Abstract
Murine B-cell development begins in bone marrow and results in the generation of immature transitional B cells that transit to the spleen to complete their maturation. It remains unclear whether the same developmental pathway takes place in humans. Using markers characteristic of human bone marrow immature B cells, we have identified a population of circulating human B cells with a phenotype most similar to mouse transitional type I (T1) B cells, although these human counterparts express CD5. These cells die rapidly in culture, and B-cell activation factor member of the tumor necrosis factor (TNF) family (BAFF) does not effect their survival regardless of B-cell receptor (BCR) stimulation. In contrast, bone marrow stromal cells or interleukin-4 (IL-4) significantly enhanced their survival. In the presence of T-cell signals provided by IL-4 or CD40 ligation, BCR stimulation can induce progression into cell cycle. Interestingly, circulating B cells that phenotypically and functionally resemble murine T2 B cells are found in cord blood and adult peripheral blood, suggesting that B-cell maturation may not be restricted to the spleen. Notably, increased proportions of T1 B cells were found in blood of patients with systemic lupus erythematosus (SLE), although bone marrow production and selection appeared to be normal.
Introduction
Primary B-cell development takes place in the bone marrow, where immature B cells must generate a functional B-cell receptor (BCR) and overcome negative selection induced by reactivity with autoantigens.1-3 In mice, approximately 10% of immature B cells survive these processes and emerge from the bone marrow expressing surface immunoglobulin M (IgM) and IgD.4 These immature “transitional” B cells transit to the spleen where they mature to become responsive to BCR-mediated signaling.5-9 Only a fraction of cells survive this process of secondary (peripheral) B-cell development.10,11 Cell surface markers have been identified that enable different stages of peripheral B-cell maturation to be characterized. In mice, heat-stable antigen (HSA, CD24) was used to distinguish immature (CD24hi) from mature (CD24lo) splenic B cells.12 Additional cell surface markers have been used to subdivide murine splenic transitional B cells into 2 distinct populations; transitional type 1 (T1; CD24hi, CD21lo, CD23lo, IgMhi, IgDlo) and transitional T2 (CD24hi, CD21hi, CD23hi, IgMhi, IgDhi).5 An alternative classification defines 3 types of transitional B cells using reactivity to a C1q receptor related protein to identify immature B cells and CD23 and IgM to distinguish T1 (CD23-, IgMhi), T2 (CD23+, IgMhi), and T3 (CD23+, IgMlo).7,11 Importantly, T1 B cells are found in the bone marrow, blood, and spleen, but not lymph nodes, whereas T2 B cells are restricted to the spleen. Moreover, in vivo transfer experiments demonstrate that T1 cells give rise to T2 cells and B cells with a mature phenotype.5,8 Most studies demonstrate that T2 cells increase in size, up-regulate survival signals, proliferate, and differentiate upon BCR ligation, whereas T1 cells die.5,9,13 Finally, B-cell activation factor member of the tumor necrosis factor (TNF) family (BAFF) plays an integral role in the survival and maturation of murine B cells, although it remains unclear if it affects T1 or T2 B cells.14
Most of the knowledge on primary and peripheral B-cell development is derived from mice. Many aspects of development in bone marrow appear similar in humans; however, our understanding of peripheral B-cell development is remarkably limited. As a first step in a systematic approach to address this issue, we have identified and characterized the phenotype and function of human transitional B cells from peripheral blood. Human transitional T1 B cells show some similarities, but some significant differences compared with their counterparts in murine spleen. Moreover, the presence of circulating B cells that resemble T2 B cells with respect to both phenotype and function suggests that human B-cell maturation may not be restricted to the spleen. Finally, we found increased proportions of T1 B cells in patients with systemic lupus erythematosus (SLE) that reflected peripheral lymphopenia rather than abnormalities in bone marrow production or selection in these patients with autoimmune disease.
Materials and methods
Clinical samples
Blood samples were obtained from healthy adult donors of various ages, sexes, and races, and from 19 patients with SLE. At the time of collection, one patient was untreated with active lupus; the others were treated with low-dose glucocorticoids with or without hydroxychloroquine and had SLE Disease Activity Index (SLEDAI) scores of 0-18. Bone marrow aspirates were obtained from healthy donors. Tonsil samples were obtained from individuals undergoing routine tonsillectomy. Cord blood was obtained from Advanced Bioscience Resources (Alameda, CA). Approval was obtained from the National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institute of Diabetes & Digestive & Kidney Diseases (NIAMS/NIDDK) Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki.
B-cell enrichment, flow cytometry, and cell sorting
B cells were enriched by negative selection from buffy coats or leukapheresis samples using RosetteSep or StemSep B cell purification antibody cocktails (Stem Cell Technology, Vancouver, BC, Canada). Peripheral blood mononuclear cells or enriched B cells were stained with various monoclonal antibody (mAb) combinations for 20 minutes on ice in staining buffer (1% bovine serum albumin [BSA], 5% fetal calf serum [FCS] in phosphate-buffered saline [PBS]). The directly conjugated antibodies used were anti-IgD–fluoroscein isothiocyanate (FITC), anti-IgD–phycoerythrin (PE), anti-kappa–FITC, anti-lambda–PE, anti-CD10–PE, anti-CD10–allophycocyanin (APC), anti-CD27–PE, anti-CD5–FITC, anti-CD5–PE, anti-CD20–FITC, anti-CD20–PeCy5 (phycoerythrin-cyanin 5), anti-CD27–PE, anti-CD40–PE, anti-CD95–PE, anti-CD69–PE, anti-CD43–FITC, anti-CD44–APC, anti-CD62L–PE, anti-CD11b–PE, anti-CD124–PE, anti-CD80–FITC, anti-CD86–FITC (Becton Dickinson Pharmingen, San Diego, CA), anti-CD19–PerCpCy5.5, anti-CD9–PE, anti-CD38–PE, anti-CD38–APC (clone HB7), anti-CD23–PE, anti-CD11b–APC, anti–mouse B220–APC (Becton Dickinson Immunocytometry, San Jose, CA), anti-IgM–PE and anti-CD32–PE (Serotec, Raleigh, NC), anti-CD24–PE, anti-CD22–PE and polyclonal anti-IgA–FITC (Caltag Laboratories, Burlingham, CA), anti-CD21–FITC, and rat IgM anti-CD77 was detected with goat anti–rat IgM–PE (Immunotech, Marseille, France). Anti-TACI (transmembrane activator and CAML-interactor)–biotin, anti-BCMA (B-cell maturation antigen)–biotin, unconjugated goat anti-BR3 (R&D Systems, Minneapolis, MN) and anti-IL6R–biotin (Bender Medsystems, San Bruno, CA) staining was detected with Streptavidin-PE, FITC, or APC (Southern Biotechnology Associates, Birmingham, AL) or rat anti-goat–FITC (Jackson ImmunoResearch, West Grove, PA). Annexin V–FITC (Becton Dickinson Pharmingen) and 7-aminoactinomycin (7-AAD; Molecular Probes, Eugene, OR) were used to assess apoptosis and cell death. Stained cells were washed and data were collected immediately using a 4-color FACScalibur (Becton Dickinson Immunocytometry) or fixed in 1% paraformaldehye and analyzed within 24 hours. Data were analyzed using FlowJo software (TreeStar, Stanford University, CA). B-cell populations were sorted using the Dako Cytomation MoFlo (Fort Collins, CO).
Tissue culture and cell-cycle analysis
B cell populations were cultured in 96-well plates at 5 × 105 cells/mL in 100 μL volumes of culture medium (10% FCS in RPMI medium with l-glutamine, penicllin-streptomycin) alone or supplemented with various cytokines and stimuli; IL-2 (10 ng/mL; Biological Research Branch, National Cancer Institute (NCI)–Frederick, MD), IL-3 (200 ng/mL), IL-4 (100 ng/mL), IL-5 (200 ng/mL), IL-7 (200 ng/mL), IL-10 (200 ng/mL), and anti-CD40 (1 μg/mL) (R&D Systems), BAFF (200 ng/mL; Apotech, San Diego, CA), goat F(ab′)2 anti-IgM (10 μg/mL; Jackson ImmunoReseach). B cells were also cultured with semiconfluent S13 mouse bone marrow stromal cells (a gift from Dr Virginia Gulino, National Cancer Institute, Bethedsa, MD). The caspase inhibitor z-VAD was used at 20μM (EMD Biosciences, San Diego, CA). Cell-cycle analysis was performed using 0.05 mg/mL propidium iodide in hypotonic sodium citrate15 with 1800 U/mL ribonuclease A (Worthington Biochemical Corp, Lakewood, NJ).
Examination of Ig heavy-chain gene sequences
Single-cell polymerase chain reaction (PCR) was used to amplify rear-ranged heavy-chain Ig genes from genomic DNA. Briefly, single cells were lysed with proteinase K, and subjected to a linear-preamplification step using random primers.16 An aliquot of this reaction was used in 2 rounds of PCR. In the first round, primers were targeted to VH3 (5′-CCATGGAGTTKGGGCTGAG-3′) and VH4 (5′-GAAACACCTGTGGTTCTTCCC-3′) leader sequences, and an intronic consensus site downstream of the JH genes (5′-ACCTGAGGAGACGGTGAC-3′). In the second, primers were targeted to the leader/FRW1 of VH3 (5′-GTCCAGTGTSAGGTGCAGC-3′) or VH4 (5′-GGTGCAGCTGCAGGAGTCG-3′) genes, and JH1/2 (5′-TGAGGAGACGGTGACCAGGGTGC-3′), JH3 (5′-TGAAGAGACGGTGACCATTGTCCC-3′), JH4/5 (3′-TGAGGAGACGGTGACCAGGGTTCC-3′) and JH6 (5′-TGAGGAGACGGTGACCGTGGTCC-3′) gene segments. PCR products were directly sequenced using Big Dye Terminator v1.1 Cycle sequencing kit with a 3100 capillary sequencer (Applied Biosystems, Foster City, CA). Sequences were compared to germline immunoglobulin genes using the web-based immunoglobulin V-gene analysis program JoinSolver (http://joinsolver.niams.nih.gov)17 to examine CDR3 characteristics and the frequency of VH mutations.
Results
Identification of immature B-cell markers
We initially examined human bone marrow to identify B-cell markers that would be useful in the characterization of the earliest bone marrow emigrants. Immature CD19+, CD21-, CD23- B cells were found to be CD10+, CD38hi, CD24hi and CD44lo compared with other bone marrow B-cell populations (Figure 1A). Consistent with the expression profile of mature circulating B cells, the majority of CD19+ CD38neg/lo bone marrow B cells were CD20+, IgM+, and IgD+ (Figure 1B). CD38hi immature B cells expressed variable amounts of CD20 and IgM consistent with an increase in expression of these markers during early B-cell development (Figure 1B). Essentially all CD20hi, CD38hi B cells expressed IgM and IgD, indicating that these cells had completed bone marrow maturation. The profile of IgD and CD38 staining and the somewhat reduced CD10 expression also suggested that IgD+ CD38hi immature B cells were immediately derived from IgD- CD38hi pre/pro B cells (Figure 1C). Interestingly, expression of IgD is also associated with expression of CD5 by these immature B cells (Figure 1C). Importantly, a similar profile of immature markers was also found on a subset of CD21lo and CD23- cord blood B cells, a population composed almost entirely of immature/naive B cells (Figure 1D).
Figure 1.
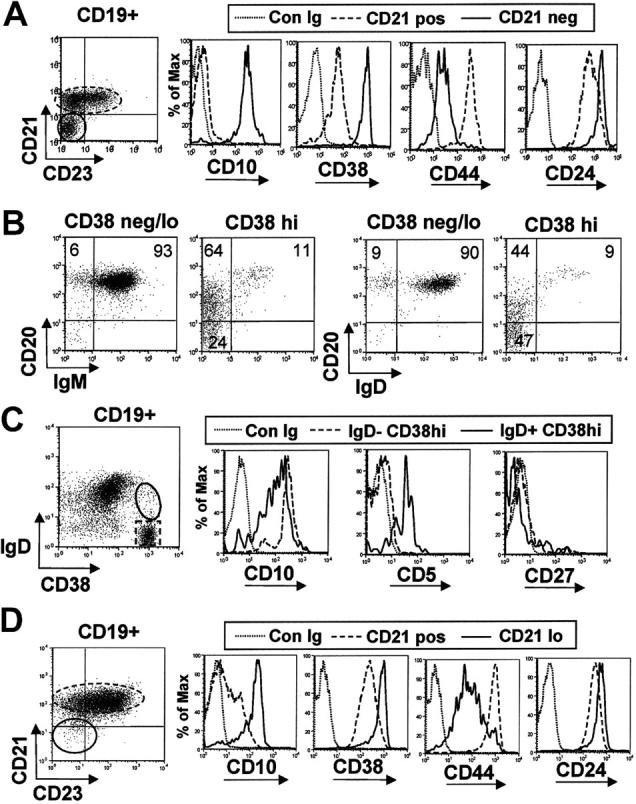
Immature B-cell markers expressed by adult bone marrow and term cord-blood B cells. (A) Bone marrow CD19+ B cells were gated into CD21- immature and CD21+ mature fractions and the differential expression of CD10, CD38, CD44, and CD24 was assessed relative to isotype matched control mAb (Con Ig). (B) Bone marrow CD19+ B cells were gated into CD38neg/lo mature B cells and a CD38hi immature subset. A fraction of the latter express CD20 more densely and both IgM and IgD. (C) Differential expression of CD5 and CD10 by bone marrow IgD- CD38hi pro/pre B cells and IgD+ CD38hi immature B cells. (D) Cord blood CD19+ B cells were gated into CD21neg/lo and CD21+ fractions, and the differential expression of immature bone marrow markers was assessed. Results are representative of 2 bone marrow aspirates and 10 cord blood samples.
A small population of peripheral B cells expresses a T1 B-cell phenotype
Immature B cells were sought in adult peripheral blood initially by using 2 of the markers characteristic of bone marrow and cord blood immature B cells, namely the expression of IgD and the increased expression of CD38 (Figure 2). The phenotype of IgD+ CD38hi B cells from peripheral blood of healthy volunteers was consistent with expression of markers found on immature B cells from bone marrow and cord blood; they were CD10+, CD44lo, and CD24hi relative to mature naive B cells (Figure 2). These immature B cells were also CD21lo, CD23lo, IgMhi, and CD62Llo (Figure 2), a phenotype that is consistent with mouse transitional type 1 (T1) B cells. A variety of other markers were also examined. Relative to naive B cells, these putative T1 B cells expressed lower levels of CD40, CD22, CD124, and BR3. They were also negative or expressed a low density of CD27, CD77, CD80, CD86, CD69, CD11b, CD95, TACI, and BCMA, and a moderate density of CD19, B220, and CD32. These cells also expressed increased densities of CD20, CD5, CD43, and CD9 (a marker also expressed on immature bone marrow B cells; data not shown) relative to naive B cells, and kappa or lambda light chains. With the notable exception of CD5 and CD43, the phenotype of these peripheral immature B cells was congruent with that of mouse splenic T1 B cells.
Figure 2.
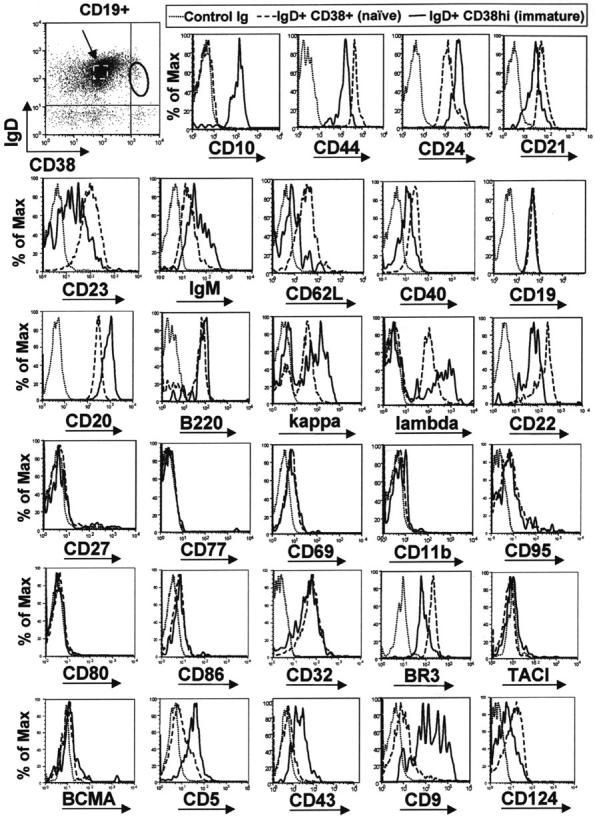
Circulating IgD+ CD38hi B cells express immature B-cell markers with an overall phenotype similar to transitional type I (T1) B cells. B cells were enriched from blood of healthy adult donors and stained with anti-CD19, anti-IgD, anti-CD38, and a fourth anti–human mAb. The differential expression of a variety of B-cell markers was compared between the IgD+ CD38hi immature population (solid line) and IgD+ CD38+ mature naive B cells (dashed line, indicated with arrow). Results are representative of data from at least 4 healthy adult donors.
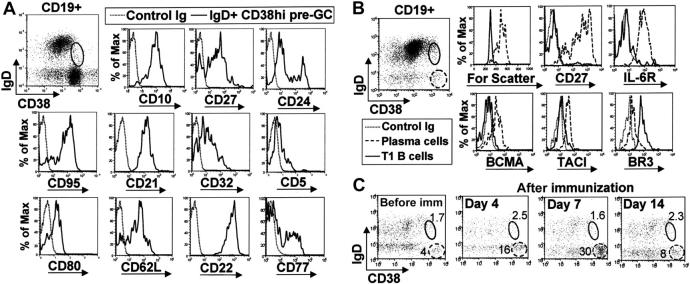
Circulating IgD+ CD38hi B cells are not pre–germinal-center B cells or plasmablasts
A small population of tonsil IgD+ B cells express a high density of CD38 and are thought to be pre–germinal center (pre-GC) B cells.18 Notably, we found that CD38 expression on tonsilar pre-GC B cells is somewhat less than on peripheral T1 B cells (Figure 3A). CD10 was expressed at similar levels on both T1 B cells and tonsil pre-GC B cells, but there were significant differences with respect to many other markers. Tonsil pre-GC B cells (and GC B cells) were mostly CD27+, CD24lo, CD95+, CD21hi, CD32lo, CD5-, CD80+, CD62Lhi, and CD22hi; 33% of these cells also expressed the GC-specific marker CD77 (Figure 3A). This is in contrast to circulating IgD+ CD38hi T1 B cells that were clearly CD24hi, CD27-, CD95lo, CD21lo, CD32hi, CD5+, CD80-, CD62Llo, CD22lo, and CD77- (Figure 2).
Figure 3.
Peripheral-blood IgD+ CD38hi T1 B cells are not circulating pre–germinal-center or plasma-cell precursors. (A) Tonsil mononuclear cells were stained with anti-CD19, anti-IgD, anti-CD38, and a fourth mAb to examine the phenotype of IgD+ CD38hi pre–germinal center B cells. (B) Enriched B cells from peripheral blood of a healthy donor were similarly stained to examine the expression of plasma cell markers on CD19+ IgD+ CD38hi T1 B cells (solid gate) and CD19lo IgD- CD38bright circulating plasma cells (dashed gate). (C) The relative frequencies of the CD19+ IgD+ CD38hi T1 B-cell population (solid gate) and CD19lo IgD- CD38bright plasma cells (dashed gate) from a healthy donor were examined before immunization and 4, 7, and 14 days after immunization with influenza vaccine.
Since T1 B cells and circulating plasma cells express high levels of CD38, we examined the expression of plasma cell markers to ensure that these IgD+ CD38hi cells are not plasma cells expressing surface IgD. T1 B cells are small and do not express CD27 or IL-6R, little or no BCMA and TACI, and significantly less CD38 and BR3 compared with peripheral circulating plasma cells (Figure 3B). Moreover, T1 B cells were CD20hi and expressed normal levels of CD19 (Figure 2), whereas both these markers were down-regulated on plasma cells. Finally, the frequency of peripheral IgD+ CD38hi T1 immature B cells did not appear to alter appreciably following immunization with influenza vaccine (in alum), whereas there was a significant increase in the proportion of peripheral plasma cells (Figure 3C). Together these results confirm that peripheral blood IgD+ CD38hi B cells are not pre-GC or plasma cells, but are likely to be the human equivalent of murine T1 B cells.
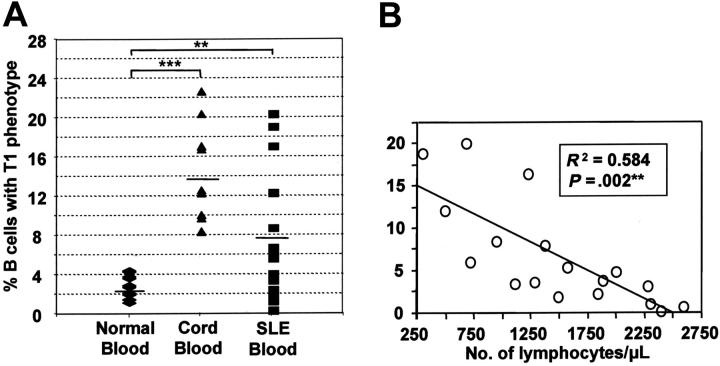
Increased frequencies of immature T1 B cells are found in cord blood and the peripheral blood of SLE patients
Since B-cell populations are perturbed in SLE patients, we examined the frequency of T1 B cells in patients' blood. SLE patients had significantly higher proportion of T1 B cells compared with healthy controls (6.7% ± 1.8 versus 2.2% ± 0.4; mean % ± SEM). The highest proportions of T1 B cells were found in cord blood (13.6 ± 4.3; Figure 4A). The T1 B-cell phenotype from each of these sources was equivalent. Among the SLE patients, the proportion of immature T1 B cells did not correlate with disease activity (SLEDAI) or other disease-associated factors such as autoantibody levels, frequency of circulating plasma cells, erythrocyte sedimentation rate, and levels of circulating complement or C-reactive protein. However, there was an inverse correlation with the total lymphocyte count (R2 = 0.584, P = .002; Figure 4B). As a result, the absolute numbers of T1 B cells were similar, or only occasionally elevated, compared with healthy controls.
Figure 4.
T1 B cells are found in increased proportions in term cord blood and systemic lupus erythematosus patients. (A) The relative frequency of T1 B cells was determined for term cord blood (n=10), and the peripheral blood of healthy adult donors (n=29) and patients with systemic lupus erythematosus (SLE; n=18). Horizontal bars indicate means. Significant differences using the nonparametric Mann-Whitney U test were detected at P <.01 (**) and P <.001(***). (B) A linear regression plot shows that the frequency of B cells with a T1 B-cell phenotype is inversely proportional to the absolute number of peripheral lymphocytes.
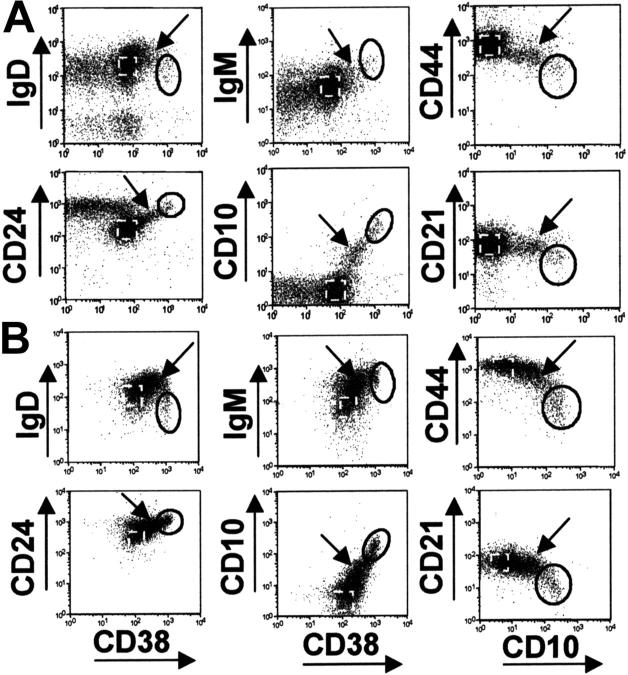
Gradation of expression of markers from T1 B cells to mature naive B cells
Cell surface staining also revealed that there was a continuum in the expression of the immature and transitional B-cell markers between T1 B cells and mature naive B cells in normal peripheral blood and cord blood (Figure 5). “Intermediate” B cells expressed higher levels of CD38, CD24, CD10, and IgM compared with naive B cells but had similar levels of CD21. Interestingly, these intermediate B cells expressed increased levels of IgD and an overall phenotype that resembled the phenotype of murine T2 B cells. These cells were more prominent in cord blood than normal peripheral blood, and were relatively infrequent in bone marrow.
Figure 5.
“Intermediate” B cells are present in cord and peripheral blood. B cells from (A) peripheral and (B) cord blood were stained with various combinations of immature and T1 B-cell markers. The T1 population (solid gate), naive B cells (dashed gate), and “intermediate B cells” (arrow) are indicated.
Survival of human T1 B cells was improved by IL-4 and stromal cells but not BAFF
Two independent sorting strategies were devised to isolate peripheral T1 B cells, naive mature B cells, and “intermediate” B cells, without using antibodies against IgD or IgM that could potentially stimulate the cells through the BCR (Figure 6A). In the absence of further stimulation, forward/side scatter characteristics and Annexin V/7-AAD staining demonstrated that the majority T1 B cells were dead or dying following 24 hours in culture, whereas mature B cells were mostly viable (Figure 6B). The pan-caspase inhibitor z-VAD could not block this rapid cell death (data not shown). Various culture conditions were examined to determine whether survival of T1 B cells could be enhanced. Soluble BAFF had no significant effect on survival of either T1 B cells, intermediate, or mature B cells after 24 hours in culture, and only modest improvements in cell viability were apparent after 3 days (Figure 6C). Notably, however, 200 ng/mL of BAFF was sufficient to improve viability of both peripheral and splenic B cells, and increase Ig production (Figure S1; see the Supplemental Figure link at the top of the online article on the Blood website). IL-4 significantly improved survival of both T1 and intermediate and mature B cells (Figure 4C), whereas IL-2, IL-3, IL-7, and IL-10 had no effect on survival of T1 B cells (data not shown). Interestingly, the viability of T1 B cells was dramatically improved when cultured with bone marrow stromal cells. This action was probably mediated through cell-to-cell contact since stromal supernatants were unable to maintain the viability of these cells (data not shown).
Figure 6.
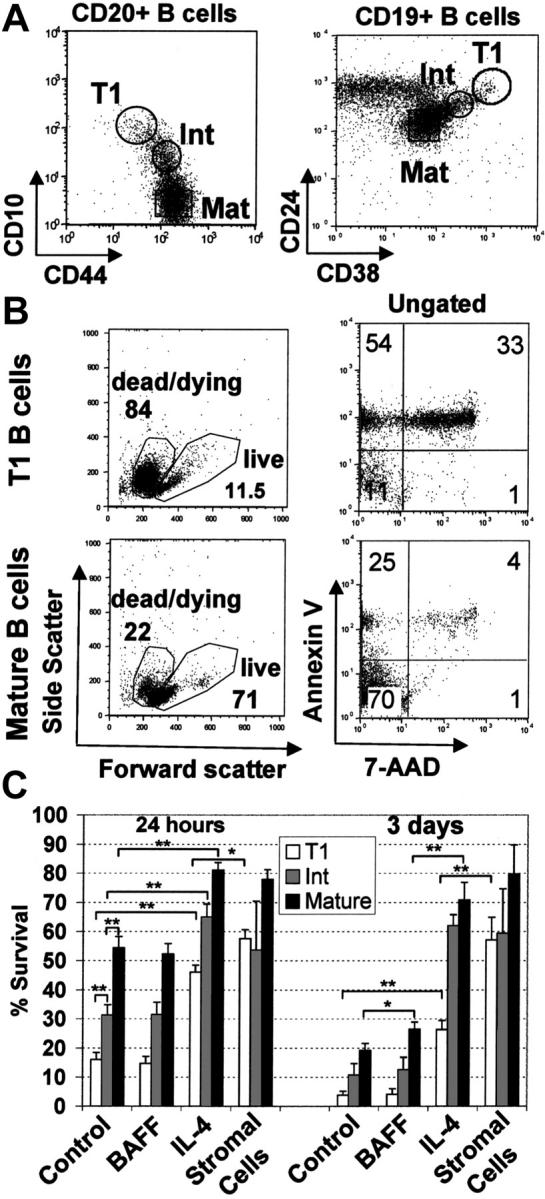
T1 B cells are short-lived in culture although survival can be improved by coculture with mouse bone marrow stromal cells or IL-4 but not BAFF. (A) Negatively selected peripheral blood B cells from healthy adult donors were stained with anti-CD20, anti-CD10, and anti-CD44 or anti-CD19, anti-CD24, and anti-CD38. T1 B cell (CD20hi CD10hi CD44lo or CD19+ CD24hi CD38hi), mature naive (Mat) (CD20+ CD10- CD44hi or CD19+, CD24lo, CD38lo) and intermediate (Int) (CD20+ CD10lo CD44hi or CD19+ CD24int, CD38int) B-cell populations were sorted as shown. Postsorting analysis indicated that each population was more than 95% pure. (B) T1 and mature B-cell populations were cultured for 24 hours and stained with annexin V and 7-AAD. (C) T1 B cells, intermediate and mature B cell populations were cultured in medium alone or with the addition of either BAFF (200 ng/mL), IL-4 (100 ng/mL), or cultured on mouse S13 bone marrow stromal cells. After 24 hours or 3 days in culture, the cells were examined for viability by flow cytometry using forward/side scatter characteristics or 7-AAD exclusion. The data show the means ± SEM of at least 4 independent experiments. Unpaired t tests were used to detect significant differences (*P <.05; **P <.01).
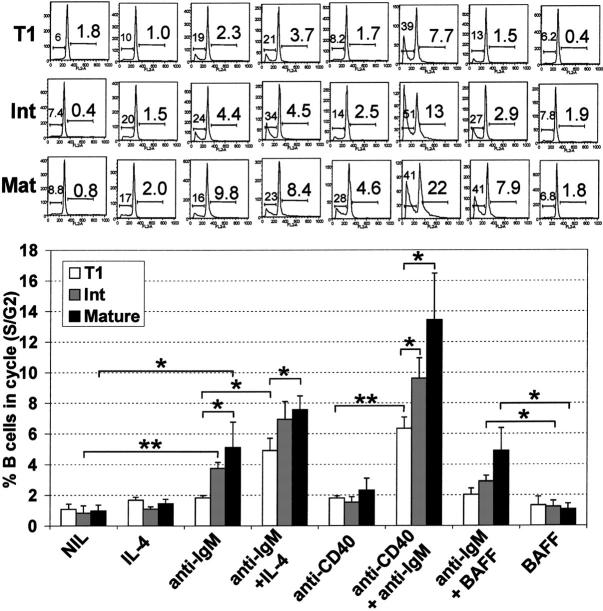
T1 B cells have reduced capacity to enter cell cycle
In the absence of stimulation, T1 B cells maintained a diploid DNA content (Figure 7), despite extensive cell death during culture (Figure 6). Notably, subdiploid DNA was not apparent at earlier timepoints (12 or 24 hours). Upon anti-IgM or anti-IgM/BAFF stimulation, T1 B cells did not significantly progress into cell cycle but apoptosis was induced, whereas both cell-cycle progression and apoptosis were induced in intermediate and mature B cells (Figure 7). However, with IL-4/anti-IgM or anti-CD40/anti-IgM stimulation, T1 B cells did enter cell cycle, although the capacity to do so was significantly less than noted with the mature B cells. Activation induced apoptosis was also associated with increased proliferation for all B-cell populations.
Figure 7.
T1 B cells have reduced capacity to enter cell cycle following B-cell receptor engagement. T1, mature, and intermediate B-cell populations (Figure 4A) were cultured alone (NIL) or stimulated with various combinations of IL-4 (100 ng/mL), anti-IgM (10 μg/mL), anti-CD40 (1 μg/mL), or BAFF (200 ng/mL) for 48 hours. Afterward, hypotonic propidium iodide staining was carried out to determine apoptotic and cycling cells. Forward/side scatter and FL2 width gating were used to gate out nuclear fragments and doublets. The FL2 area was examined to identify the frequency of sub G0/G1 apoptotic cells (left number) and S/G2 cycling cells (right number). A representative experiment is shown in the top panel, and the means ± SEM of 4 independent experiments is shown in the bottom panel. Unpaired t tests were used to detect significant differences (*P <.05; **P <.01).
T1 B cells have unmutated Ig genes
We next examined characteristics of Ig heavy chain genes from bone marrow, and peripheral blood B cell populations from an SLE patient and a healthy control. Since there were no differences within peripheral B cell subsets with respect to gene usage or CDR3 characteristics between the control and the SLE donor, these data were pooled. Bone marrow pre/pro B cells, bone marrow, and peripheral T1 B cells as well as intermediate B cells had essentially unmutated VH genes, whereas mutations were readily detected in the majority of bone marrow plasma cells and peripheral memory B cells (Table 1). The repertoire of V/D/J segments of T1 B cells was unremarkable and typical of peripheral B cells, except for a small increase in JH6 usage (Figure S2). However, T1 B cells were significantly different from bone marrow pro/pre B cells with respect to CDR3 length and the frequency of positively charged arginine residues in the CDR3 (Table 1). There was no evidence of additional selection occurring between the T1 B-cell subset and the intermediate or mature B cells, but CD27+ memory B cells did have a shorter CDR3.
Table 1.
Characteristics of Ig heavy-chain genes from bone marrow and peripheral-blood B-cell populations
| Source/B-cell subset* | No. sequences† | Mean VH mismatches | CDR3 length (nucleotide)‡ | N-addition V-D‡ | N-addition D-J‡ | Arginine residues in CDR3‡ |
|---|---|---|---|---|---|---|
| Bone Marrow | ||||||
| Total NP | 17 | 1.1 ± 1.0 | 65.6 ± 4.1§ | 15.1 ± 2.2§ | 9.4 ± 1.4 | 2.4 ± 0.4§ |
| Pre/pro | 32 | 0.0 | 57.4 ± 3.1 | 9.1 ± 1.2 | 10.0 ± 1.4 | 1.7 ± 0.2 |
| T1 | 26 | 0.0 | 50.0 ± 1.8¶ | 7.3 ± 1.0 | 7.6 ± 1.2∥ | 1.5 ± 0.2∥ |
| Plasma cells | 14 | 18.2 ± 2.5 | 44.4 ± 3.3 | 12.8 ± 4.3 | 7.1 ± 1.6 | 1.4 ± 0.2 |
| Peripheral B cells | ||||||
| Total NP | 30 (19) | 2.1 ± 1.2 | 56.6 ± 3.4§ | 12.0 ± 1.6§ | 7.9 ± 1.7 | 1.7 ± 0.2§ |
| T1 | 44 (21) | 0.1 ± 0.0 | 49.0 ± 2.0∥ | 7.8 ± 0.8 | 5.6 ± 0.7∥ | 1.0 ± 0.1∥ |
| Intermediate | 72 (37) | 0.0 | 48.6 ± 1.5 | 7.5 ± 0.7 | 5.9 ± 0.9 | 1.1 ± 1.1 |
| Mature | 28 (28) | 0.8 ± 0.5 | 49.6 ± 2.4 | 8.8 ± 1.4 | 5.8 ± 0.9 | 1.3 ± 0.2 |
| Memory | 50 (50) | 14.1 ± 1.2 | 42.9 ± 1.4¶ | 7.2 ± 0.7 | 6.1 ± 0.7 | 1.0 ± 0.1 |
Data for productive heavy chain V-D-J rearrangements was derived from pre/pro B cells (CD19+, CD20-/lo, CD38++), T1 B cells (CD19+, CD20hi, CD38++) and plasma cells (CD19-/lo, CD20-, CD38+++) from bone marrow, and T1 B cells (CD20hi, CD10hi, CD44lo), intermediate B cells (CD20hi, CD10+, CD44lo), mature B cells (CD20+, CD10-, CD44hi) and memory B cells (CD19+, CD27+) from peripheral blood. Nonproductive (NP) V-D-J rearrangement data were for the bone marrow B- cell subsets and the peripheral blood B-cell subsets
The number of sequences in brackets were derived from an SLE patient with an abnormally high proportion (17%) and absolute number of T1 B cells. Since there were no significant differences between the healthy donor and the SLE patient with respect to the repertoire or the characteristics of the CDR3, these data were pooled
The mean ± SEM for the CDR3 length, N-addition, and the number of arginines in the CDR3 are given. N-addition was only determined from sequences where a D segment with at least 9 consecutive base matches could be identified. Significant differences were determined using unpaired t tests
The CDR3 length and the mean N-addition at the V-D join of nonproductively rearranged immunoglobulin genes were significantly greater (P < .05) than the productive rearrangements from the combined B-cell subsets
The difference in the CDR3 length between the pro/pre and T1 bone marrow B-cell subsets was not significant (P = .054). However, if the T1 B cells from the bone marrow and peripheral blood were pooled, the CDR3 length and number of arginine residues in the CDR3 were significantly less (P < .05) in the T1 B-cell subset compared with the pro/pre B cells
The CDR3 length was significantly smaller in the memory B-cell population than all other peripheral B-cell subsets
Discussion
In mice, transitional B cells bridge primary B-cell development in bone marrow and B-cell maturation in the spleen, and are the target of negative selection.19 One difficulty identifying human immature transitional B-cell subsets is that no single unique phenotypic marker has been found. Moreover, a number of markers expressed by immature B cells are up-regulated on human B cells following activation or differentiation, thereby making their precise identification of transitional B cells problematic.
To assist the identification of human transitional B cells, we found that CD10hi, CD44lo, CD24hi, and CD38hi expression was characteristic of all immature bone marrow B cells. CD38, CD10, and CD24 have been previously identified as immature B-cell markers in human bone marrow,20-23 whereas the lower expression of CD44 was noted for the first time here. Importantly, about 8%-10% of these cells also expressed IgD indicating that these markers could identify immature B cells in the bone marrow and recent bone marrow emigrants. Consistent with this interpretation was the finding that CD10+ B cells are the first to emerge in the periphery 7 weeks after bone marrow transplantation.23 Further phenotyping revealed that approximately 14% of cord blood B cells and 2% of peripheral B cells expressed these immature B-cell markers and a phenotype similar to that described for murine transitional type I (T1) B cells: these cells are CD19+, CD24hi, CD21lo, CD23neg/lo, IgMhi, IgDlo/int, and CD62Llo.5 The increased expression of CD20 and reduced expression of CD40 and CD22 was found on these human B cells have also been reported on murine T1 B cells.5,8,24,25 The lack or diminished expression of activation markers, such as CD69, CD11b, CD80, CD86, and CD95, compared with memory or recently activated B cells indicates that these cells are unlikely to have responded to antigen recently. The absence of both CD27 expression and Ig somatic hypermutation demonstrates that these cells are not memory B cells.26
There were differences between murine T1 B cells and their putative human counterparts. Human T1 B cells expressed CD38 brightly and more CD23 than murine splenic T1 B cells, but less CD44 and CD95. In human peripheral blood, we noted that higher levels of CD44 were found on naive and activated B cells, and increased CD95 expression was restricted to memory B cells and circulating plasma cell populations. The most notable difference between murine and human T1 B cells was the expression of CD5 on the latter. CD5 expression is associated with a subset of murine B cells known as B1a cells that are predominately found in the peritoneum and express low affinity BCRs with autoreactive specificity.27,28 The immature markers on T1 B cells, particularly the increased expression of CD24, low expression of CD11b, and their poor survival, distinguishes them from murine B1a B cells.29,30 Moreover, Ig gene usage and substantial nontemplated nucleotide addition are not characteristic of either murine peritoneal B1a cells or human fetal B-cell repertoires.31-33 Notably, the initial B cells emerging from bone marrow following human stem cell transplantation are CD5+.34 Therefore, we conclude that CD5-expressing immature B cells represent T1 B cells rather than B1a cells, and we suggest that these cells are the precursors of conventional B2 cells.
Using a recent classification of human peripheral B cells,18 T1 B cells could be confused with “pre–germinal center” (pre-GC) B cells since they are both IgD+ CD38hi. The pre-GC designation was based on previous classifications of tonsil B cells.35,36 As previously reported, we demonstrated that the expression of CD27, CD77, and somatic hypermutation of Ig genes of peripheral blood IgD+ CD38hi B cells was different from tonsil IgD+ CD38hi B cells.18,26,35,37 The expression of CD38 was higher on peripheral T1 B cells compared with tonsil pre-GC cells, and many other significant phenotypic differences were observed. A small proportion of tonsil IgD+ CD38hi B cells could be T1 B cells since 5%-10% are either CD27-, CD5+, or express higher levels of CD24. However, using 4-color flow cytometry we were unable to determine conclusively whether this small proportion of tonsil B cells expresses the profile of T1 markers concurrently; they could also represent blood contaminants. Since T1 B cells express low levels of the adhesion molecules CD62L and CD44 compared with mature B cells, we assume that their capacity to exit the circulation and enter secondary lymphoid tissue is limited, and their presence in tonsil is therefore unlikely. Nevertheless, the expression profile of circulating T1 B cells and the relative stability of this population following immunization distinguish these cells from pre-GC and plasma cell precursors.
In agreement with our data, a recent review article indicated that human transitional B cells are CD19+, CD24hi, CD38hi, CD27-.38 However, it was also concluded that transitional B cells do not express CD10, and no data on CD21 and CD23 expression were provided.38 This is in contrast to our data, and that of others who have found that peripheral blood IgD+ CD38hi B cells express CD10.18,39,40 Moreover, we also demonstrated that peripheral IgDlo/int, CD38hi B cells, besides expressing high levels of CD10 and CD24, manifest a phenotypic profile characteristic of mouse T1 B cells. Suggestions that CD24hi CD10+ B cells are circulating pro/pre B cells38 are unfounded since they express surface IgD and Ig light chains, but not Rag1/2 mRNA (data not shown), and their heavy chain Ig repertoire is significantly different from pre/pro B cells. A population of CD10-, CD24hi B cells is present in blood and tonsil, but these cells are CD38-/lo and CD27+ and therefore are memory B cells. In summary, the data are most consistent with the conclusion that CD10 expression is a characteristic of human T1 B cells.
Consistent with functional studies in mice, it was noted that BCR stimulation induced some apoptosis of human T1 B cells rather than proliferation.5,8,9,13 However, human T1 B cells died rapidly in culture without any stimulation, a feature common to mouse splenic T1 B cells.6,9,13 Despite positive staining for Annexin V, the process of this cell death did not appear to be apoptotic since it was not associated with DNA fragmentation and was not blocked by a broad caspase inhibitor z-VAD, consistent with necrotic cell death by neglect or G1 arrest. Therefore, circulating human T1 B cells appear more predisposed to passive cell death than BCR-mediated negative selection. This may represent a fundamental difference from murine splenic transitional B cells, in which spontaneous cell death is apoptotic,41 or this difference could relate to contributions from the splenic environment.
Since B cell development is perturbed in BAFF- and BR3-deficient mice with an apparent block at the T1 B-cell stage,42-47 the role of BAFF on human circulating transitional B cells was examined. Soluble BAFF was unable to enhance survival of human T1 B cells or prevent apoptosis induced by anti-IgM stimulation. This result is consistent with murine studies that have shown BAFF induced survival and differentiation of T2 B cells but not of their T1 precursors,6,43 but in contrast to a recent report that found murine T1 B cells could be matured after 3 days in culture with BAFF followed by anti-IgM stimulation.48 In our experiments, even using 1 μg/mL BAFF, which is beyond normal physiologic or pathogenic levels,49 the frequency of surviving cells was so low after this period in culture that we were unable to determine whether the few remaining cells had matured or were contaminants. The lower expression of BR3 on human T1 B cells may in part account for the lack of responsiveness to BAFF. This is compatible with murine data where lower levels of BR3 were also found on T1 B cells compared with T2 and marginal zone B cells, both of which responded to BAFF.25,50 Other studies on human B cells have also shown that BAFF has little effect on resting peripheral blood B cells but can improve the survival of plasmablasts and myeloma cells.6,51,52 This is consistent with the increased survival and production of IgG that we noted from splenic B cells cultured with BAFF. Notably, the effects of BAFF on T1 B cells and naive peripheral B cells appeared to be relatively minor compared with the enhanced survival provided by bone marrow stromal cells, and the potent effects of IL-4.
IL-4 significantly improved survival of T1 B cells, even though IL-4R was expressed at low levels. Importantly, we also found that IL-4 and anti-CD40 enhanced BCR-mediated cell-cycle progression of T1 B cells. These data are consistent with murine studies that have shown that T-cell signals can overcome BCR-mediated apoptosis of immature B cells and induce proliferation.3,41,53,54 The increased BCR-mediated proliferation fostered by T-cell help was also associated with apoptosis, and may be comparable to the activation-induced cell death of human tonsil B cells.55
The increased BCR-mediated proliferation with IL-4 may relate to the significant improvement in survival afforded by IL-4R ligation. Intriguingly, preliminary results suggest that improved survival also coincides with down regulation of CD5 on T1 B cells (data not shown). These results are consistent with previous findings that IL-4 down-regulates CD5 expression on B cells,56 CD5 can act as a negative regulator of BCR and TCR signaling,57-59 and IL-4 enhances BCR stimulation by improving viability and by increasing the sensitivity of B cells to BCR signaling.60 These results are all consistent with the conclusion that IL-4 and/or stromal cell influences play the major role in sustaining human T1 B cell viability and may promote their maturation.
Interestingly, there appears to be a continuum in expression of surface markers between T1 B cells and mature naive B cells in cord blood and peripheral blood of humans. The phenotype of these “intermediate” B cells is similar to murine T2 B cells.5 Moreover, their improved survival and capacity to enter cell cycle following anti-IgM stimulation compared with T1 B cells is also indicative of T2 B cells in most murine studies.5,9,13 The presence of these cells in peripheral blood suggests that B cells can mature beyond the T1 stage outside the spleen or that splenic T2 cells are not necessarily retained in the spleen but may exit into the blood to complete their maturation. Since congenitally asplenic humans have normal numbers of mature B cells,61 we favor the former explanation. Extrasplenic maturation of B cells would also explain the prominence of T1 and “intermediate” B cells in cord blood, since fetal bone marrow is the major site for B cell lymphopoiesis from midgestation22 yet the spleen is not fully functional until weeks after birth. Soluble factors such as IL-4 and cellular interactions with stromal cells may contribute to the survival and maturation of T1 B cells to naive B cells outside the spleen. Extrasplenic B-cell maturation may also occur in mice, since mice with congenital or experimentally induced asplenia have grossly normal B2 cells.62
In SLE patients, the homeostasis of the peripheral B-cell subsets is clearly disturbed with increased frequencies of circulating IgD- CD38hi CD27hi plasma cells, and decreased IgD+ naive and memory B cells.40,63-65 In this study we also found that SLE patients have increased proportions of immature T1 B cells, with some patients having extremely high frequencies. In support of our data, a recent study reported increased frequencies of CD21lo, CD24hi, CD38hi B cells in SLE patients.40 Increased frequencies of IgD+ CD38hi B cells were also found in the peripheral blood of patients with primary Sjögren syndrome.18 Almost 20 years ago, increased frequencies of “immature” B cells were also reported in 1/16 common variable immunodeficiency (CVID) patients and most X-linked agammaglobulinemia (XLA) patients, using a monoclonal antibody HB7 (which was later found to bind CD38).66 More recently, a CD19+, IgMhi, CD24hi, CD38hi population (described as “pre-GC” B cells) was found in blood of CVID patients,67 and “immature” CD38hi CD10+ B cells were also reported in blood of patients with immunodeficiency, centromeric region instability, and facial abnormalities disease (ICF).39 Although the precise phenotype of these cells was not analyzed, it is likely that each of these represented T1 B cells and that this population is elevated in selected autoimmune diseases and immunodeficiency syndromes.
We initially considered the possibility that increased T1 B cells in SLE patients might result from dysfunctional bone marrow production that in turn could be responsible for the generation of B cells with autoreactive specificities. Although the proportions of B cells with a T1 B-cell phenotype were increased in SLE, the absolute numbers were comparable with controls, indicating that the bone marrow production in SLE patients is normal. The productive Ig heavy chain gene repertoire from SLE T1 B cells was similar to T1 B cells from control blood and bone marrow. However, there was a clear repertoire shift between bone marrow pre/pro B cells and T1 B cells that suggests selection, whether positive for a functional Ig heavy and light chain pairing or negative against autoreactive specificities, is intact in the SLE patient we examined. It was recently found that most polyreactive B cells are removed in the bone marrow in normals, but 40% of IgM+ CD10+ B cells (which would include T1 B cells and “intermediate” B cells) and 20% of mature naive B cells are autoreactive.68 However, these B cells are likely to have low affinity receptors and it is not known whether they can respond to antigen or if they are functionally anergic. We did not detect a shift in the repertoire between T1 and naive B cells, suggesting either transitional B cells were not targeted for negative selection, or perhaps changes were too subtle to detect. However, we did detect further change in the CDR3 length once B cells had responded to antigen, up-regulated CD27, and undergone somatic hypermutation. It is likely that abnormalities at later stages of B-cell differentiation are more important in contributing to the generation of autoantibodies in patients with SLE.
Supplementary Material
Acknowledgments
We thank Dr Peiman Hematti for bone marrow aspirates, Colleen Satorius for DNA sequencing, and Luisheng He, James Simone, and Derek Hewgill of the NIAMS flow cytometry facility for sorting B-cell subsets and advice on cell-cycle analysis.
Prepublished online as Blood First Edition Paper, February 8, 2005; DOI 10.1182/blood-2004-11-4284.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid-tissues of self-reactive lymphocytes-B recognizing membrane-bound antigens. Nature. 1991;353: 765-769. [DOI] [PubMed] [Google Scholar]
- 2.Nemazee DA, Burki K. Clonal deletion of lymphocyte-B in a transgenic mouse bearing anti-Mhc class-I antibody genes. Nature. 1989;337: 562-566. [DOI] [PubMed] [Google Scholar]
- 3.Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B-lymphocytes results in death by apoptosis. J Immunol. 1995;154: 4404-4413. [PubMed] [Google Scholar]
- 4.Forster I, Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone-marrow: Proc Natl Sci U S A. 1990;87: 4781-4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loder F, Mutschler B, Ray RJ, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190: 75-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batten M, Groom J, Cachero TG, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192: 1453-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three non-proliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167: 6834-6840. [DOI] [PubMed] [Google Scholar]
- 8.Chung JB, Sater RA, Fields ML, Erikson J, Monroe JG. CD23 defines two distinct subsets of immature B cells which differ in their responses to T cell help signals. Int Immunol. 2002;14: 157-166. [DOI] [PubMed] [Google Scholar]
- 9.Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277: 48009-48019. [DOI] [PubMed] [Google Scholar]
- 10.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B-cell maturation, 2: heat-stable antigen(Hi) splenic B-cells are an immature developmental intermediate in the production of long-lived marrow-derived B-cells. J Immunol. 1993;151: 4431-4444. [PubMed] [Google Scholar]
- 11.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28: 3738-3748. [DOI] [PubMed] [Google Scholar]
- 12.Allman DM, Ferguson SE, Cancro MP. Peripheral B-cell maturation, 1: immature peripheral B-cells in adults are heat-stable antigen(Hi) and exhibit unique signaling characteristics. J Immunol. 1992;149: 2533-2540. [PubMed] [Google Scholar]
- 13.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168: 2101-2110. [DOI] [PubMed] [Google Scholar]
- 14.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2: 465-475. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow-cytometry. J Immunol Methods. 1991;139: 271-279. [DOI] [PubMed] [Google Scholar]
- 16.Brezinschek HP, Brezinschek RI, Lipsky PE. Analysis of the heavy-chain repertoire of human peripheral B-cells using single-cell polymerase chain-reaction. J Immunol. 1995;155: 190-202. [PubMed] [Google Scholar]
- 17.Souto-Carneiro MM, Longo NS, Russ DE, Sun HW, Lipsky PE. Characterization of the human Ig heavy chain antigen binding complementarity determining region 3 using a newly developed software algorithm, JOINSOLVER. J Immunol. 2004;172: 6790-6802. [DOI] [PubMed] [Google Scholar]
- 18.Bohnhorst JO, Bjorgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J Immunol. 2001;167: 3610-3618. [DOI] [PubMed] [Google Scholar]
- 19.Carsetti R, Kohler G, Lamers MC. Transitional B-cells are the target of negative selection in the B-cell compartment. J Exp Med. 1995;181: 2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tedder TF, Clement LT, Cooper MD. Discontinuous expression of a membrane antigen (Hb-7) during lymphocyte-B differentiation. Tissue Antigens. 1984;24: 140-149. [DOI] [PubMed] [Google Scholar]
- 21.Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human-bone marrow, 2: normal lymphocyte-B development. Blood. 1987;70: 1316-1324. [PubMed] [Google Scholar]
- 22.Nunez C, Nishimoto N, Gartland GL, et al. B cells are generated throughout life in humans. J Immunol. 1996;156: 866-872. [PubMed] [Google Scholar]
- 23.Meffre E, Casellas R, Nussenzweig MC. Antibody regulation of B cell development. Nature Immunol. 2000;1: 379-385. [DOI] [PubMed] [Google Scholar]
- 24.Uchida J, Lee Y, Hasegawa M, et al. Mouse CD20 expression and function. Int Immunol. 2004;16: 119-129. [DOI] [PubMed] [Google Scholar]
- 25.Gorelik L, Cutler AH, Thill G, et al. Cutting edge: BAFF regulates CD21/35 and CD23 expression independent of its B cell survival function (vol 172, pg 762, 2004). J Immunol. 2004;172: 4646-4646. [DOI] [PubMed] [Google Scholar]
- 26.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M(+)IgD(+) peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188: 1679-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantor A. A new nomenclature of B cells. Immunol Today. 1991;12: 388-391. [DOI] [PubMed] [Google Scholar]
- 28.Kantor AB, Herzenberg LA. Origin of murine B-cell lineages. Annu Rev Immunol. 1993;11: 501-538. [DOI] [PubMed] [Google Scholar]
- 29.Wortis HH, Berland R. Cutting edge commentary: Origins of B-1 cells. J Immunol. 2001;166: 2163-2166. [DOI] [PubMed] [Google Scholar]
- 30.Rothstein TL. Cutting edge commentary: two B-1 or not to be one. J Immunol. 2002;168: 4257-4261. [DOI] [PubMed] [Google Scholar]
- 31.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V-H-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158: 1175-1186. [PubMed] [Google Scholar]
- 32.Zemlin M, Bauer K, Hummel M, et al. The diversity of rearranged immunoglobulin heavy chain variable region genes in peripheral blood B cells of preterm infants is restricted by short third complementarity-determining regions but not by limited gene segment usage. Blood. 2001;97: 1511-1513. [DOI] [PubMed] [Google Scholar]
- 33.Bauer K, Zemlin M, Hummel M, et al. Diversification of Ig heavy chain genes in human preterm neonates prematurely exposed to environmental antigens. J Immunol. 2002;169: 1349-1356. [DOI] [PubMed] [Google Scholar]
- 34.Waddick KG, Uckun FM. Cd5 antigen-positive B-lymphocytes in human B-cell ontogeny during fetal development and after autologous bone-marrow transplantation. Exp Hematol. 1993;21: 791-798. [PubMed] [Google Scholar]
- 35.Pascual V, Liu YJ, Magalski A, Debouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in 5 B-cell subsets of human tonsil. J Exp Med. 1994;180: 329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156: 111-126. [DOI] [PubMed] [Google Scholar]
- 37.Lebecque S, deBouteiller O, Arpin C, Banchereau J, Liu YJ. Germinal center founder cells display propensity for apoptosis before onset of somatic mutation. J Exp Med. 1997;185: 563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carsetti R, Rosado MM, Wardemann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197: 179-191. [DOI] [PubMed] [Google Scholar]
- 39.Blanco-Betancourt CE, Moncla A, Milili M, et al. Defective B-cell–negative selection and terminal differentiation in the ICF syndrome. Blood. 2004;103: 2683-2690. [DOI] [PubMed] [Google Scholar]
- 40.Wehr C, Eibel H, Masilamani M, et al. A new CD21 low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113: 161-171. [DOI] [PubMed] [Google Scholar]
- 41.Sater RA, Sandel PC, Monroe JG. B cell receptor-induced apoptosis in primary transitional murine B cells: signaling requirements and modulation by T cell help. Int Immunol. 1998;10: 1673-1682. [DOI] [PubMed] [Google Scholar]
- 42.Lentz VM, Hayes CE, Cancro MP. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J Immunol. 1998;160: 3743-3747. [PubMed] [Google Scholar]
- 43.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293: 2111-2114. [DOI] [PubMed] [Google Scholar]
- 44.Schneider P, Takatsuka H, Wilson A, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194: 1691-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293: 2108-2111. [DOI] [PubMed] [Google Scholar]
- 46.Yan MH, Brady JR, Chan B, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11: 1547-1552. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173: 2245-2252. [DOI] [PubMed] [Google Scholar]
- 48.Rolink AG, Tschopp J, Schneider P, Melchers F. BAFF is a survival and maturation factor for mouse B cells. Eur J Immunol. 2002;32: 2004-2010. [DOI] [PubMed] [Google Scholar]
- 49.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J Clin Invest. 2002;109: 59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng LG, Sutherland APR, Newton R, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173: 807-817. [DOI] [PubMed] [Google Scholar]
- 51.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112: 286-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103: 3148-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsubata T, Wu J, Honjo T. B-cell apoptosis induced by antigen receptor cross-linking is blocked by a T-cell signal through Cd40. Nature. 1993;364: 645-648. [DOI] [PubMed] [Google Scholar]
- 54.Parry SL, Hasbold J, Holman M, Klaus GGB. Hypercross-linking surface Igm or Igd receptors on mature B-cells induces apoptosis that is reversed by costimulation with Il-4 and anti-Cd40. J Immunol. 1994;152: 2821-2829. [PubMed] [Google Scholar]
- 55.Berard M, Casamayor-Palleja M, Billian G, Bella C, Mondiere P, Defrance T. Activation sensitizes human memory B cells to B-cell receptor-induced apoptosis. Immunology. 1999;98: 47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeFrance T, Vanbervliet B, Durand I, Banchereau J. Human interleukin-4 down-regulates the surface expression of CD5 on normal and leukemic B-cells. Eur J Immunol. 1989;19: 293-299. [DOI] [PubMed] [Google Scholar]
- 57.Tarakhovsky A, Kanner SB, Hombach J, et al. A role for Cd5 in Tcr-mediated signal-transduction and thymocyte selection. Science. 1995;269: 535-537. [DOI] [PubMed] [Google Scholar]
- 58.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274: 1906-1909. [DOI] [PubMed] [Google Scholar]
- 59.Hippen KL, Tze LE, Behrens TW. CD5 maintains tolerance in anergic B cells. J Exp Med. 2000;191: 883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodgkin PD, Go NF, Cupp JE, Howard M. Interleukin-4 enhances anti-Igm stimulation of B-cells by improving cell viability and by increasing the sensitivity of B-cells to the anti-Igm signal. Cell Immunol. 1991;134: 14-30. [DOI] [PubMed] [Google Scholar]
- 61.Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197: 939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195: 771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167: 2361-2369. [DOI] [PubMed] [Google Scholar]
- 64.Jacobi AM, Odendahl M, Reiter K, et al. Correlation between circulating CD27(high) plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48: 1332-1342. [DOI] [PubMed] [Google Scholar]
- 65.Odendahl M, Jacobi A, Hansen A, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165: 5970-5979. [DOI] [PubMed] [Google Scholar]
- 66.Tedder TF, Crain MJ, Kubagawa H, Clement LT, Cooper MD. Evaluation of lymphocyte differentiation in primary and secondary immunodeficiency diseases. J Immunol. 1985;135: 1786-1791. [PubMed] [Google Scholar]
- 67.Warnatz K, Wehr C, Drager R, et al. Expansion of CD19(hi)CD21(lo/neg) B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology. 2002;206: 502-513. [DOI] [PubMed] [Google Scholar]
- 68.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301: 1374-1377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.