Abstract
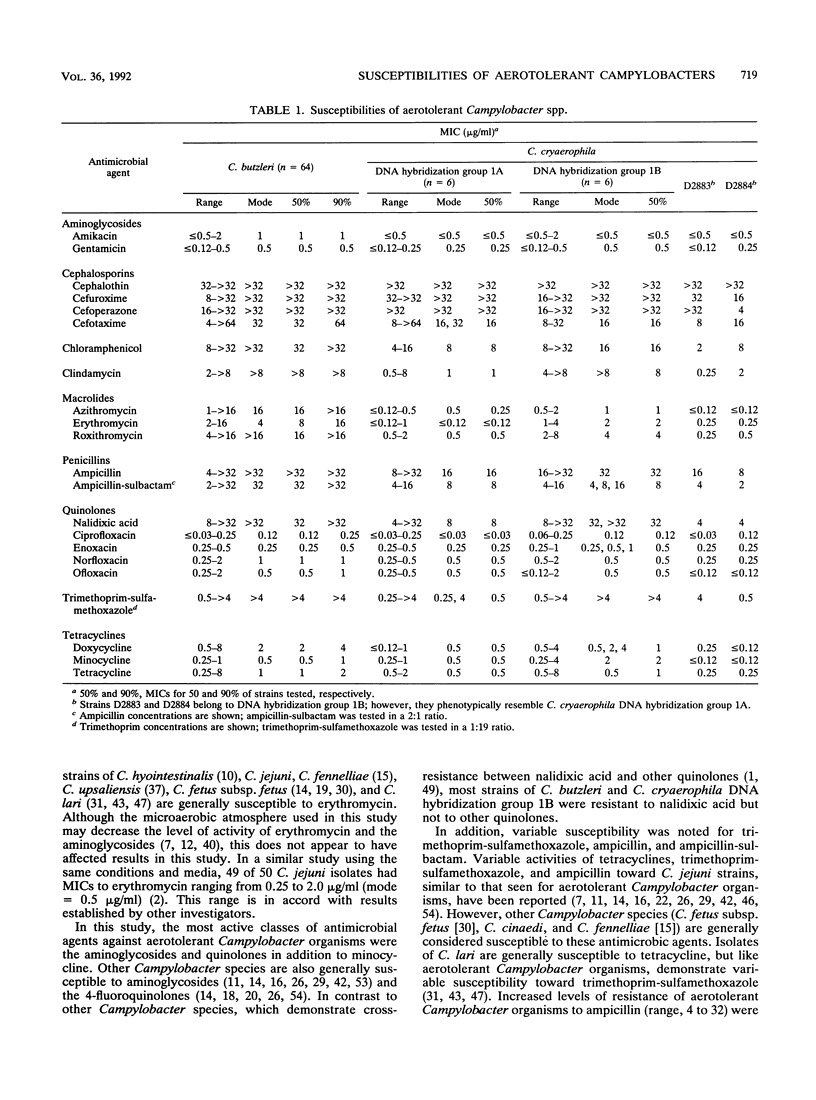
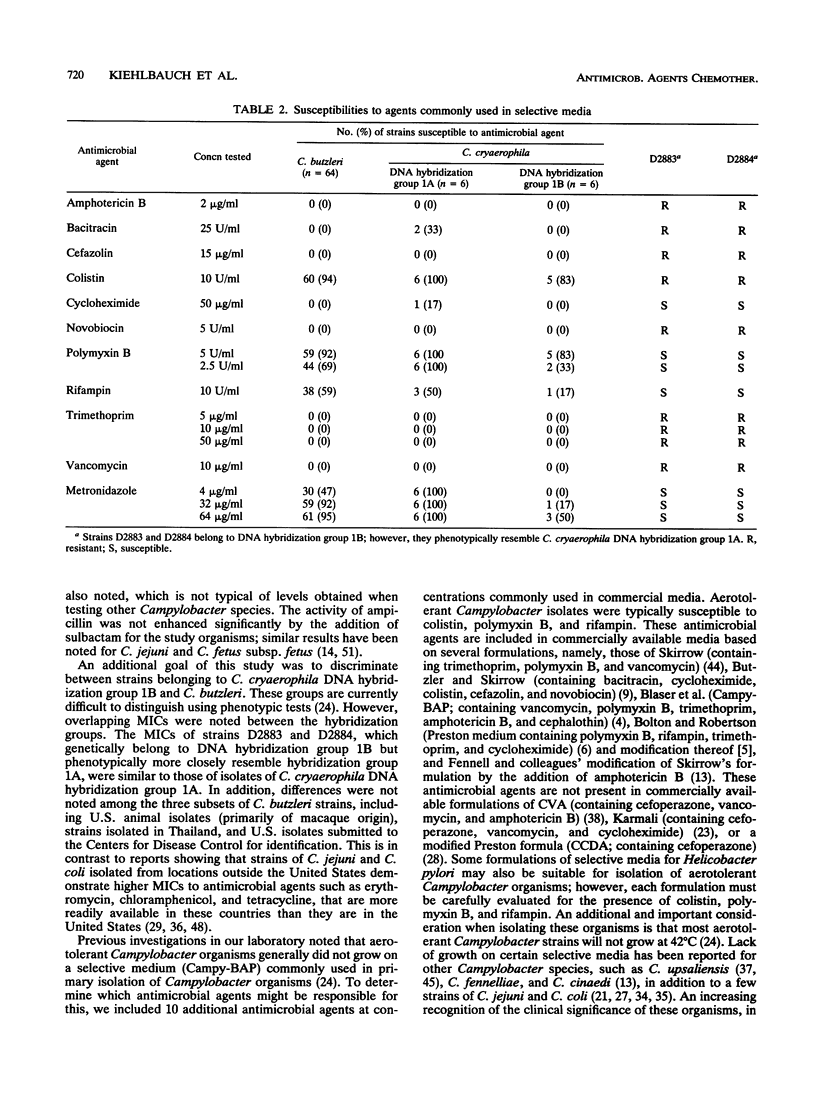
We evaluated the in vitro activities of 22 antimicrobial agents against 78 human and animal isolates belonging to two aerotolerant Campylobacter species, C. cryaerophila and C. butzleri, using a broth microdilution technique. An additional 10 antimicrobial agents were included at concentrations found in selective Campylobacter media. Strains of C. cryaerophila belonged to two DNA hybridization groups: DNA hybridization group 1A, which includes the type strain of C. cryaerophila, and DNA hybridization group 1B. The aminoglycosides, fluoroquinolones, and one tetracycline (minocycline) demonstrated the most activity against all DNA hybridization groups (C. cryaerophila DNA groups 1A and 1B and C. butzleri). Most isolates were resistant to cephalosporin antibiotics, with the exception of cefotaxime, and were variably susceptible to trimethoprim-sulfamethoxazole. C. cryaerophila DNA hybridization group 1A isolates were generally susceptible to the tetracyclines, chloramphenicol, nalidixic acid, azithromycin, erythromycin, and roxithromycin and moderately susceptible to clindamycin, trimethoprim-sulfamethoxazole, ampicillin, and ampicillin-sulbactam. The MICs of tetracyclines were higher for C. butzleri and C. cryaerophila DNA hybridization group 1B isolates than for C. cryaerophila DNA hybridization group 1A isolates, but most strains were still susceptible to doxycycline and tetracycline; all isolates were susceptible to minocycline. C. butzleri and C. cryaerophila DNA hybridization group 1B isolates were generally resistant to the macrolide antibiotics (including erythromycin), chloramphenicol, clindamycin, nalidixic acid, ampicillin, and trimethoprim-sulfamethoxazole. Differences in antimicrobial susceptibility between aerotolerant Campylobacter species and more common Campylobacter species, e.g., C. jejuni, suggest that different treatment strategies may be necessary. Strains of all three DNA hybridization groups of aerotolerant Campylobacter isolates were susceptible to colistin, polymyxin B, and rifampin at concentrations commonly used in selective media. These results suggest that primary isolation methods for Campylobacter species may need to be modified to include aerotolerant Campylobacter strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altwegg M., Burnens A., Zollinger-Iten J., Penner J. L. Problems in identification of Campylobacter jejuni associated with acquisition of resistance to nalidixic acid. J Clin Microbiol. 1987 Sep;25(9):1807–1808. doi: 10.1128/jcm.25.9.1807-1808.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson C. A., Segreti J., Beaudette F. E., Hines D. W., Goodman L. J., Kaplan R. L., Trenholme G. M. In vitro activity of A-56268 (TE-031), a new macrolide, compared with that of erythromycin and clindamycin against selected gram-positive and gram-negative organisms. Antimicrob Agents Chemother. 1987 Feb;31(2):328–330. doi: 10.1128/aac.31.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Berkowitz I. D., LaForce F. M., Cravens J., Reller L. B., Wang W. L. Campylobacter enteritis: clinical and epidemiologic features. Ann Intern Med. 1979 Aug;91(2):179–185. doi: 10.7326/0003-4819-91-2-179. [DOI] [PubMed] [Google Scholar]
- Bolton F. J., Coates D. Development of a blood-free Campylobacter medium: screening tests on basal media and supplements, and the ability of selected supplements to facilitate aerotolerance. J Appl Bacteriol. 1983 Feb;54(1):115–125. doi: 10.1111/j.1365-2672.1983.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Bolton F. J., Robertson L. A selective medium for isolating Campylobacter jejuni/coli. J Clin Pathol. 1982 Apr;35(4):462–467. doi: 10.1136/jcp.35.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. E., Kelly M. T. Susceptibility testing of Campylobacter fetus subsp. jejuni, using broth microdilution panels. Antimicrob Agents Chemother. 1982 Feb;21(2):274–277. doi: 10.1128/aac.21.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge R., Warren C., Phillips I. Macrolide, lincosamide and streptogramin resistance in Campylobacter jejuni/coli. J Antimicrob Chemother. 1986 Mar;17(3):315–321. doi: 10.1093/jac/17.3.315. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Edmonds P., Patton C. M., Griffin P. M., Barrett T. J., Schmid G. P., Baker C. N., Lambert M. A., Brenner D. J. Campylobacter hyointestinalis associated with human gastrointestinal disease in the United States. J Clin Microbiol. 1987 Apr;25(4):685–691. doi: 10.1128/jcm.25.4.685-691.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elharrif Z., Megraud F. Sensibilité de Campylobacter jejuni/coli à onze antibiotiques. Pathol Biol (Paris) 1984 Jun;32(5 Pt 2):536–539. [PubMed] [Google Scholar]
- Fennell C. L., Totten P. A., Quinn T. C., Patton D. L., Holmes K. K., Stamm W. E. Characterization of Campylobacter-like organisms isolated from homosexual men. J Infect Dis. 1984 Jan;149(1):58–66. doi: 10.1093/infdis/149.1.58. [DOI] [PubMed] [Google Scholar]
- Fliegelman R. M., Petrak R. M., Goodman L. J., Segreti J., Trenholme G. M., Kaplan R. L. Comparative in vitro activities of twelve antimicrobial agents against Campylobacter species. Antimicrob Agents Chemother. 1985 Mar;27(3):429–430. doi: 10.1128/aac.27.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores B. M., Fennell C. L., Holmes K. K., Stamm W. E. In vitro susceptibilities of Campylobacter-like organisms to twenty antimicrobial agents. Antimicrob Agents Chemother. 1985 Aug;28(2):188–191. doi: 10.1128/aac.28.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Dzink J. L., Ackerman J. I. Antibiotic sensitivity patterns of Campylobacter jejuni/coli isolated from laboratory animals and pets. Lab Anim Sci. 1984 Jun;34(3):264–267. [PubMed] [Google Scholar]
- Gebhart C. J., Ward G. E., Kurtz H. J. In vitro activities of 47 antimicrobial agents against three Campylobacter spp. from pigs. Antimicrob Agents Chemother. 1985 Jan;27(1):55–59. doi: 10.1128/aac.27.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. J., Fliegelman R. M., Trenholme G. M., Kaplan R. L. Comparative in vitro activity of ciprofloxacin against Campylobacter spp. and other bacterial enteric pathogens. Antimicrob Agents Chemother. 1984 Apr;25(4):504–506. doi: 10.1128/aac.25.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., Coignau H., Vlaes L., Butzler J. P. In-vitro evaluation of antibiotic combinations against Campylobacter fetus. J Antimicrob Chemother. 1989 Aug;24(2):195–201. doi: 10.1093/jac/24.2.195. [DOI] [PubMed] [Google Scholar]
- Goossens H., De Mol P., Coignau H., Levy J., Grados O., Ghysels G., Innocent H., Butzler J. P. Comparative in vitro activities of aztreonam, ciprofloxacin, norfloxacin, ofloxacin, HR 810 (a new cephalosporin), RU28965 (a new macrolide), and other agents against enteropathogens. Antimicrob Agents Chemother. 1985 Mar;27(3):388–392. doi: 10.1128/aac.27.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gun-Munro J., Rennie R. P., Thornley J. H., Richardson H. L., Hodge D., Lynch J. Laboratory and clinical evaluation of isolation media for Campylobacter jejuni. J Clin Microbiol. 1987 Dec;25(12):2274–2277. doi: 10.1128/jcm.25.12.2274-2277.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., De Grandis S., Fleming P. C. Antimicrobial susceptibility of Campylobacter jejuni with special reference to resistance patterns of Canadian isolates. Antimicrob Agents Chemother. 1981 Apr;19(4):593–597. doi: 10.1128/aac.19.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Simor A. E., Roscoe M., Fleming P. C., Smith S. S., Lane J. Evaluation of a blood-free, charcoal-based, selective medium for the isolation of Campylobacter organisms from feces. J Clin Microbiol. 1986 Mar;23(3):456–459. doi: 10.1128/jcm.23.3.456-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehlbauch J. A., Brenner D. J., Nicholson M. A., Baker C. N., Patton C. M., Steigerwalt A. G., Wachsmuth I. K. Campylobacter butzleri sp. nov. isolated from humans and animals with diarrheal illness. J Clin Microbiol. 1991 Feb;29(2):376–385. doi: 10.1128/jcm.29.2.376-385.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Lariviere L. A., Gaudreau C. L., Turgeon F. F. Susceptibility of clinical isolates of Campylobacter jejuni to twenty-five antimicrobial agents. J Antimicrob Chemother. 1986 Dec;18(6):681–685. doi: 10.1093/jac/18.6.681. [DOI] [PubMed] [Google Scholar]
- Lovett J., Francis D. W., Hunt J. M. Isolation of Campylobacter jejuni from raw milk. Appl Environ Microbiol. 1983 Aug;46(2):459–462. doi: 10.1128/aem.46.2.459-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino F. J., Agulla A., Villasante P. A., Díaz A., Saz J. V., Velasco A. C. Comparative efficacy of seven selective media for isolating Campylobacter jejuni. J Clin Microbiol. 1986 Sep;24(3):451–452. doi: 10.1128/jcm.24.3.451-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J., Rogol M., Dickman D. Susceptibility of clinical isolates of Campylobacter jejuni to sixteen antimicrobial agents. Antimicrob Agents Chemother. 1983 May;23(5):796–797. doi: 10.1128/aac.23.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morooka T., Oda T., Shigeoka H. In vitro evaluation of antibiotics for treatment of meningitis caused by Campylobacter fetus subspecies fetus. Pediatr Infect Dis J. 1989 Sep;8(9):653–654. doi: 10.1097/00006454-198909000-00022. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Stowell C., Skalina D., Jones A. M., Hoop R. M., 2nd, Smibert R. M. Campylobacter laridis causing bacteremia in an immunosuppressed patient. Ann Intern Med. 1984 Jul;101(1):55–57. doi: 10.7326/0003-4819-101-1-55. [DOI] [PubMed] [Google Scholar]
- Ng L. K., Stiles M. E., Taylor D. E. Inhibition of Campylobacter coli and Campylobacter jejuni by antibiotics used in selective growth media. J Clin Microbiol. 1985 Oct;22(4):510–514. doi: 10.1128/jcm.22.4.510-514.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L. K., Taylor D. E., Stiles M. E. Characterization of freshly isolated Campylobacter coli strains and suitability of selective media for their growth. J Clin Microbiol. 1988 Mar;26(3):518–523. doi: 10.1128/jcm.26.3.518-523.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Maldonado N. R., Morales-De la Vega A., Resano-Pérez F., Landa L. Isolation of Campylobacter fetus ss jejuni in adult patients with diarrheal syndrome. Arch Invest Med (Mex) 1984 Jul-Sep;15(3):245–253. [PubMed] [Google Scholar]
- Patton C. M., Shaffer N., Edmonds P., Barrett T. J., Lambert M. A., Baker C., Perlman D. M., Brenner D. J. Human disease associated with "Campylobacter upsaliensis" (catalase-negative or weakly positive Campylobacter species) in the United States. J Clin Microbiol. 1989 Jan;27(1):66–73. doi: 10.1128/jcm.27.1.66-73.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E., Schoenknecht F. Effect of several components of anaerobic incubation on antibiotic susceptibility test results. Antimicrob Agents Chemother. 1972 May;1(5):433–440. doi: 10.1128/aac.1.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker S., Sorrell T. C. Susceptibility of Campylobacter jejuni to twenty-three antimicrobial agents. Pathology. 1983 Jan;15(1):61–63. doi: 10.3109/00313028309061403. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Wilcox L. Enteritis associated with Campylobacter laridis. J Clin Microbiol. 1987 Jan;25(1):10–12. doi: 10.1128/jcm.25.1.10-12.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. W., Sangster N., Lanser J. A. DNA relatedness and biochemical features of Campylobacter spp. isolated in central and South Australia. J Clin Microbiol. 1985 Jul;22(1):71–74. doi: 10.1128/jcm.22.1.71-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedhem A., Kaijser B., Sjögren E. Antimicrobial susceptibility of Campylobacter jejuni isolated from humans with diarrhoea and from healthy chickens. J Antimicrob Chemother. 1981 Mar;7(3):301–305. doi: 10.1093/jac/7.3.301. [DOI] [PubMed] [Google Scholar]
- Tauxe R. V., Patton C. M., Edmonds P., Barrett T. J., Brenner D. J., Blake P. A. Illness associated with Campylobacter laridis, a newly recognized Campylobacter species. J Clin Microbiol. 1985 Feb;21(2):222–225. doi: 10.1128/jcm.21.2.222-225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Ng L. K., Lior H. Susceptibility of Campylobacter species to nalidixic acid, enoxacin, and other DNA gyrase inhibitors. Antimicrob Agents Chemother. 1985 Nov;28(5):708–710. doi: 10.1128/aac.28.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. N., Blaser M. J., Echeverria P., Pitarangsi C., Bodhidatta L., Wang W. L. Erythromycin-resistant Campylobacter infections in Thailand. Antimicrob Agents Chemother. 1987 Mar;31(3):438–442. doi: 10.1128/aac.31.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Swenson J. M., Baker C. N., McDougal L. K., Stocker S. A., Hill B. C. Methods for determining susceptibility of fastidious and unusual pathogens to selected antimicrobial agents. Diagn Microbiol Infect Dis. 1988 Mar;9(3):139–153. doi: 10.1016/0732-8893(88)90024-7. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Scorneaux B. In vitro susceptibility of Campylobacter jejuni to 27 antimicrobial agents and various combinations of beta-lactams with clavulanic acid or sulbactam. Antimicrob Agents Chemother. 1985 Jul;28(1):37–40. doi: 10.1128/aac.28.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandamme P., Falsen E., Rossau R., Hoste B., Segers P., Tytgat R., De Ley J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int J Syst Bacteriol. 1991 Jan;41(1):88–103. doi: 10.1099/00207713-41-1-88. [DOI] [PubMed] [Google Scholar]
- Vanhoof R., Gordts B., Dierickx R., Coignau H., Butzler J. P. Bacteriostatic and bactericidal activities of 24 antimicrobial agents against Campylobacter fetus subsp. jejuni. Antimicrob Agents Chemother. 1980 Jul;18(1):118–121. doi: 10.1128/aac.18.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoof R., Hubrechts J. M., Roebben E., Nyssen H. J., Nulens E., Leger J., De Schepper N. The comparative activity of pefloxacin, enoxacin, ciprofloxacin and 13 other antimicrobial agents against enteropathogenic microorganisms. Infection. 1986 Nov-Dec;14(6):294–298. doi: 10.1007/BF01643966. [DOI] [PubMed] [Google Scholar]
- Wang W. L., Reller L. B., Blaser M. J. Comparison of antimicrobial susceptibility patterns of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1984 Sep;26(3):351–353. doi: 10.1128/aac.26.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]


