Abstract
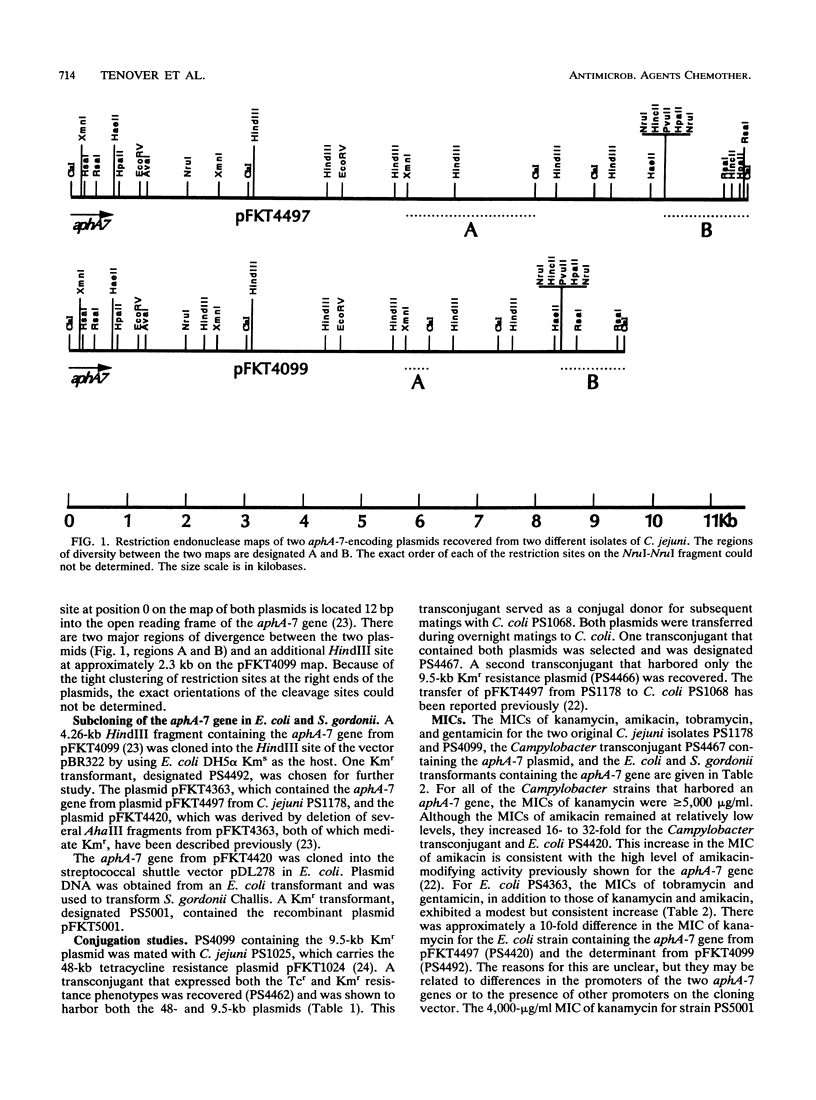
Two small plasmids of 11.5 and 9.5 kb, each carrying an aphA-7 kanamycin phosphotransferase gene, were studied. The MICs of kanamycin for the two human Campylobacter jejuni isolates harboring the plasmids were 10,000 and 5,000 micrograms/ml, while the MICs of amikacin were 32 and 8 micrograms/ml, respectively. The MICs of gentamicin and tobramycin were less than or equal to 2 micrograms/ml for both isolates. The restriction endonuclease maps of the plasmids were similar, with the larger plasmid showing two discrete regions of additional DNA. When the aphA-7 gene from each plasmid was cloned into pBR322, the aphA-7 gene expressed the kanamycin resistance phenotype in Escherichia coli. For transformants containing the cloned aphA-7 gene, kanamycin MICs were greater than or equal to 128 micrograms/ml. The aphA-7 gene was also subcloned from the plasmid pFKT4420 into the E. coli-Streptococcus shuttle vector pDL278 and was transformed into Streptococcus gordonii Challis. For streptococcal transformants containing the novel plasmid, kanamycin MICs were 4,000 micrograms/ml. In the presence of a tetracycline resistance plasmid, both small plasmids could be mobilized during conjugal matings to Campylobacter coli recipients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Tn1545: a conjugative shuttle transposon. Mol Gen Genet. 1987 Feb;206(2):259–264. doi: 10.1007/BF00333582. [DOI] [PubMed] [Google Scholar]
- Kotarski S. F., Merriwether T. L., Tkalcevic G. T., Gemski P. Genetic studies of kanamycin resistance in Campylobacter jejuni. Antimicrob Agents Chemother. 1986 Aug;30(2):225–230. doi: 10.1128/aac.30.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Lee L. N., Titmas B. M., Smith C. J., Tenover F. C. Nucleotide sequence analysis of tetracycline resistance gene tetO from Streptococcus mutans DL5. J Bacteriol. 1988 Aug;170(8):3618–3626. doi: 10.1128/jb.170.8.3618-3626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette M., Gerbaud G., Lambert T., Courvalin P. Acquisition by a Campylobacter-like strain of aphA-1, a kanamycin resistance determinant from members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1987 Jul;31(7):1021–1026. doi: 10.1128/aac.31.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ounissi H., Derlot E., Carlier C., Courvalin P. Gene homogeneity for aminoglycoside-modifying enzymes in gram-positive cocci. Antimicrob Agents Chemother. 1990 Nov;34(11):2164–2168. doi: 10.1128/aac.34.11.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J. Nucleic acids in the classification of Campylobacters. Eur J Clin Microbiol. 1983 Aug;2(4):367–377. doi: 10.1007/BF02019473. [DOI] [PubMed] [Google Scholar]
- Papadopoulou B., Courvalin P. Dispersal in Campylobacter spp. of aphA-3, a kanamycin resistance determinant from gram-positive cocci. Antimicrob Agents Chemother. 1988 Jun;32(6):945–948. doi: 10.1128/aac.32.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L. The genus Campylobacter: a decade of progress. Clin Microbiol Rev. 1988 Apr;1(2):157–172. doi: 10.1128/cmr.1.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M. J., Castillo J., Martin C., Navarro M., Gomez-Lus R. Aminoglycoside-phosphotransferases APH(3')-IV and APH(3") synthesized by a strain of Campylobacter coli. J Antimicrob Chemother. 1986 Aug;18(2):153–158. doi: 10.1093/jac/18.2.153. [DOI] [PubMed] [Google Scholar]
- Sagara H., Mochizuki A., Okamura N., Nakaya R. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli with special reference to plasmid profiles of Japanese clinical isolates. Antimicrob Agents Chemother. 1987 May;31(5):713–719. doi: 10.1128/aac.31.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Courvalin P. Mechanisms of antibiotic resistance in Campylobacter species. Antimicrob Agents Chemother. 1988 Aug;32(8):1107–1112. doi: 10.1128/aac.32.8.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Bronsdon M. A., Gordon K. P., Plorde J. J. Isolation of plasmids encoding tetracycline resistance from Campylobacter jejuni strains isolated from simians. Antimicrob Agents Chemother. 1983 Feb;23(2):320–322. doi: 10.1128/aac.23.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Elvrum P. M. Detection of two different kanamycin resistance genes in naturally occurring isolates of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1170–1173. doi: 10.1128/aac.32.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Gilbert T., O'Hara P. Nucleotide sequence of a novel kanamycin resistance gene, aphA-7, from Campylobacter jejuni and comparison to other kanamycin phosphotransferase genes. Plasmid. 1989 Jul;22(1):52–58. doi: 10.1016/0147-619x(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Tenover F. C., Williams S., Gordon K. P., Nolan C., Plorde J. J. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1985 Jan;27(1):37–41. doi: 10.1128/aac.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3'5"-aminoglycoside phosphotransferase type III. Gene. 1983 Sep;23(3):331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Gerbaud G., Lambert T., Courvalin P. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 1985 Dec 16;4(13A):3583–3587. doi: 10.1002/j.1460-2075.1985.tb04120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


