 6 days PE, female mosquitoes were given a 10% sugar solution supplemented with either 25 mg/ml vitamin C or 1 mg/ml uric acid and were blood fed at 8 days PE. Antioxidant supplemention to sugar continued for 72 h PBM until oviposition. Fecundity assays were repeated as before. A separate experiment checked that female appetite was not affected by antioxidants by using a modified hemoglobinometry method (36) and 30 G3 females for each treatment (sugar with and without antioxidants; data not shown).
3,3′-DAB Activity of Ova.
Ova were dissected from mosquitoes after blood feeding and fixed in 0.5% glutaraldehyde for 10 min at room temperature. Samples were then incubated in 2.5 mM DAB (Sigma, St. Louis, MO) and 2.5 mM H2O2 (Sigma) in 0.1M Tris·HCl (pH 10.0) for 10 min. The high pH favors catalase compared with peroxidases (22). Samples were then removed, washed in PBS, and fixed for 1 h in 4% paraformaldehyde. To establish whether the DAB staining was catalase-dependent, samples were subjected to preincubation for 10 min with 60 mM AT, a catalase inhibitor, or 60 mM sodium azide (NaN3), a catalase and peroxidase inhibitor, in 0.1 M Tris·HCl before adding DAB and H2O2. In addition, some samples preincubated with AT were rinsed three times in PBS to remove the inhibitor and then were subjected to the DAB assay to determine whether AT inhibition was reversible.
DNA Extraction, Amplification, and Sequencing.
DNA was extracted from individual mosquitoes, 10 of each strain, and the two exons of the A. gambiae catalase gene were amplified using primers located outside the exons, and then directly sequenced. For primer sequences, see supporting information (SI) Table 2.
SNP Assay for Catalase Alleles.
A TaqMan SNP assay was designed using the CustomSNP services of Applied Biosystems. The assay was designed to distinguish between two alleles (C/G) in the fifth nucleotide position of the first exon (for primer and probe sequences, see SI Table 2).
Ovarian Catalase Activity.
Ovaries were collected from six female mosquitoes at 24, 48, and 72 h PBM. Samples were homogenated in PBS containing a protease inhibitor mixture and centrifuged at 4°C, placed on ice for 15 min, vortexed, and centrifuged, and then the supernatant was collected. Five microliters of supernatant was incubated with 40 μM H2O2 for 10 min at 28°C. Each assay was run in triplicate, both in the presence and absence of AT (100 μg/ml), and the H2O2 consumption that could be inhibited by AT was considered to be catalase-specific. The level of H2O2 consumption was determined as described in refs. 1 and 37.
Quantitation of Ovarian Catalase mRNA.
Ovaries were collected in RNAlater (Ambion, Austin, TX) from 10–15 mosquitoes at 12, 24, 48, and 72 h PBM, as well as from unfed controls, in duplicate. Total RNA was extracted by using an RNAeasy kit (Qiagen, Chatsworth, CA), which was used as template for cDNA synthesis using a Quantitect cDNA synthesis kit (Qiagen). Relative amounts of catalase mRNA from each were determined by quantitative PCR with cDNA as template (276-bp fragment). Quantitations were normalized to the housekeeping gene S7 ribosomal protein. For all primer sequences, see SI Table 2.
Recombinant Protein Expression.
Primers were designed to amplify the full catalase gene from a full-length cDNA of catalase from a G3 fourth instar larvae cDNA library (38), with forward primers incorporating the nonsynonymous difference in the second codon and the reverse primer lacking the stop codon. Each allele was cloned into a pCRT7/CT-TOPO plasmid (Invitrogen, San Diego, CA) and expressed in BL21(DE3)pLysE E. coli (Invitrogen). The expressed proteins included a C-terminal His-tag and were purified using Ni-NTA columns (Bio-Rad, Hercules, CA) by an imidazole gradient. At a concentration of 200 mM of imidazole, each protein was eluted without any other protein bands visible in a Coomassie-stained SDS/PAGE gel.
Recombinant Catalase Activity.
The concentration of each protein was measured in the linear range of the MicroBCA kit (Pierce, Rockford, IL), in triplicate. Identical amounts of purified protein were used for activity assays, incubating with H2O2 for 2 min, and consumption of H2O2 was measured with a colorimetric assay (CATK100; Sigma). The specific activity of each recombinant allele was determined in a broad range of pH and temperatures. The rate of consumption was also determined in a range of substrate (H2O2) concentrations to estimate Km of each recombinant enzyme. Analyses and calculations for Km and Vmax were completed by using enzymology functions in SigmaPlot (SPSS, Chicago, IL). Additionally, native-condition electrophoresis was used to check for differences in the equilibrium of subunit association by using a 3–8% Tris-acetate gel and 4 h of electrophoresis at 20 mA and 4°C in 0.5× TAE buffer (pH 4.5), as in ref. 29.
Catalase Knockdown by RNAi.
A 495-bp DNA fragment was amplified by RT-PCR, and cloned into pCRII-TOPO vector (Invitrogen) and used as template in PCR using primers complementary to the vector near the cloning site that includes a Xho site and a T7 promoter sequence. The PCR product was used in an in vitro dsRNA synthesis using the MEGAscript RNAi kit (Ambion, Austin, TX). Similarly, the control dsRNA was generated by amplifying the LacZ gene from the pCRII-TOPO vector, cloning it, and amplifying a 658-bp product by using M13 primers with a Xho site and T7 promoter sequence, which was then used in dsRNA synthesis. dsRNA was cleaned and concentrated to 3 μg/ul with Microcon YM-100 filters (Millipore, Billerica, MA) and 69 nl was injected into 1-day PE G3 females by using a Nanoject II (Drummond, Broomall, PA). Mosquitoes were fed on a single mouse at 5-days PE, and a fecundity assay was performed as above on four groups of 11 to 12 mosquitoes for each treatment. Additionally, two sets of 10 mosquitoes each were collected at 48 h PBM to check for the efficacy of RNAi by using quantitative PCR (qPCR). Primers used for qPCR amplify a catalase fragment nonoverlapping with the fragment used for RNAi.
Supplementary Material
Acknowledgments
We thank Andre Laughinghouse, Kevin Lee, Tovi Lehman, and Robert Gwadz for insectary support and Jose Ribeiro and Jesus Valenzuela for comments that improved the manuscript. Anopheles gambiae, collected from natural African populations, were kindly provided by Tovi Lehman (Laboratory of Malaria and Vector Research, National Institutes of Health). This research was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- AT
3-amino-1,3,4-triazole
- DAB
diaminobenzidine
- PBM
post-blood meal
- PE
post-emergence
- ROS
reactive oxygen species
- SOD
superoxide dismutase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. DQ980199–DQ980210 and DQ986315).
This article contains supporting information online at www.pnas.org/cgi/content/full/0608407104/DC1.
References
- 1.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. Proc Natl Acad Sci USA. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman D. Antioxid Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 4.Orr WC, Sohal RS. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 5.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 6.Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 7.Sampayo JN, Olsen A, Lithgow GJ. Aging Cell. 2003;2:319–326. doi: 10.1046/j.1474-9728.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 8.Mockett RJ, Bayne AC, Kwong LK, Orr WC, Sohal RS. Free Radical Biol Med. 2003;34:207–217. doi: 10.1016/s0891-5849(02)01190-5. [DOI] [PubMed] [Google Scholar]
- 9.Orr WC, Mockett RJ, Benes JJ, Sohal RS. J Biol Chem. 2003;278:26418–26422. doi: 10.1074/jbc.M303095200. [DOI] [PubMed] [Google Scholar]
- 10.Keaney M, Matthijssens F, Sharpe M, Vanfleteren J, Gems D. Free Radical Biol Med. 2004;37:239–250. doi: 10.1016/j.freeradbiomed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. J Biol Chem. 1998;273:7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- 12.Parkes TL, Kirby K, Phillips JP, Hilliker AJ. Genome. 1998;41:642–651. [PubMed] [Google Scholar]
- 13.Nallella KP, Sharma RK, Allamaneni SS, Agarwal A. Clinics. 2005;60:317–324. doi: 10.1590/s1807-59322005000400010. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal A, Gupta S, Sharma RK. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Agarwal A, Sharma RK. Obstet Gynecol Surv. 2005;60:807–816. doi: 10.1097/01.ogx.0000193879.79268.59. [DOI] [PubMed] [Google Scholar]
- 16.Ho YS, Xiong Y, Ma W, Spector A, Ho DS. J Biol Chem. 2004;279:32804–32812. doi: 10.1074/jbc.M404800200. [DOI] [PubMed] [Google Scholar]
- 17.Griswold CM, Matthews AL, Bewley KE, Mahaffey JW. Genetics. 1993;134:781–788. doi: 10.1093/genetics/134.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay WJ, Bewley GC. Genetics. 1989;122:643–652. doi: 10.1093/genetics/122.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Attardo GM, Hansen IA, Raikhel AS. Insect Biochem Mol Biol. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Pumpuni CB, Demaio J, Kent M, Davis JR, Beier JC. Am J Trop Med Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- 21.Hancock JT, Desikan R, Neill SJ. Biochem Soc Trans. 2001;29:345–350. doi: 10.1042/0300-5127:0290345. [DOI] [PubMed] [Google Scholar]
- 22.Fahimi HD, Baumgart E. J Histochem Cytochem. 1999;47:1219–1232. doi: 10.1177/002215549904701001. [DOI] [PubMed] [Google Scholar]
- 23.Chelikani P, Ramana T, Radhakrishnan TM. Indian J Clin Biochem. 2005;20:131–135. doi: 10.1007/BF02867412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chelikani P, Fita I, Loewen PC. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokolova MI. J Vector Ecol. 1995;20:121–128. [Google Scholar]
- 26.Detinova TS. Annu Rev Entomol. 1968;13:427–450. [Google Scholar]
- 27.Ueda M, Kinoshita H, Maeda SI, Zou W, Tanaka A. Appl Microbiol Biotechnol. 2003;61:488–494. doi: 10.1007/s00253-003-1251-5. [DOI] [PubMed] [Google Scholar]
- 28.Sevinc MS, Switala J, Bravo J, Fita I, Loewen PC. Protein Eng. 1998;11:549–555. doi: 10.1093/protein/11.7.549. [DOI] [PubMed] [Google Scholar]
- 29.Wang DH, Tsutsui K, Sano K, Masuoka N, Kira S. Biochim Biophys Acta. 2001;30:217–220. doi: 10.1016/s0167-4781(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 30.Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, Collins WE, Campbell CC, Gwadz RW. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Kim SR, Li J. Insect Biochem Mol Biol. 2004;34:1195–1203. doi: 10.1016/j.ibmb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Zheng L, Cornel AJ, Wang R, Erfle H, Voss H, Ansorge W, Kafatos FC, Collins FH. Science. 1997;276:425–428. doi: 10.1126/science.276.5311.425. [DOI] [PubMed] [Google Scholar]
- 33.Takken W, Stuke K, Klowden MJ. Entomol Exp App. 2002;103:83–89. [Google Scholar]
- 34.Takken W, Klowden MJ, Chambers GM. J Med Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- 35.Femandes L, Briegel H. J Vector Ecol. 2005;30:11–26. [PubMed] [Google Scholar]
- 36.Menge DM, Guda T, Zhong D, Pai A, Zhou G, Beier JC, Gouagna L, Yan G. Malar J. 2005;4:44. doi: 10.1186/1475-2875-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang ZY, Hunt JV, Wolff SP. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 38.Barillas-Mury C, Charlesworth A, Gross I, Richman A, Hoffmann JA, Kafatos FC. EMBO J. 1996;15:4691–4701. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.