Abstract
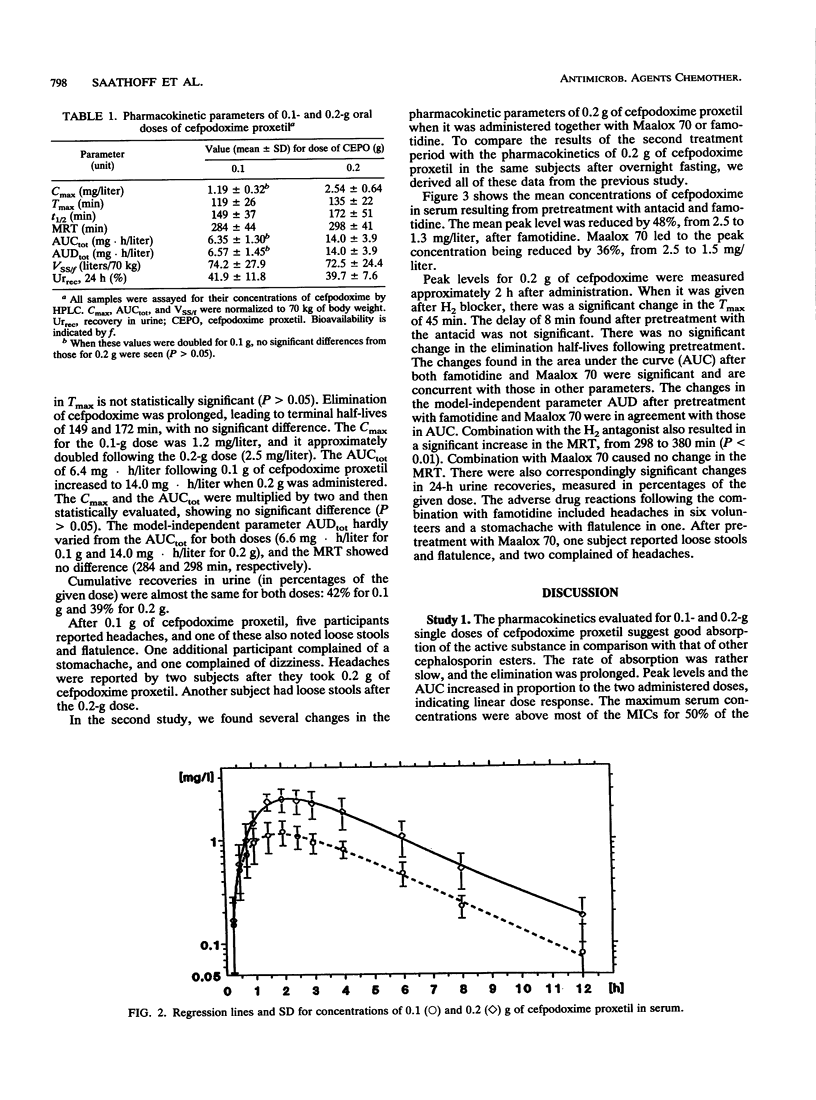
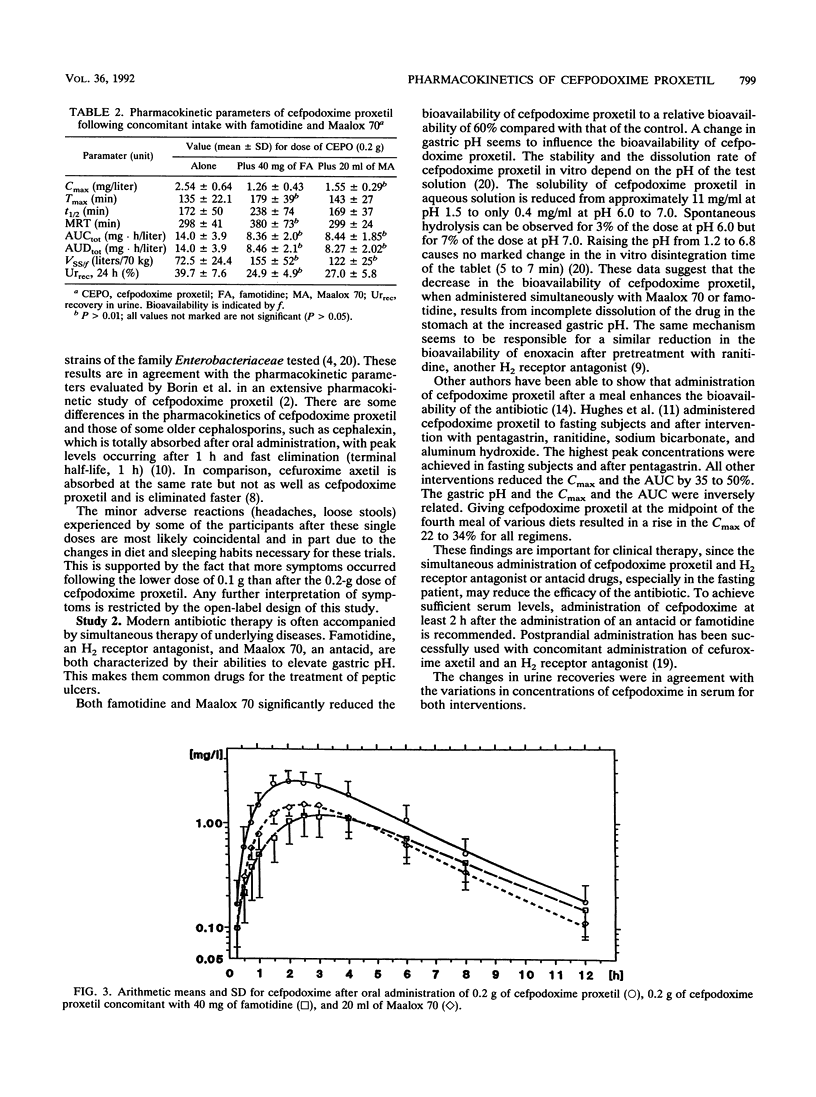
Cefpodoxime proxetil is a new oral esterified cephem antibiotic with a broad antibacterial spectrum. The dissolution of cefpodoxime proxetil is pH dependent. The objectives of this study were to characterize the pharmacokinetics of cefpodoxime proxetil in two different oral doses and to examine possible interactions with an antacid, aluminum magnesium hydroxide (Maalox 70), and an H2 receptor antagonist, famotidine. Two studies involving the same 10 healthy volunteers were performed. In the first study, cefpodoxime proxetil was administered in two doses, 0.1 and 0.2 g. In the second study, two interventions were performed in a randomized crossover design. For one intervention, the volunteers were pretreated with 40 mg of famotidine 1 h before 0.2 g of cefpodoxime proxetil was administered. In the second trial, participants were given 10 ml of Maalox 70 2 h and 10 ml of Maalox 70 15 min before they received 0.2 g of cefpodoxime proxetil. Serum and urine concentrations were determined by high-performance liquid chromatography. For the statistical evaluation, these data were tested by using the pharmacokinetics of 0.2 g of cefpodoxime proxetil from the first study. The maximum concentrations were 1.19 +/- 0.32 mg/liter after 0.1 g of cefpodoxime proxetil and 2.54 +/- 0.64 mg/liter after 0.2 g of cefpodoxime proxetil. The elimination half-lives were 149 min for 0.1 g and 172 min for 0.2 g of cefpodoxime proxetil. The total increase in the area under the concentration-time curve (AUC) was dose dependent. Combination with Maalox 70 caused a reduction in the AUC from 14.0 +/- 3.9 to 8.44 +/- 1.85 mg.h/liter. After famotidine, the AUC decreased to 8.36 +/- 2.0 mg . h/liter. Corresponding changes were registered for the maximum concentration of drug in serum, 24-h urine recovery, and the time to maximum concentration of drug serum. Cefpodoxime proxetil was well tolerated without any seriously adverse drug reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borin M. T., Hughes G. S., Spillers C. R., Patel R. K. Pharmacokinetics of cefpodoxime in plasma and skin blister fluid following oral dosing of cefpodoxime proxetil. Antimicrob Agents Chemother. 1990 Jun;34(6):1094–1099. doi: 10.1128/aac.34.6.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. In vitro activity of an oral iminomethoxy aminothiazolyl cephalosporin, R-3746. Antimicrob Agents Chemother. 1988 May;32(5):671–677. doi: 10.1128/aac.32.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppermann K. M., Lode H., Höffken G., Tschink G., Kalz C., Koeppe P. Influence of ranitidine, pirenzepine, and aluminum magnesium hydroxide on the bioavailability of various antibiotics, including amoxicillin, cephalexin, doxycycline, and amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1989 Nov;33(11):1901–1907. doi: 10.1128/aac.33.11.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro activity of U-76,252 (CS-807), a new oral cephalosporin. Antimicrob Agents Chemother. 1988 Jul;32(7):1082–1085. doi: 10.1128/aac.32.7.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K., Ishihara S., Yanagisawa H., Ide J., Nakayama E., Nakao H., Sugawara S., Iwata M. Studies on orally active cephalosporin esters. J Antibiot (Tokyo) 1987 Mar;40(3):370–384. doi: 10.7164/antibiotics.40.370. [DOI] [PubMed] [Google Scholar]
- Ginsburg C. M., McCracken G. H., Jr, Petruska M., Olson K. Pharmacokinetics and bactericidal activity of cefuroxime axetil. Antimicrob Agents Chemother. 1985 Oct;28(4):504–507. doi: 10.1128/aac.28.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasela T. H., Jr, Schentag J. J., Sedman A. J., Wilton J. H., Thomas D. J., Schultz R. W., Lebsack M. E., Kinkel A. W. Inhibition of enoxacin absorption by antacids or ranitidine. Antimicrob Agents Chemother. 1989 May;33(5):615–617. doi: 10.1128/aac.33.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith R. S. The pharmacology of cephalexin. Postgrad Med J. 1983;59 (Suppl 5):16–27. [PubMed] [Google Scholar]
- Hughes G. S., Heald D. L., Barker K. B., Patel R. K., Spillers C. R., Watts K. C., Batts D. H., Euler A. R. The effects of gastric pH and food on the pharmacokinetics of a new oral cephalosporin, cefpodoxime proxetil. Clin Pharmacol Ther. 1989 Dec;46(6):674–685. doi: 10.1038/clpt.1989.204. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Antimicrobial activity and disk diffusion susceptibility testing of U-76,253A (R-3746), the active metabolite of the new cephalosporin ester, U-76,252 (CS-807). Antimicrob Agents Chemother. 1988 Apr;32(4):443–449. doi: 10.1128/aac.32.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp C. C., Sierra-Madero J., Washington J. A. Antibacterial activities of cefpodoxime, cefixime, and ceftriaxone. Antimicrob Agents Chemother. 1988 Dec;32(12):1896–1898. doi: 10.1128/aac.32.12.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langtry H. D., Grant S. M., Goa K. L. Famotidine. An updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in peptic ulcer disease and other allied diseases. Drugs. 1989 Oct;38(4):551–590. doi: 10.2165/00003495-198938040-00005. [DOI] [PubMed] [Google Scholar]
- Peck C. C., Sheiner L. B., Nichols A. I. The problem of choosing weights in nonlinear regression analysis of pharmacokinetic data. Drug Metab Rev. 1984;15(1-2):133–148. doi: 10.3109/03602538409015060. [DOI] [PubMed] [Google Scholar]
- Sommers D. K., van Wyk M., Moncrieff J., Schoeman H. S. Influence of food and reduced gastric acidity on the bioavailability of bacampicillin and cefuroxime axetil. Br J Clin Pharmacol. 1984 Oct;18(4):535–539. doi: 10.1111/j.1365-2125.1984.tb02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of CS-807, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Jul;31(7):1085–1092. doi: 10.1128/aac.31.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]


