Abstract
The outcome of various osteoprogenitor-cell transplantation protocols was assessed using Col1a1-GFP reporter transgenic mice. The model requires the recipient mice to undergo lethal total body irradiation (TBI) followed by rescue with whole bone marrow. When the mice are rescued with total bone marrow from a Col1a1-GFP transgenic mouse, green fluorescence protein (GFP)-positive donor cells can be observed on most endosteal and trabecular bone surfaces. Although the cells express an osteoblast-restricted GFP, they fail to progress to osteocytes, do not form a mineralized matrix, and do not generate bone nodules in vitro. However when calvarial progenitor cells derived from the same transgenic mice are injected into the bone marrow space, osteogenesis by the donor cells is observed. Using different GFP colors that distinguish the donor and recipient osteoblasts, commingling of the 2 cells types is observed along the mineralizing osteoblast surface as well as within the osteocyte population of the endosteal bone. Despite the ability of the injected progenitor cells to produce bone within the injected bone, they lack the ability to form mineralized bone nodules when explanted to primary osteoblast culture. These reagents and imaging protocols will be useful in evaluating other cells having a better progenitor potential than calvarial-derived stromal cells.
Introduction
The effectiveness of systemic transplantation of osteoblast progenitor cells for meaningful treatment of heritable diseases of bone remains uncertain. Reports of successful engraftment after transplantation of total bone marrow (TBM),1,2 enriched side population cells,3 or bone marrow stromal cells in mouse4-8 and human subjects9 suggest that this strategy will ultimately be successful. These studies generally report levels of engraftment thought to be insufficient to ameliorate the underlying bone disease and are often based on criteria that do not unequivocally demonstrate that the engrafted cells are osteoblast lineage cells. The recent experience of investigators studying transplantation of myocardial,10 neural,11 and vascular endothelial progenitor cells12 points to the importance of a cell-specific green fluorescence protein (GFP) reporter construct for evaluating the fate of a transplanted progenitor population. In these cases, use of a nonspecific reporter such as Rosa βgal shows engraftment by donor cells, whereas no evidence is observed when a tissue-specific GFP reporter is used. The conclusion suggests that many of the studies demonstrating engraftment using a tissue nonspecific marker represent either a wide distribution of engrafted cells within the myeloid lineages13,14 or fusion of the engrafted cell with a host cell.15-17 A similar outcome might be anticipated for transplantation of osteoblast progenitor cells.
With the development of transgenic mice each harboring different type I collagen GFP reporter constructs that mark various levels of differentiation within the osteoprogenitor lineage,18,19 we were in a position to evaluate various transplantation protocols with a more specific criteria for assessing outcome. The pOBCol3.6GFP transgene has been shown to activate at the preosteoblast level of differentiation and to continue to be strongly expressed in osteoblasts that line the bone surface. However the transgene is not specific to bone and can be observed in other type I collagen-producing cells such as dermal fibroblasts and smooth muscle cells. In contrast, pOBCol2.3GFP does appear to be osteoblast and osteocyte specific. The other technical development that made this transplantation study feasible was the preservation of a strong GFP signal in frozen sections of adult bone.20
In the study reported here, 2 combinations of GFP colors are used to distinguish either the donor and host cells or 2 different populations of bone lining cells within the bone.1 Total bone marrow (TBM) derived from pOBCol3.6GFPcyan (3.6-blue) transgenic mice is systemically transplanted into a pOBCol3.6GFPtpz (3.6-green) or pOBCol2.3GFPemd (2.3-green) recipient.2 Calvarial progenitor cells derived from a 3.6-green or 2.3-green donor mouse are mixed with TBM derived from a 3.6-blue donor mouse for direct injection into the bone marrow space of a nontransgenic recipient. Both transplantation protocols yield significant engraftment of bone lining cells, but the homing and differentiation properties are very different.
Materials and methods
Transgenic mice
Established transgenic mouse lines bearing the pOBCol3.6GFPtpz (3.6-green) and pOBCol2.3GFPemd (2.3-green) expression constructs have been characterized previously.18 A new line of mice transgenic for pOBCol3.6GFPcyan (3.6-blue) has a similar expression profile as the 3.6-green version.21 No attempt was made to standardize the sex of the donor or recipient mice. Donor mice were approximately 2 months old, whereas recipient mice ranged from 2 to 10 months of age but were of the same age within a specific experiment. In one series of experiments, the Col2.3Δtk were used as recipient mice. This model of conditional ablation of active osteoblasts during gangciclovir treatment has been previously described.22,23 The mice received 8 mg/kg/d by intraperitoneal injection for 16 days and received transplants at days 1, 7, 14, 21, and 28 after the drug was discontinued. All mice were studied on a CD1 background.
TBI and transplantation
Recipient mice received a lethal dose of 900 cGy total body gamma irradiation using a 137Cs source (Nordion Gammacell 40 Irradiator; Atomic Energy of Canada, Ottawa, Canada), with a dose rate of 68.2 cGy/min. Without an infusion of whole bone marrow cells, the mice died within 2 weeks after treatment. Two transplantation strategies were used to introduce whole bone marrow cells (5.0 × 106) either alone or mixed with calvarial progenitor cells (1.0 × 106). Systemic transplantation was performed by infusion of donor cells into the tail vein or retro-orbital sinus. Recipient mice were anesthetized with a ketamine/xylazine mixture (135 mg/kg and 15 mg/kg) to facilitate placing the 26-gauge needle along the medial aspect of the retro-orbital space. Cells were introduced in a volume up to 100 μL. Local injection into the medullary space of the tibia or femur was performed in ketamine/xylazine-anesthetized recipient mice. The knee was flexed to 90° and the proximal end of the tibia or distal side of the femur was drawn to the anterior. A 26-gauge needle was inserted into the joint surface of the femur or tibia through the patellar tendon and then inserted into the medullary space. The needle was removed and another 26-gauge needle attached to a 1-mL syringe was inserted into the needle track and extended into the bone marrow space. Donor cells in a total volume of 30 μL were slowly injected into the marrow cavity as the needle was being withdrawn from the intramedullary space. The mice that received a transplant were fed with regular animal chow that was not supplemented with antibiotics. Sufficient numbers of mice were subjected to this protocol so that a minimum of 3 mice would be available to be killed at various days after transplantation. In some experiments the mice received a 100 μL intraperitoneal injection of freshly prepared xylenol orange (XO; 30 mg/mL, 90 mg/kg). The protocol for total body irradiation (TBI) and whole bone marrow transplantation (WBMT) of the recipient mice and cell preparations from the donor mice was approved by the institutional animal care committee (ACC 2001-103).
Preparation of cells for transplantation
TBM was obtained from freshly isolated femora and tibiae and placed into α modified Eagle minimum essential medium (αMEM) containing 10% fetal bovine serum (FBS). The cell suspension was aspirated and expelled by a syringe and an 18-gauge needle, filtered through a 70-μm cell strainer, counted, and centrifuged at 300g for 8 minutes. The cells were adjusted to 5.0 × 107 cells/mL for transplantation. Freshly isolated calvarial cells for direct transplantation were obtained from 6-day-old mouse calvaria by sequential trypsin/ethylenediaminetetraacetic acid (EDTA)/collagenase P digestion as previously described.18 The pooled cell suspension from digest fractions 2 to 4 was passed through a 70-μm cell strainer, pelleted, and resuspended at a cell density of 3.0 × 107/mL. Calvarial progenitor cells for transplantation were initiated from the freshly isolated calvarial cells that were plated at a density of 1 × 106 cells per 100-mm plate and grown for 5 to 6 days in Dulbecco modified Eagle medium (DMEM) containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells at a preconfluent stage of osteoblast differentiation were harvested in 0.25% trypsin/EDTA, collected by centrifugation, and resuspended in DMEM containing 10% FBS at a concentration of 3.0 × 107/mL.
Analysis of the transplanted bone
Bones used for histologic analysis were fixed and decalcified as previously described.21 Decalcified and nondecalcified bones were embedded in Cryomatrix (Thermo Shandon, Pittsburgh, PA) and cryostat sections were prepared using a Leica CM1900 Cryostat (Leica; D-69226; Nussloch, Germany) outfitted with the Cryojane (Instrumedics, Hackensack, NJ) tape transfer system.21 The bone sections were examined on a Zeiss Axiovert 200M (Carl Zeiss, Thornwood, NY) equipped for fluorescence imaging and a computer-controlled mechanical stage as previously described.21 A Zeiss W-PL 10 ×/23 numeric aperture (NA) eyepiece and Fluar 2.5 ×/0.12 NA, Fluar 5 ×/0.25 NA, Plan-Apochromatic 10 ×/0.45 NA, and Plan-Apochromatic 20 ×/0.75 NA objectives were used. All images were taken through air and recorded on a Zeiss Axiocam color digital camera. Image processing was performed using Openlab (Improvision, Lexington, MA), GraphicConverter (Lemke Software, Peine, Germany), and Adobe Photoshop (Adobe Systems, San Jose, CA).
The in vitro differentiation potential of the transplanted TBM or stromal cells was assessed by initiating marrow stromal-cell cultures from marrow explants of the engrafted bone. The procedure was modified from a standard marrow stromal-cell culture18 to inoculate a single 35-mm well of a 6-well plate from a single femur explant. The development of donor- and recipient-derived bone nodules was assessed by the Axiovert/Improvision fluorescent microscope as previously described.24
The in vitro differentiation potential of the engrafted osteoblast population was determined by initiating osteoblast cultures from bone chips derived from the transplanted bone.25,26 The dissected bone was cleared of adherent muscle and ligamentous attachments, opened at either end by removal of the epiphysis, and cleaned of marrow by flushing with phosphate-buffered saline. The cortical bone and remaining trabeculae were cut into 1-mm chips and placed in a solution containing 0.02% trypsin-EDTA, 250 units/mL collagenase type II, and 1.6 × 10-6 Na-tosyl-lys chloromethyl ketone hydrochloride for 30 minutes at 37°C with 200 rpm shaking to remove adherent cells. The chips from a single transplanted bone were placed in a 35-mm plate in 2 mL of αMEM containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin to allow the cells within the bone to grow onto the plastic. By 10 to 14 days the plastic adherent cells were harvested by trypsin digestion, separated from the remaining chips by filtration through a 70-μm cell strainer filter, and replated in 35-mm wells at a density of 0.5 × 106 cells/well. The adherent cells were expanded for 7 days in nondifferentiating medium after which they were grown in differentiating medium for 21 days.18 The acquisition of GFP fluorescence was monitored by the Zeiss/Improvision microscope with XO staining in the late stages of the culture as discussed for the stromal cell cultures.24
Results
Expression of the GFP reporter genes in the donor mice
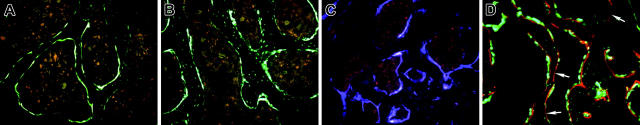
The pattern of GFP expression within donor bone is presented to assist in the interpretation of engrafted bone lining cells. Figure 1A illustrates 3.6-green in the osteoblastic cells that line the bone surface and extend into the outer layers of bone matrix. The low level of autofluorescence from bone marrow has a pink coloration. The 2.3-green (Figure 1B) is strong in cells on the bone surface and extends to most of the osteocytes within the bone matrix. Figure 1C shows 3.6-blue, which is prominent in cells on the bone surface and in osteocytes near the bone surface. Figure 1D is a nondecalcified section derived from a 3.6-green mouse that was labeled with XO 2 days before it was killed. This in vivo fluorescent stain will identify the surfaces of bone that are actively depositing new mineralizing osteoid. In most cases there are parallel 3.6-green and XO lines, indicating that the GFP-positive cells are associated with recent mineral deposition. However there are areas of XO labeling that are not associated with the 3.6-green cells (Figure 1D, arrows), indicating that this marker does not identify all matrix-depositing cells.
Figure 1.
Patterns of GFP expression in the bones of donor mice. (A) Within trabecular bone, 3.6-green is strongly expressed on the bone surface and can extend into regions of recently formed bone. The pink cellular regions surrounded by the GFP-positive cells are bone marrow. (B) The 2.3-green is strongly expressed in bone surface cells and in the osteocytes throughout the bone. (C) The 3.6-blue expression resembles 3.6-green. (D) Nondecalcified trabecular bone of trabecular from a 3.6-green donor mouse labeled with XO 2 days before it was killed. The red label is closely associated with the majority of the GFP-positive cells although there are regions of XO labeling without overlying GFP-positive cells (arrows). (A-D) Original magnification, × 200.
Systemic transplantation of marrow stromal cells
We initially used this TBI model to assess the capability of in vitro-expanded marrow stromal cells from GFP-transgenic donors to engraft after systemic coinjection with nontransgenic TBM. Whether the irradiated mice were injected via the orbital plexus, tail vein, or left ventricle, no evidence for engraftment of 2.3-green- or 3.6-green-labeled donor cells could be demonstrated in the long bone. Occasionally 3.6-green cells were found in the lung. Engraftment could not be observed whether the cells were expanded in fibroblast growth factor 2 or parathormone or by multiple passages in nondifferentiating medium. The potential for opening osteoblast niches was examined by performing a transplantation on the gangciclovir-treated Col2.3Δtk mouse.22 This murine model of inducible osteoblast destruction undergoes a recovery phase of new osteoblast proliferation once the gangciclovir treatment is discontinued. We hypothesized that this recovery response might be an optimal time for engraftment of circulating precursor cells; however, no evidence of engraftment was observed in this model (data not shown).
Systemic and local transplantation of whole bone marrow
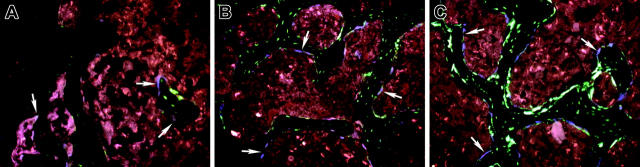
The osteogenic potential of whole bone marrow cells was examined using tail vein or retro-orbital plexus injection of TBM cells obtained from a 3.6-blue donor mouse into irradiated 3.6-green recipient mice. This time 3.6-blue donor cells (Figure 2 A-D, arrows) could be found along the surface of the bone next to 3.6-green host cells (Figure 2A femur; Figure 2B tibia). The highest number was found on trabecular surfaces within the metaphyseal region. Similar results were obtained in experiments using the 3.6-blue donor and a 2.3-green recipient (not shown). Injection of TBM cells from a 3.6-blue donor into the bone marrow space of the tibia of an irradiated 3.6-green or 2.3-green recipient resulted in a similar finding of cell-surface engraftment with 3.6-blue cells both in the injected tibia (Figure 2C arrows) and a contralateral bone (Figure 2D femur, arrows). Local and peripheral bone surface engraftment by TBM-derived 3.6-green donor cells was also demonstrated after intramedullary transplantation, indicating that our findings with the 3.6-blue donor were not unique to a specific marker transgene (Figure 3A tibia; Figure 3B femur). A time series was performed using 3.6-blue as the donor of TBM cells and 2.3-green as the irradiated host. The 3.6-blue cells begin to appear at 14 days after transplantation (Figure 4A), increase to their maximal number by day 28 (Figure 4B), and remain at this level for at least 78 days (Figure 4C).
Figure 2.
Engraftment of bone lining cells after transplantation with TBM from 3.6-blue donor mice. (A-B) An image of frozen decalcified bone (A, femur; B, tibia) from a 3.6-green recipient rescued with systemic TBM from a 3.6-blue donor. The 3.6-blue cells (arrows) are present on the bone surface admixed with 3.6-green cells. Note that the 3.6-blue cells do not extend into the osteocyte layer. (C-D) An image of the bone from a 2.3-green host that received an intramedullary injection of TBM from a 3.6-blue mouse. The 3.6-blue cells line the bone surface whereas the host cells express 2.3-green on the bone surface and within osteocytes. (C) Injected tibia. (D) The contralateral femur also demonstrates 3.6-blue cells, indicating engraftment by a systemic route. (A-D) Original magnification, × 200.
Figure 3.
Engraftment of bone lining cells after transplantation with TBM from 3.6-green donor mice. (A-B) Engraftment of 3.6-green donor-derived TBM cells. The recipient was a 3.6-blue mouse. (A) Injected tibia. (B) Ipsilateral femur. Now 3.6-green cells (arrows) line the bone but do not extend into the bone matrix. (C-D) Frozen nondecalcified section of bone from a nontransgenic recipient rescued by an intramedullary injection of TBM from a 3.6-green mouse. The recipient mouse was injected with XO 2 days prior to being killed. The injected femur (C) and the contralateral tibia (D) show bone surface 3.6-green cells but no associated XO labeling. (A-D) Original magnification, × 200.
Figure 4.
Temporal sequence of engraftment of donor-derived 3.6-blue bone lining cells in a 2.3-green recipient. Mice from the same TBI/rescue experiment were examined at days 7, 14, 21, 28, 56, and 78 after systemic transplantation. Shown here are representative images at day 14 (A, when 3.6-blue cells first appear, arrows), day 28 (B), and day 78 (C). Digital thresholding and counting of the number of 3.6-blue cells in each image was (A) 28, (B) 37, and (C) 48. (A-C) Original magnification, × 200.
Although this was our first solid evidence of bone surface engraftment by donor cells after systemic injection of TBM, there were features of the GFP-positive cells that were inconsistent with a cell having osteoblastic properties. First, GFP-positive cells were always on the surface of bone. It was difficult to find examples of GFP-positive cells that appeared to have entered the bone matrix. Part of this could be explained by the fact that 3.6-blue expression is limited to cells in the outer layers of bone matrix (Figure 1A,C-D). Second, examination of nondecalcified sections of transplanted bone labeled with XO 2 days before the mice were killed failed to demonstrate the red fluorescent labeling line in association with the 3.6-green donor cells (Figure 3C-D) as would be expected if they had osteoblastic properties (Figure 1D). The third observation casting doubt on the osteoblastic properties of the GFP-positive bone surface cells came from primary marrow stromal-cell cultures derived from mice that received a transplant. Figure 5A shows that the irradiated 3.6-blue host mice do generate a limited number of 3.6-blue mineralizing nodules relative to nonirradiated controls (not shown), which is consistent with diminished marrow-derived osteoprogenitor-cell activity after recovery from TBI.27 Even though the 3.6-blue mice can form 3.6-blue nodules after irradiation (Figure 5A), there is no evidence of 3.6-blue mineralizing nodules derived from mice that received a transplant of TBM carrying this marker gene. This observation is illustrated in Figure 5B in which the host is transgenic for 2.3-green cells and 2.3-green mineralizing nodules do develop. This internal control for bone-cell differentiation indicates that the culture conditions were sufficient for progenitor cells to achieve bone nodule development irrespective of their source of origin. Repeat experiments in which the donor TBM cells carried the 3.6-green or 2.3-green marker failed to generate bone nodules expressing the marker transgene (not shown). However 3.6-blue cells are present in the culture. By 10 days after plating, scattered blue cells appear in a loose cluster that is distinctly different from the cells that form a bone nodule (Figure 5Ci-iv). Over time these clusters of cells expand in area (but not density) and gradually develop distorted elongated syncytial-like processes (Figure 5Di-iii; days 10, 21, and 28 in culture). These cells do not deposit mineral and do not develop into a multilayered nodule (Figure 5Civ). Similar types of cells can be demonstrated in marrow cultures derived from 3.6-green donor mice that received a transplant but not the 2.3-green donor mice (not shown). Thus the engrafted cells that develop from a WBMT acquire an expression pattern from a type I collagen GFP reporter construct that is inconsistent with its expression in donor mice. Although the nature of these cells is still unclear, they do not appear to have the properties of a member of the osteoprogenitor lineage.
Figure 5.
Fluorescent images of marrow stromal-cell culture grown in a 6-well plate. Each panel is formed from × 25 to × 2.5 original magnification images that are tiled together, and represents approximately 60% of the surface area of a cell-culture well. The same well is recorded at increasing days of culture (days 10-28) and the fluorescent spots that develop over time are produced by the strong GFP expression within the bone nodule. At day 21 the nodules that have mineralized are visualized by the XO staining that colocalized with the GFP nodules (Aiv,Biv). (A) Profile of developing bone nodules from an irradiated 3.6-blue transgenic mouse to illustrate the bone nodule-forming potential of the osteoprogenitor population in a bone marrow stromal culture. Images from days 14 (Ai), 18 (Aii), and 21 (Aiii) show nodule progression and the extent of mineralization at day 21 is shown by XO staining (Aiv). (B) Stromal-cell culture derived from a 2.3-green recipient mouse that was rescued by WBMT from a 3.6-blue donor. Although the number of host-derived 2.3-green bone nodules is diminished relative to nonirradiated controls (not shown), only host-derived nodules are observed. Images from days 14 (Bi), 18 (Bii), and 21 (Biii) show nodule progression and the extent of mineralization at day 21 is shown by XO staining (Biv). (C) Fluorescent image (original magnification, × 50) of a 2.3-green nodule as seen in the × 25 (original magnification) tiled image in panel B. The 2.3-green nodule (Cii) shows strong mineralization (Ciii). Panel Ci shows the cluster of 3.6-blue donor-derived cells (arrowheads) that happened to form adjacent to this 2.3-green nodule. It has a distinctly different morphology than the bone nodule (BN) that in this image is blue in color because the 2.3-green signal seen in panel Cii spills through the GFPcyan filter. Panel Civ shows the composite image of the 3.6-blue cell cluster and 2.3-green/XO bone nodule. (D) Fluorescent and phase-contrast image (original magnification, × 100) of 3.6-blue cells that develop in a stromal-cell culture derived from a recipient mouse rescued with TBM from a 3.6-blue donor mouse. The expanding cluster of cells does not become a multilayered nodule and does not stain with XO. Images from days 10 (Di), 21 (Dii), and 28 (Diii) show the cellular progression. The overlay (Div) with the phase-contrast image shows the 3.6-blue cells relative to the nonfluorescent cells in the field of view.
Intramedullary transplantation of calvarial progenitor cells
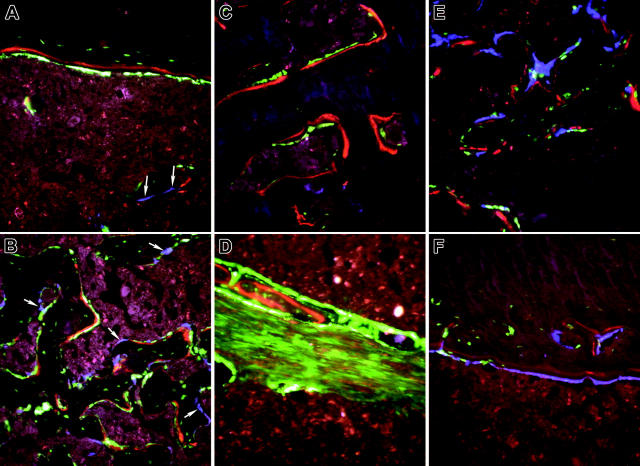
When expanded calvarial progenitor cells from a 2.3-green donor were injected simultaneously with TBM derived from a 3.6-blue donor mouse into the bone marrow space of the femur of nontransgenic recipients, blue cells were again seen along the surface of bone (Figure 6A, arrows) but now green cells were found both on the endosteal and trabecular surface in association with XO labeling (Figure 6A endosteal bone from the diaphyseal region; Figure 6B new trabecular bone from the metaphyseal area). A similar level of engraftment of cells capable of entering bone matrix was observed with freshly isolated cells from newborn calvaria (not shown). In both cases, bone surfaces making matrix as judged by XO incorporation have overlying donor-derived 2.3-green cells; however, no label is found under the 3.6-blue cells. In addition, 2.3-green cells are visible within the bone matrix, indicating that they achieved an osteocyte level of differentiation. When 3.6-green was used as the donor stromal cell, 3.6-green cells were also present on the bone surface (Figure 6C). Most of these 3.6-green donor cells are associated with XO label but only a few extend into the bone matrix. This pattern is consistent with the donor-derived expression of 3.6-green marking early osteoblasts but not extending into mature osteocytes (Figure 1A,C). The 3.6-green reporter transgene revealed another population of elongated fibroblastic-like cells within the bone marrow space that is never observed in the donor mouse bone (Figure 6D). Presumably these cells represent a precursor population at a preosteoblast level of differentiation that is deposited along the needle tract (NT; Figure 7B). Figure 6D suggests that osteoblastic-cell differentiation occurs in cells at the perimeter of the tract.
Figure 6.
High-power images of bone from nontransgenic recipients of an intramedullary injection of calvarial stromal cells. (A-B) Calvarial progenitor cells from a 2.3-green donor mouse injected into a nontransgenic recipient and rescued with TMB from a 3.6-blue donor. Selected images (original magnification, × 200) show engraftment of diaphyseal bone (A) and formation of new trabecular bone (B) and illustrate the association of XO label with 2.3-green cells. Because the 2.3-green marker gene remains active in osteocytes, marked donor cells become incorporated into the host bone and within the newly formed trabecular bone. In contrast, the 3.6-blue cells (arrows) remain on the bone surface and are not associated with the XO label. These images are obtained from the scan shown in Figure 7A. (C-D) Calvarial progenitor cell from a 3.6-green donor mouse injected into a nontransgenic recipient that was rescued TBM from a 3.6-blue donor. Selected images (original magnification, × 200) show engraftment of pre-existing bone (C) and formation of new trabecular bone (D) that appears to be derived from the fibroblastic tract. Both structures exhibit donor-derived osteoblasts (strong GFP signal) in association with the XO label. The fibroblastic-shaped cells along the injection tract are not associated with bone mineralization and run between regions that have produced trabecular bone. These images are obtained from the scan shown in Figure 7B. (E-F) Nondecalcified sections of a femur showing engraftment of 3.6-blue donor-derived calvarial cells after injection into a 3.6-green host that was rescued with nontransgenic TBM. (E) Trabecular region. (F) Cortical region. In both images there is commingling of 3.6-blue donor and 3.6-green host cells on the bone surface that is associated with the XO label.
Figure 7.
Histology from a nontransgenic recipient mouse that received a transplant via intramedullary injection of TBM from a 3.6-blue donor and expanded calvarial progenitor cells from a 3.6-green or 2.3-green donor. The recipient mice were injected with XO 2 days prior to being killed. (A) Full-length section of bone transplanted with calvarial progenitor cells from a 2.3-green donor mouse and TBM from a 3.6-blue donor. The needle tract (NT) is less obvious with the 2.3-green marker gene because it only visualizes mature osteoblasts. Distal engraftment is apparent in the femoral neck (FN) region. (B) Full-length section of bone transplanted with calvarial progenitor cells from a 3.6-green donor mouse and TBM from a 3.6-blue donor. The needle tract (NT) is composed of newly formed trabeculae and a core of fibroblastic-shaped progenitor cells. Bone lining cells of donor origin are present at sites distal to the injected cells such as the femoral neck region.
There was no evidence for systemic engraftment by the donor progenitor cells in contrast to the TBM-derived donor cells. Osteoblast differentiation was only observed in the bone that was injected. However, the cells migrated well beyond the needle tract. The full-length femur section reveals the distribution of the donor progenitor-cell population (Figure 7A engrafted with 2.3-green; Figure 7B engrafted with 3.6-green). Donor-derived osteoblasts are found along the needle tract (entered through the left of the image), adjacent endosteal surfaces, and at distal sites, particularly in the femoral neck (FN) region. This experiment suggests that the progenitor donor cells do have osteogenic capability when injected locally where they can distribute to many sites contiguous with the bone marrow.
The relative contribution of host and donor osteoblasts to new bone formation was determined by injecting calvarial progenitor cells derived from a 3.6-blue donor into a 3.6-green or 2.3-green recipient. The proportion of donor and host osteogenic cells within new bone along the needle tract (Figure 6E trabecular bone) or at an engrafted site of preexisting bone (Figure 6F endocortical bone) can be regionally variable. However both colors can be commingled in the same regions of bone formation and both are associated with XO-labeled matrix.
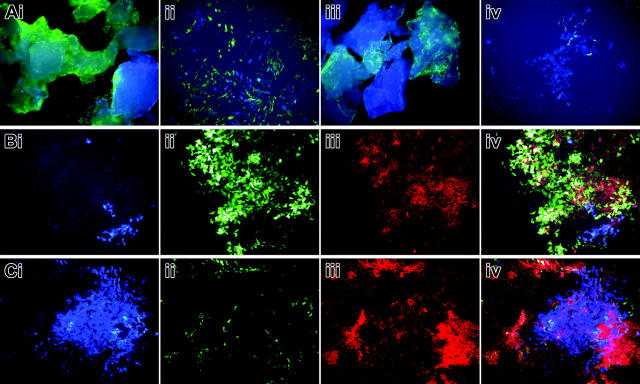
The in vitro differentiation potential of the progenitor cells that were injected into the marrow space was investigated by initiating osteoblast cultures from bone chips of the injected host bone. This protocol is designed to eliminate the bone surface or contaminating bone marrow as the source of cells that grow out from the chips25,26 so as to enrich for progenitor cells from within the bone matrix. The initial outgrowth cells are used to initiate a secondary culture that is grown under osteogenic conditions devoid of the chips (see “Analysis of the transplanted bone”). The presence of donor and host cells within the processed chips can be appreciated with the fluorescent microscope (Figure 8Ai,iii). Many of the host cells that migrate from the chips to the culture dish surface continue to express a strong 3.6-green signal but not 2.3-green because they have not been induced to osteoblast differentiation. Donor-derived 3.6-blue cells are evident in the initial cell outgrowth but are difficult to demonstrate at low power. During the period when the chip-free secondary culture is grown under nondifferentiating conditions, clusters of donor 3.6-blue and host 3.6-green fibroblastic-like cells develop independently or in overlapping groups (Figure 8Aii). Cultures derived from 2.3-green recipient mice did not show activity during this time (Figure 8Aiv). However 7 to 10 days after the culture was switched to osteogenic medium, nodules developed with strong expression of the 3.6-green, characteristic of host cells that acquire osteoblastic differentiation, and deposited mineral that was stained with XO (Figure 8Bii-iii). However, the few 3.6-blue donor-derived nodules that did develop failed to show XO labeling (Figure 8Bi,iv). Because this is a secondary culture, distinct bone nodules are not as clearly defined as observed in a primary culture, and regions of XO labeling develop in regions that are not obviously 3.6-green (Figure 8Cii-iii). Note that the 3 regions of 2.3-green-associated mineralization surround the cluster of 3.6-blue donor cells (Figure 8Ci) that is not associated with deposited mineral (Figure 8Civ). Despite this problem with the culture model, the analysis indicates that the host-derived areas of strong transgene expression also deposit mineral but areas of donor-derived transgene expression do not mineralize.
Figure 8.
Primary osteoblast cultures derived from bone chips of a 2.3-green or 3.6-green recipient mouse that received a transplant of calvarial progenitor cells from a 3.6-blue donor. This scenario is as illustrated in Figure 6E-F. (A) Bone chips after 7 days in primary culture and in early secondary culture showing the 2 colors of cells embedded within and growing from the bone fragments. The 3.6-green chips (Ai) show 3.6-green cells on the plate surface. The 3.6-blue cells that grow out from these chips can be appreciated at higher power in the secondary culture (Aii). The 2.3-green chips (Aiii) show the 2 colors of GFP within the bone. No 2.3-green cells are seen in the early outgrowth cells (Aiii-iv) because the transgene is not activated prior to bone nodule formation. At high power in the early secondary culture the 3.6-blue cells are evident (Aiv). (B) Mineralizing bone nodule (Biii) derived from the 3.6-green recipient mouse (Bii) that is adjacent to a cluster of 3.6-blue cells (Bi). An overlay of the 3 images (Biv) shows XO staining of the 3.6-green cells but not the 3.6-blue cells. (C) Fate of a donor-derived 3.6-blue cell cluster (Ci) that is surrounded by host-derived 2.3-green cells (Cii) that are producing a mineralized matrix (Ciii). The overlay image (Civ) shows faint XO staining over the 3.6-blue cells relative to the strong staining over the 2.3-green cells. (A-C) Original magnification, × 100.
Discussion
The use of cell-differentiation-specific GFP markers to distinguish the source and function of cells in a transplantation experiment is particularly important in studies of solid tissues, especially bone, where traditional morphology criteria do not always equate to cellular function. Because myeloid elements appear to be widely distributed throughout most tissues, use of a ubiquitously expressed molecular marker to assess a transplantation experiment can be misinterpreted as evidence of successful engraftment within a solid tissue.28 In addition, evidence of engraftment based solely on immunologic or enzymatic criteria is highly dependent on variables of tissue fixation and technical experience, making the evaluation of transplantation outcomes difficult to replicate. With the demonstration that multiple colors of GFP expression can be maintained in frozen sections of bone as well as the preservation of tissue integrity with the tape transfer process,21 it is now possible to evaluate the outcome of a transplantation experiment in a rapid and technically unbiased manner. The use of the dual-color strategy of donor and host has the additional advantage of excluding the possibility of cell fusion as the explanation of engraftment.15-17 Unlike the mixed color patterns produced when similar promoters but different GFP color transgenes are bred into the mouse,21 the transplantation experiments gave rise to cells that were clearly of donor or host origin and no examples of mixed GFP expression were observed.
The results obtained in the present study may explain the results of previous reports of successful engraftment of bone surface lining cells but limited effect on bone matrix production. The donor-derived cells that reside on the bone surface after WBMT are not osteoblasts. Although they express a promoter-GFP marker gene on the bone surface, a feature that we previously strongly associated with acquiring an osteoblast level of differentiation, it now appears that this is an improper interpretation. Three properties that would be expected of a progenitor cell with the ability to differentiate into osteoblasts were not met. First, the engrafted Col3.6-positive donor cells remain on the bone surface and do not become incorporated into the bone matrix as would be expected when osteoblasts become osteocytes. In the donor mice, the same Col3.6GFP cells are found on the cell surface and the most recently embedded osteocytes. Second, the engrafted Col3.6GFP donor cells are not producing a mineralizing bone matrix. Systemic injection of XO will stain mineralizing bone that can be associated with GFP-positive cells in the frozen nondecalcified sections. This approach readily labels matrix lying beneath GFP-positive cells of host origin. However, there is no evidence of matrix labeling beneath donor-derived GFP-positive cells transplanted as whole bone marrow. Third, progenitor cells that engraft the host marrow should be capable of initiating bone nodule formation. Although host progenitor cells do survive TBI, there is a reduction in the number of nodules due to either a direct effect of the irradiation on the progenitor population or a secondary effect on the hematopoietic system that is required for osteogenic nodules to develop. In either case, the reduction of progenitor-cell activity after TBI makes assessment of donor-derived progenitor cells more difficult to evaluate. Despite this problem, the only mineralizing nodules that developed from TBI and whole bone marrow-rescued mice developed from host progenitor cells. Thus the nature of the GFP-positive cells that engraft the bone surface after systemic transplantation of TBM remains uncertain.
In contrast to TBM, calvarial progenitor cells and primary calvarial digest cells are able to differentiate into osteoblasts when directly injected into the marrow space. These cells can form bone de novo within the bone marrow space and participate with resident osteoblasts to maintain endosteal bone formation. The donor-derived osteoblasts can deposit new matrix and can progress to the osteocyte level of differentiation. The cells appear to develop from a reservoir of fibroblastic progenitor cells that expand within the transplanted marrow and have the ability to migrate well beyond the needle tract of injected progenitor cells. They can maintain the population of differentiated osteoblasts for at least 5 months. Thus the cells appear to have osteogenic function similar to stromal cells that have been used for repair of nonunion of fracture or critical size defects in calvaria.29,30 Similar to these studies, the engrafted cells appear to function locally and do not show evidence of systemic circulation either from the engrafted site or when injected systemically.
Despite the osteogenic progenitor properties of the injected progenitor calvarial cells, they do not appear to retain this attribute after transplantation. Although proliferating fibroblastic-like cells of donor origin can be recovered either by explant from bone chips or by stromal-cell culture of the transplanted marrow (data not shown), they can no longer generate a mineralizing bone nodule, a property that is maintained by the progenitor cells of the host that received a transplant. This property would suggest that the cells used in the transplantation have progressed sufficiently down the lineage to differentiate into osteoblasts when injected in sufficient numbers locally but lose this ability to generate a mineralized nodule in vitro possibly because the early progenitors necessary for cellular expansion and differentiation no longer existed in the donor-cell population.
The murine transgenic reagents to distinguish donor and host cells and the histologic techniques to distinguish different colors of GFP and to colocalize them with traditional stains used in bone biology provide a rapid and unequivocal method for evaluating the differentiation potential of a progenitor population. With experience it is possible to harvest, process, and evaluate the results of the transplantation experiment within 7 days after the mice were killed. Although the studies reported here were performed in outbred CD1 mice requiring TBI to guard against graft rejection, it will be possible to migrate this model to inbred C57Bl/6 mice as the transgenes are developed in this mouse strain. Identifying the optimal source of progenitor cells and transplantation protocols for successful osteoblast engraftment, either locally or systemically, will require empirically testing many experimental variables. We believe that the methods presented in this study provide a rapid and quantitative tool for identifying robust progenitor populations and effective transplantation protocols.
Prepublished online as Blood First Edition Paper, August 4, 2005; DOI 10.1182/blood-2005-02-0582.
Supported by grants from the Public Health Department; National Institutes of Health (NIH) PO1-AR038933, P20 GM65764, and U01-DK63478; and the Michael Geisman Fellowship of the Osteogenesis Imperfecta Foundation (Z.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Hou Z, Nguyen Q, Frenkel B, et al. Osteoblast-specific gene expression after transplantation of marrow cells: implications for skeletal gene therapy. Proc Natl Acad Sci U S A. 1999;96: 7294-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson SK, Dooner MS, Weier HU, et al. Cells capable of bone production engraft from whole bone marrow transplants in nonablated mice. J Exp Med. 1999;189: 729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olmsted-Davis EA, Gugala Z, Camargo F, et al. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc Natl Acad Sci U S A. 2003;100: 15877-15882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira RF, O'Hara MD, Laptev AV, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95: 1142-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onyia JE, Clapp DW, Long H, Hock JM. Osteoprogenitor cells as targets for ex vivo gene transfer. J Bone Miner Res. 1998;13: 20-30. [DOI] [PubMed] [Google Scholar]
- 6.Niyibizi C, Wallach CJ, Mi Z, Robbins PD. Approaches for skeletal gene therapy. Crit Rev Eukaryot Gene Expr. 2002;12: 163-173. [DOI] [PubMed] [Google Scholar]
- 7.Shen FH, Visger JM, Balian G, Hurwitz SR, Diduch DR. Systemically administered mesenchymal stromal cells transduced with insulin-like growth factor-I localize to a fracture site and potentiate healing. J Orthop Trauma. 2002;16: 651-659. [DOI] [PubMed] [Google Scholar]
- 8.Dominici M, Pritchard C, Garlits JE, Hofmann TJ, Persons DA, Horwitz EM. Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. Proc Natl Acad Sci U S A. 2004;101: 11761-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99: 8932-8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428: 664-668. [DOI] [PubMed] [Google Scholar]
- 11.Wehner T, Bontert M, Eyupoglu I, et al. Bone marrow-derived cells expressing green fluorescent protein under the control of the glial fibrillary acidic protein promoter do not differentiate into astrocytes in vitro and in vivo. J Neurosci. 2003;23: 5004-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machein MR, Renninger S, de Lima-Hahn E, Plate KH. Minor contribution of bone marrow-derived endothelial progenitors to the vascularization of murine gliomas. Brain Pathol. 2003;13: 582-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297: 2256-2259. [DOI] [PubMed] [Google Scholar]
- 14.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116: 639-648. [DOI] [PubMed] [Google Scholar]
- 15.Camargo FD, Chambers SM, Goodell MA. Stem cell plasticity: from transdifferentiation to macrophage fusion. Cell Prolif. 2004;37: 55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg LM, Eisenberg CA. Stem cell plasticity, cell fusion, and transdifferentiation. Birth Defects Res Part C Embryo Today. 2003;69: 209-218. [DOI] [PubMed] [Google Scholar]
- 17.Shi D, Reinecke H, Murry CE, Torok-Storb B. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood. 2004;104: 290-294. [DOI] [PubMed] [Google Scholar]
- 18.Kalajzic I, Kalajzic Z, Kaliterna M, et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res. 2002;17: 15-25. [DOI] [PubMed] [Google Scholar]
- 19.Kalajzic I, Staal A, Yang WP, et al. Expression profile of osteoblast lineage at defined stages of differentiation. J Biol Chem. 2005;280: 24618-24626. [DOI] [PubMed] [Google Scholar]
- 20.Jiang X, Kalajzic Z, Maye P, et al. Histological analysis of GFP expression in murine bone. J Histochem Cytochem. 2005;53: 593-602. [DOI] [PubMed] [Google Scholar]
- 21.Bilic-Curcic I, Kronenberg M, Bellizzi J, Jiang X, Gardiner E, Rowe D. Visualizing levels of osteoblast differentiation by a two color promoter-GFP strategy: type I collagen-GFPcyan and osteocalcin-GFPtpz. Genesis. 2005;43: 87-98. [DOI] [PubMed] [Google Scholar]
- 22.Visnjic D, Kalajzic I, Gronowicz G, et al. Conditional ablation of the osteoblast lineage in Col2.3deltatk transgenic mice. J Bone Miner Res. 2001;16: 2222-2231. [DOI] [PubMed] [Google Scholar]
- 23.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103: 3258-3264. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y-H, Liu Y, Buhl K, Rowe D. Assessment of the action of transient and continuous PTH on primary osteoblast cultures expressing differentiation stage-specific GFP. J Bone Min Res. 2005;20: 5-11. [DOI] [PubMed] [Google Scholar]
- 25.Chen XD, Qian HY, Neff L, Satomura K, Horowitz MC. Thy-1 antigen expression by cells in the osteoblast lineage. J Bone Miner Res. 1999;14: 362-375. [DOI] [PubMed] [Google Scholar]
- 26.Robey PG, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985;37: 453-460. [PubMed] [Google Scholar]
- 27.Galotto M, Berisso G, Delfino L, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27: 1460-1466. [DOI] [PubMed] [Google Scholar]
- 28.Jackson KA, Snyder DS, Goodell MA. Skeletal muscle fiber-specific green autofluorescence: potential for stem cell engraftment artifacts. Stem Cells. 2004;22: 180-187. [DOI] [PubMed] [Google Scholar]
- 29.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63: 1059-1069. [DOI] [PubMed] [Google Scholar]
- 30.Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66: 1272-1278. [DOI] [PubMed] [Google Scholar]