Abstract
Raf kinases play an integral role in the classic mitogen-activated protein (MAP) kinase (Raf/MEK/extracellular signal-related kinase [ERK]) intracellular signaling cascade, but their role in specific developmental processes is largely unknown. Using a genetic approach, we have identified a role for B-Raf during hematopoietic progenitor cell development and during megakaryocytopoiesis. Fetal liver and in vitro embryonic stem (ES) cell–derived myeloid progenitor development is quantitatively impaired in the absence of B-Raf. Biochemical data suggest that this phenotype is due to the loss of a normally occurring rise in B-Raf expression and associated ERK1/2 activation during hematopoietic progenitor cell formation. However, the presence of B-raf–/– ES cell–derived myeloid progenitors in the bone marrow of adult chimeric mice indicates the lack of an obligate cell-autonomous requirement for B-Raf in myeloid progenitor development. The lack of B-Raf also impairs megakaryocytopoiesis. Thrombopoietin (Tpo)–induced in vitro expansion of ES cell–derived megakaryocyte-lineage cells fails to occur in the absence of B-Raf. Moreover, this quantitative in vitro defect in megakaryocyte-lineage expansion is mirrored by chimeric mice data that show reduced B-raf–/– genotype contribution in megakaryocytes relative to its contribution in myeloid progenitors. Together, these data suggest that B-Raf plays a cell-autonomous role in megakaryocytopoiesis and a permissive role in myeloid progenitor development.
Introduction
Cytokines play critical roles during hematopoietic development.1 Some, such as stem cell factor (SCF), regulate early hematopoiesis through their action on multipotent progenitors.1 Others, such as erythropoietin (Epo), regulate later stages of hematopoiesis through their effects on specific, lineage-committed cells.1 Thrombopoietin (Tpo), while first identified as a key cytokine regulator of megakaryocyte-lineage development,2-5 also plays a vital role in hematopoietic progenitor or stem cell biology.6,7 Cytokine effects are mediated by the activation of specific intracellular signaling pathways following binding to their cognate receptors.
While the intracellular signaling pathways activated by most hematopoietic cytokines have been identified, their biologic significance remains poorly understood. The classic mitogen-activated protein (MAP) kinase (Raf/MEK/extracellular signal-related kinase [ERK]) intracellular pathway can affect cell proliferation, apoptosis, and differentiation in various cell lines and systems,8,9 yet its role in hematopoiesis remains undefined. Moreover, since the MAP kinase cascade is activated by various cytokines, one would predict that its function is context dependent.
As an entry point to the classic MAP kinase pathway, Raf kinases play a key role in controlling the signal flow from activated cytokine receptors by activating MAP kinase/ERK kinase 1/2 (MEK 1/2), which in turn activates ERK 1/2, the pathway's key known effector.10 There are 3 known vertebrate Raf isoforms, A-Raf, B-Raf, and Raf-1.11 We recently reported that Raf-1 is not required for establishment of the myeloid progenitor compartment in murine bone marrow, megakaryocytopoiesis, or Tpo-induced ERK phosphorylation.12 As the Raf/MEK/ERK pathway is activated by a number of cytokines, including Tpo, and our Raf-1 findings raised questions about previously reported roles for Raf-1 in megakaryocytopoiesis,13 we have extended our genetic approach to better understand the role of other Raf family members in myeloid development, including megakaryocytopoiesis.
While Raf-1 is the most studied Raf family member, B-Raf is more widely expressed than originally thought and is possibly the most potent MEK/ERK activator among the Raf isoforms.11,14 Recent reports suggest an important role for B-Raf in a number of pathologic and physiologic events including melanoma, thyroid, ovarian, and colon cancer15; sensory and motor neuron survival16; and midgestational mouse embryogenesis.17-19 Moreover, the classic MAP kinase cascade is frequently activated in leukemia. This is most often due to Ras activating mutations,20 but primary B-raf mutations have been identified in acute leukemias and malignant lymphomas.21-23 Despite the important role of Ras activating mutations in hematopoietic pathology, and the recent entry of Raf inhibitors into clinical trails,24-26 the role of B-Raf in hematopoiesis remains undefined.
In this study, we characterized the role of B-Raf in myelopoiesis using B-raf–/– embryos, B-raf–/– embryonic stem (ES) cells cultured in vitro, and chimeric mice. The results indicate a role for B-Raf in myeloid progenitor cell formation and in megakaryocytopoiesis.
Materials and methods
Mice and genotyping
The generation of B-raf+/– mice was previously described.19 The mice were backcrossed one generation to an MF1 background, and B-raf–/– embryos were obtained by mating the backcrossed B-raf+/– males and females. Genotyping of fetuses was performed as previously described.19 Tissue samples were obtained at 12.5 to 14.5 days after conception (E12.5-E14.5), and littermates were used as wild-type controls. All procedures were performed in accordance with UCSF animal care guidelines.
Cell lines
Murine OP9 stromal cells, derived from the calvaria of newborn macrophage colony-stimulating factor (M-CSF)–deficient B6C3F1-op/op mice,27 and murine B-raf+/+ (R1) and B-raf–/– ES cells were maintained as previously described.19,28 ROSA-II B-raf–/– ES cells and ROSA-III B-raf+/– ES cells were maintained on irradiated mouse embryonic fibroblast feeder cells in complete Dulbecco modified Eagle medium (DMEM; GIBCO/Invitrogen, Carlsbad, CA) supplemented with 1000 units/mL mouse leukemia inhibitory factor (mLIF; Chemicon, Temecula, CA).
Fetal liver harvest
Fetal livers were removed using a sterile needle, passed through a 70-mm nylon mesh, and resuspended in red blood cell lysis buffer (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM EDTA [ethylenediaminetetraacetic acid], pH 7.4). Following isolation, cells were washed once in calcium- and magnesium-free phosphate-buffered saline (PBS-CMF) and a viable cell count was determined using a trypan blue dye exclusion assay. For morphologic examinations, isolated fetal liver cells were cytospun onto slides, air dried, and stained with Diff-Quick (Baxter, Westchester, PA).
Terminal deoxynucleotidyl transferase (TdT)–-mediated deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) analysis and bromo-deoxyuridine (BrdU) incorporation
TUNEL analysis was performed as previously described,12 with minor modification. Briefly, freshly isolated fetal liver cells were incubated with phycoerythrin (PE) anti-TER119 antibody (Pharmingen, San Diego, CA) before fixation, and TUNEL assays were performed using the In Situ Cell Death Detection Kit Fluorescein (Roche, Indianapolis, IN) according to the manufacturer's instructions. TER119-positive erythrocytes were analyzed by 2-color flow cytometry (FACScan; Becton Dickinson, San Jose, CA). For BrdU incorporation assays, isolated fetal liver cells were incubated in Iscoves modified Dulbecco medium (IMDM) media containing 10% fetal calf serum (FCS), 2 U/mL recombinant human erythropoietin (rhEPO; Ortho Biotech, Raritan, NJ) and 10 μM BrdU for one hour. The cells with incorporated BrdU were detected by flow cytometry (FACScan), using the In Situ Cell Proliferation Kit (Roche) according to the manufacturer's instructions.
Hematopoietic progenitor cell colony-forming assays
Colony-forming assays were performed in 35-mm plates at a density of 2 × 104 fetal liver cells/plate or 1 × 104 ES-derived hematopoietic cells/plate in methylcellulose media (Methocult 3234; Stem Cell Technologies, Vancouver, BC) supplemented with 50 ng/mL murine stem cell factor (mSCF; Peprotech, Rocky Hill, NJ), 10 ng/mL murine interleukin-3 (mIL-3; Peprotech), 4 U/mL rhEPO, and 50 ng/mL human TPO (hTPO; Genentech, South San Francisco, CA). The plates were incubated at 37°C in 5% CO2, and colonies were identified by microscopy after 7 to 10 days. In each experiment, 50 to 100 colonies were plucked, cytospun onto slides, and stained with Diff-Quick (Baxter) to determine the colony type.
Chimeric mice analysis
Chimeric mice were obtained by injection of ROSA-II B-raf–/– ES cells or ROSA-III B-raf+/– ES cells into blastocysts from C57BL6 mice. Peripheral blood was obtained by retro-orbital puncture using a capillary tube and EDTA anticoagulation, and complete blood counts were analyzed using a HEMAVET HV850 (CDC Technologies, Oxford, CT) hematology analyzer. To determine the genetic contribution by injected ES cells, genomic DNA was extracted from selected tissues using the DNeasy kit (Qiagen, Valencia, CA) and subjected to real-time polymerase chain reaction (PCR) to determine the relative B-raf+/– and B-raf–/– contribution. To determine the contribution in bone marrow myeloid progenitors, bone marrow mononuclear cells (BMMNCs) were isolated from each chimeric mouse using Isoprep (specific gravity 1.077; Robbins Scientific, Sunnyvale, CA), and plated at 1 × 104 cell/mL in methylcellulose media. Genotyping was performed on DNA extracted from each individual colony on day 10 of culture. To determine the ES cell genetic contribution to megakaryocytes, BMMNCs were cultured for 4 days in serum-free media29 supplemented with 50 ng/mL hTPO. Mature megakaryocytes were isolated by 2 rounds of discontinuous bovine serum albumin (BSA) density gradient (1.5%/3%) separation. Genomic DNA was extracted from the purified megakaryocytes and subjected to real-time PCR.
Real-time PCR for B-Raf genotype
Real-time PCR was performed in 96-well plates using a GeneAmp 5700 system (Applied Biosystems, Foster City, CA) essentially as described.30 DNA was obtained from selected tissues using the DNeasy kit (Qiagen) for animal cells per the manufacturer's recommendations. Ten-fold serial dilutions (103 to 100 copies/reaction) of DNA, obtained from white blood cells from known B-raf+/– mice, were used to generate standard curves for total DNA and B-Raf null DNA. Standard curves, calculated by the system's software using linear regression, were performed in triplicate in the same plate as the test samples to minimize interassay variability. Total DNA and B-Raf null DNA in each test sample was quantified by interpolation of the samples onto the standard curve. The amount of B-Raf null DNA was divided by the amount of total genomic DNA to determine the contribution of B-Raf null DNA in each sample. PCR reactions included 5 μL of a 1:10 dilution of the DNA lysate added to 10 μL PCR mixture consisting of 15 mM KCl, 5 mM Mg, 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 8.0), BSA (0.1 mg/mL), Triton X-100 (1%), 1 mM deoxynucleoside triphosphates (dNTPs; Roche Diagnostics, Mannheim, Germany), 1 μM of each primer (Integrated DNA Technologies, Coralville, IA), SYBR Green (18.75 Units/reaction; BioWhittaker Molecular Applications, Rockland, ME), and Amplitaq Gold (1.5 Units/reaction; Applied Biosystems). Real-time PCR was performed using the following cycle conditions: 10 minutes at 95°C followed by 45 cycles of 30 seconds at 95°C, 30 seconds at 60°C, and 45 seconds at 72°C for both B-Raf null and total genomic DNA. Dissociation curves were reviewed to confirm signal data. Primers used include the following: for detecting total DNA (murine H2K), 5′-ggcgcctcctccgcgggtac-3′ and 5′-tcaccagcctgctcccac-3′; for detecting B-Raf null DNA, 5′-gcctatgaagagtacaccagcaagctagatgccc-3′ and 5′-taggtttctgtggtgacttggggttgttccgtga-3′.19
In vitro differentiation of ES cells
B-raf+/+ and B-raf–/– ES cells were dissociated with 0.25% trypsin/EDTA and allowed to settle for 75 minutes to deplete adherent feeder cells. ES cells (1 × 104) were then seeded into each well of a 6-well plate containing confluent OP9 stromal cells and cultured in α–modified essential medium (α-MEM) medium (GIBCO/Invitrogen) supplemented with 20% FBS.28 After 5 days in culture, the cells were dissociated and seeded at 2 × 105 cells/well onto a fresh OP9 layer in the same culture medium supplemented with or without 50 ng/mL hTPO. Hematopoietic clusters were counted under a microscope after an additional 3 days of culture (ie, 8 days total). The following day, after 9 total days of culture, loosely adherent and nonadherent cells were either reseeded onto a new OP9 layer or used in methylcellulose colony-forming assays. After the day-9 replating, viable nonadherent cell number was determined using a trypan blue dye exclusion assay. Nonadherent cells were also cytospun for May-Giemsa staining, and differential counts were performed on 100 cells in each of 3 or more representative visual fields.
Cell surface marker and DNA ploidy analysis
For fetal liver cell surface marker analysis, freshly isolated fetal liver cells were incubated with PE-TER119 or fluorescein isothiocyanate (FITC)–CD45 antibody (Pharmingen) on ice for 45 minutes and analyzed by flow cytometry (FACScan). For DNA ploidy analysis, ES-derived day-12 nonadherent cells were stained with anti-CD41 antibody (Pharmingen) on ice for 45 minutes. Cells were then fixed in 100% methanol for 30 minutes on ice, washed, resuspended in PBS containing 100 U/mL DNAse-free RNase (Roche), incubated for one hour at 37°C; 50 μg/mL propidium iodide (Sigma) was then added and the cells were analyzed by 2-colored flow cytometry (FACScan) to determine DNA content of CD41+ cells.
Immunoblotting
Undifferentiated ES cells were collected after 75 minutes of settling to deplete adherent feeder cells, and starved in serum-free α-MEM medium for 3 hours. Cells were then stimulated with 20% FCS for 0 to 60 minutes. Differentiating ES cells on day 5 of coculture were collected after 60 minutes settling to deplete adherent OP9 cells, starved in serum-free α-MEM medium for 3 hours, and then stimulated with 20% FCS for 0 to 60 minutes. Protein lysates were generated and Western blots performed essentially as described.12 For quantification for ERK phosphorylation, the immunoblots were visualized by chemiluminescence and the images detected on X-ray films were quantified using NIH image software (Rockville, MD).
Statistics
Comparison between any 2 groups was performed by unpaired student t test for normally distributed data or alternate Welch t test for nonnormally distributed data.
Results
Impaired fetal hematopoiesis in the absence of B-Raf
B-raf–/– mice on a C57BL/6 background die in utero at days E9.5 to E11.5 with endothelial cell apoptosis and vascular leakage, precluding an opportunity to study fetal or adult hematopoiesis.19 To extend their survival, the C57BL/6 B-raf+/– mice were crossed one generation onto the outbred MF1 background. Interbreeding of the mixed background B-raf+/– mice generated B-raf–/– fetuses that live until day E14.5 with a normal Mendelian distribution, allowing us to obtain fetal liver tissue for hematopoiesis studies. The B-raf–/– fetuses were smaller than their littermates and manifested a bleeding tendency similar to that previously described on a C57BL/6 background,19 but it was delayed a few days.
Fetal livers from B-raf+/– × B-raf+/– matings were obtained on days E12.5, E13.5, and E14.5 to assess fetal hematopoiesis. No gross differences were seen between heterozygous (B-raf+/–) and wild-type (B-raf+/+) fetuses. In contrast, only 85% of B-raf–/– embryos contained an identifiable liver under the dissecting microscope at day E12.5, which declined to 45% by day E14.5 (Figure 1A). Moreover, the B-raf–/– fetal livers observed were consistently smaller, paler, and more fragile then their wild-type and heterozygous littermates. The visible size difference correlated with a significant difference in the number of viable cells obtained from B-raf–/– and B-raf+/+ fetal livers (Figure 1B), and quantification of TER119+ and CD45+ cells confirmed a reduced number of B-raf–/– hematopoietic cells (Figure 1C).
Figure 1.
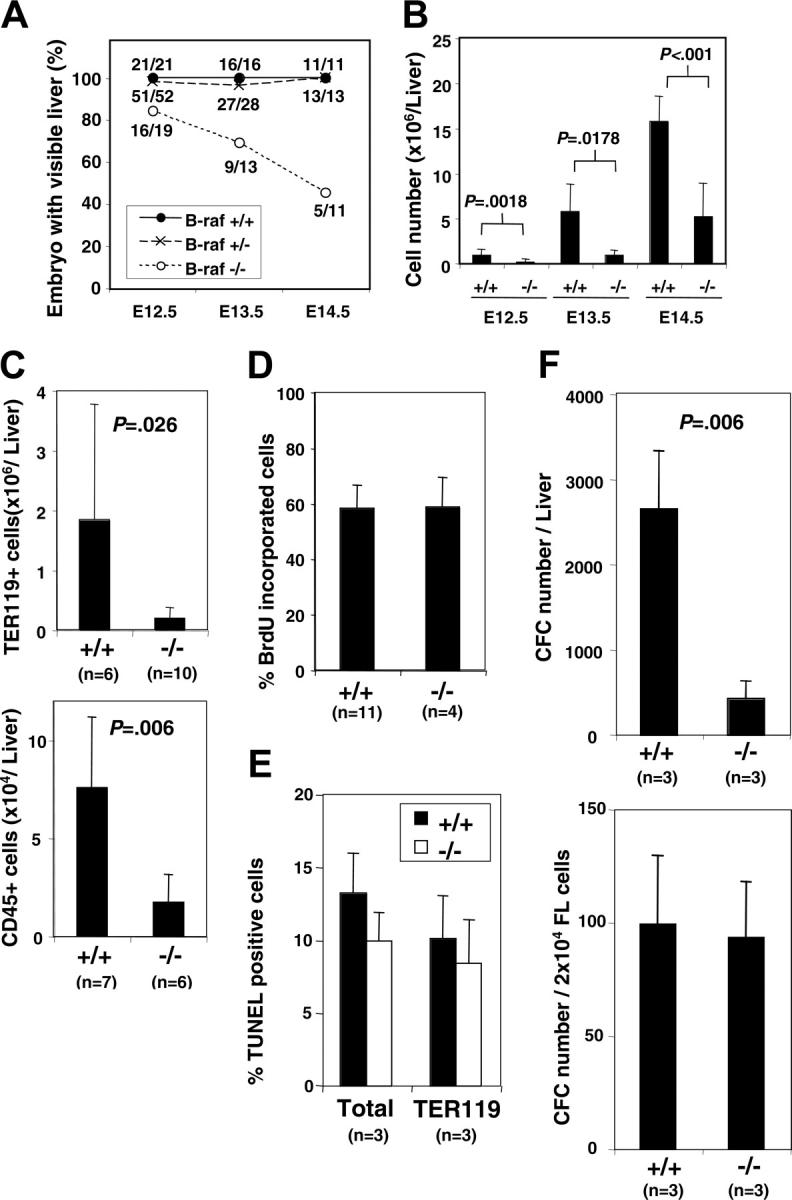
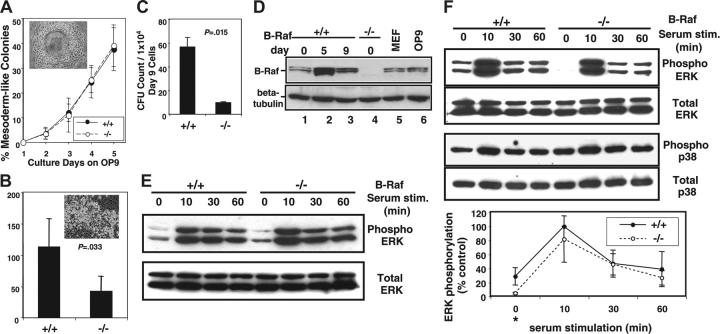
Fetal liver hematopoiesis in the absence of B-Raf. (A) The percentage of embryos with identifiable livers at gestation days E12.5, E13.5, and E14.5 is graphed for each indicated B-Raf genotype. The total number of embryos (denominator) and the number with identifiable livers (numerator) are indicated for each day. (B) The number of viable (trypan blue exclusion) fetal liver cells is presented for wild-type (+/+) and B-raf–/– (–/–) fetuses with a visible liver on gestation days E12.5, E13.5, and E14.5. (C) The number of TER119-positive (top) and CD45+ (bottom) cells identified by fluorescence-activated cell sorter (FACS) analysis is indicated for wild-type (+/+) and B-raf–/– (–/–) E12.5 fetal liver. (D) The percentage of freshly harvested E12.5 wild-type (+/+) and B-raf–/– (–/–) fetal liver cells incorporating BrdU is presented. Cells were incubated in IMDM media containing 10% FCS, 2 U/mL rhEPO, and 10 μM BrdU for one hour prior to detection of BrdU incorporation. (E) The percentage of TUNEL-positive cells determined by FACS analysis is reported for freshly harvested E12.5 total fetal liver cells (left), and for the predominant hematopoietic cell population, TER 119–positive cells (right). Differences are not statistically significantly different. (F) Methylcellulose colony-forming assays performed on freshly harvested E12.5 wild-type (+/+) and B-raf–/– (–/–) fetal liver cells. The total number of myeloid progenitors per fetal liver (FL) (top) and per 2 × 104 cells (bottom) is presented for wild-type (+/+) and B-raf–/– (–/–) cells. The data in panels B to F represent the average ± SD for the indicated number (n) of samples. B-raf–/– embryos without detectable livers are not included in the analyses in panels B to F. CFC indicates colony-forming cells.
Raf family members have been shown to play a role in cell proliferation and survival.9,31 BrdU and TUNEL assays were therefore performed to understand the mechanism underlying the abnormally low number of B-raf–/– fetal liver hematopoietic cells. Surprisingly, B-raf–/– cells did not have reduced BrdU uptake (Figure 1D) or increased TUNEL positivity (Figure 1E), implying that the quantitative defect in the B-raf–/– hematopoietic cells is not due to an abnormal net expansion of cells within the fetal liver itself. In addition, B-raf–/– fetal liver erythroid cells, the largest hematopoietic population within the fetal liver, were morphologically indistinguishable from wild-type erythroid cells (data not shown).
Taken together, these findings turned our attention to the myeloid progenitor pool. Using methylcellulose colony assays, we found that the total number of myeloid progenitor cells was decreased in the B-raf–/– fetal livers (Figure 1F, upper panel). However, the relative progenitor frequency as a fraction of the total number of fetal liver cells was not affected by the absence of B-Raf (Figure 1F, lower panel). Moreover, B-raf–/– fetal liver cells generated a full spectrum of myeloid colonies, including granulocyte-erythrocyte-megakaryocyte-macrophage colony-forming unit (CFU-GEMM) colonies containing all myeloid-lineage cells (data not shown). Together, these data suggest that the small fetal livers in B-raf–/– embryos reflect, at least in part, a failure to establish the proper number of myeloid progenitors in the absence of B-Raf. However, these findings do not distinguish a primary defect in hematopoiesis from a secondary defect related to impaired hepatogenesis or other environmental factors.
B-raf–/– ES cells have a quantitative defect in myeloid progenitor cell formation in vitro
To circumvent the confounding variable of hepatic development, and thereby better understand the role of B-Raf in the generation of myeloid progenitor cells, we characterized hematopoietic development of B-raf–/– and B-raf+/+ ES cells in vitro using the OP9 coculture system.27 Murine ES cells cultured on OP9 stromal cells without added cytokines differentiate to form mesodermal colonies by day 5. Disrupting and replating these colonies generates hematopoietic cell clusters on day 8 composed of small hematopoietic progenitor cells loosely adherent to the OP9 cells, providing an in vitro model system for hematopoietic progenitor cell generation.27
B-raf–/– ES cells cocultured with OP9 cells generate day-5 mesodermal colonies with the same frequency as wild-type ES cells (Figure 2A). However, when equal numbers of day-5 mesodermal colony cells are replated onto fresh OP9 cells, B-raf–/– cells generate significantly fewer day-8 hematopoietic clusters than do B-raf+/+ cells (Figure 2B). Moreover, the number of myeloid colony-forming progenitor cells generated is significantly reduced in the absence of B-Raf (Figure 2C), indicating a quantitative defect in progenitor cell development that mirrors the data presented for B-raf–/– fetal livers.
Figure 2.
Myeloid progenitor development by B-raf–/– ES cells cocultured with OP9 stromal cells in vitro. (A) The percentage of mesodermal colonies developed each day after plating equal numbers of undifferentiated ES cells on OP9 cells is indicated for days 1 to 5 of coculture. Inset figure shows the morphology of a typical mesodermal colony observed using a phase-contrast microscope (original magnification, × 100). The data represent the average ± SD for 3 independent experiments. (B) The number of day-8 hematopoietic clusters generated per 1 × 105 day-5 cells plated is presented for wild-type (+/+) and B-raf–/– (–/–) ES cells (P = .033). Inset figure shows a typical hematopoietic cluster (original magnification, × 200). Data are the average ± SD for 4 independent experiments. Images in panels A and B were obtained using a Nikon Eclipse TE300 microscope (Nikon, Melville, NY), IPLab Spectrum imaging software (Scanalytics, Fairfax, VA), and Photoshop (Adobe Systems, San Jose, CA). (C) The bar graph shows the number of myeloid progenitors obtained after plating 1 × 104 day-9 loosely adherent and floating cells generated from wild-type (+/+) and B-raf–/– (–/–) ES cells (P = .015). Data are the average ± SD for 3 independent experiments. (D) Western blot analysis of B-Raf expression. Cell lysates were prepared from undifferentiated ES cells (day 0) and OP9 cocultured differentiating ES cells (day 5 and day 9), fractionated by SDS-PAGE and immunoblotted with a B-Raf–specific antibody. Cell lysates from undifferentiated (day 0) B-raf–/– ES cells, mouse embryo fibroblasts (MEF), and OP9 cells serve as controls. The membrane was reblotted with β-tubulin–specific antibody to confirm equal loading. (E) Western blot analysis of serum-stimulated (20%; 0 to 60 minutes) undifferentiated day-0 ES cells. Cell lysates were fractionated by SDS-PAGE and immunoblotted for phosphorylated ERK1/2 and total ERK1/2. (F, top) Western blot analysis of serum-stimulated (20%; 0-60 minutes) differentiating ES cells on day 5. Cell lysates were fractionated by SDS-PAGE and immunoblotted for phosphorylated ERK1/2 and total ERK1/2. (Middle) Western blot for phospho- and total p38 performed as in the top panel. (Bottom) Quantification of ERK phosphorylation in serum-stimulated day-5 mesodermal colony cells is presented. The immunoblots were visualized using chemiluminescence and x-ray film, and the bands were quantified using NIH image software. Relative intensity of serum-induced ERK phosphorylation is expressed as a percentage of the peak (10-minute stimulation) level of wild-type cells (100%). The data are the average ± SD of 4 independent experiments. Basal ERK phosphorylation level in B-raf–/– cells was significantly decreased compared with wild-type cells (*P = .042).
B-Raf expression and ERK 1/2 activation in early hematopoietic progenitor cell formation
Western blots were performed to determine if there is a link between B-Raf expression, ERK phosphorylation, and the quantitative defect in myeloid progenitor development from B-raf–/– ES cells. Low-level B-Raf expression was found in undifferentiated (day 0) wild-type ES cells following adhesive cell selection to remove contaminating MEFs (Figure 2D). The absence of detectable B-Raf in similarly treated B-raf–/– ES cells demonstrates the effectiveness of the MEF removal (Figure 2D lanes 1,4-5). B-Raf expression is markedly up-regulated in the day-5 mesodermal colony cells, and then declines by the time mesodermal colony cells develop into hematopoietic clusters that contain the hematopoietic progenitors and more mature hematopoietic cells (Figure 2D lanes 1-3). The rise in B-Raf expression is readily seen by day 3 and is maximal by day 4 of the ES-OP9 coculture (data not shown). In light of the quantitative defect in hematopoietic progenitor cell formation (Figure 2C), the B-Raf developmental expression kinetics suggest a role for B-Raf in the establishment of day-5 mesodermal colony cells that are fully capable of generating myeloid progenitors.
Given the fact that B-Raf is a potent MEK activator,11,14 we next determined the extent of ERK phosphorylation in undifferentiated (day 0) ES cells and in day-5 mesodermal colony cells. The absence of B-Raf did not alter basal or serum-stimulated ERK 1/2 phosphorylation in undifferentiated (day 0) ES cells (Figure 2E). In contrast, basal ERK1/2 phosphorylation in B-raf–/– day-5 mesodermal cells was significantly decreased, by nearly 90%, compared with wild-type cells (Figure 2F). A similar loss of baseline activation is not seen for p38, a different MAP kinase family member that is not thought to be downstream of B-Raf (Figure 2F). In contrast with baseline activity, ERK1/2 phosphorylation following acute serum stimulation of B-raf–/– day-5 mesodermal cells was not statistically significantly affected, averaging 80% (range, 45%-108%; n = 4) that of wild-type cells (Figure 2F). Of note, the absolute differences in ERK phosphorylation at baseline and maximal stimulation are similar, suggesting that B-Raf is normally active at both time points, and that its activity is at most minimally modified by the serum stimulation. The B-Raf expression kinetics (Figure 2D) and the basal ERK activity in day-5 mesodermal cells (Figure 2F) correlate well with the quantitative defect in hematopoietic progenitor cell formation (Figure 2C).
B-Raf and Mpl signaling
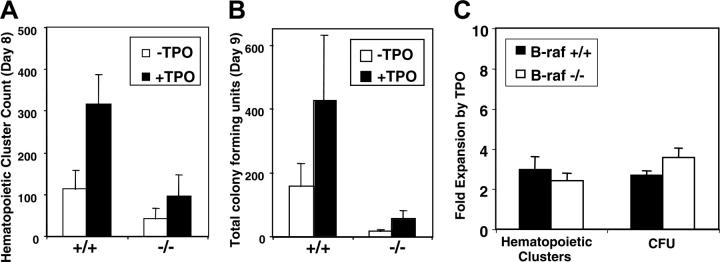
Tpo and its cognate receptor, Mpl, play a significant role in the generation and/or maintenance of hematopoietic stem and myeloid progenitor cells.6,32,33 The effect of Tpo on the generation of hematopoietic clusters and myeloid progenitors in B-raf–/– and wild-type ES cells was therefore determined. While B-raf–/– ES cells produce fewer hematopoietic clusters and fewer colony-forming cells than do B-raf+/+ ES cells (Figure 3A-B), Tpo-induced relative expansion of the hematopoietic clusters and colony-forming cells is not significantly affected by the absence of B-Raf (Figure 3C). Tpo is known to activate the classic MAP kinase pathway in megakaryocyte-lineage cells,12,34 but Tpo-induced ERK1/2 phosphorylation was not detectable in wild-type or B-raf–/– mesodermal colony cells (data not shown). It is not clear if this reflects a true absence of ERK phosphorylation downstream of Mpl at this hematopoietic developmental stage, or simply the fact that too small a fraction of day-5 cells are Tpo responsive. Regardless, these data demonstrate that while B-Raf is required for normal levels of myeloid progenitor formation (Figure 2B-C), Tpo-induced progenitor cell expansion is B-Raf independent (Figure 3C).
Figure 3.
Tpo-induced expansion of hematopoietic clusters and myeloid progenitors derived from B-raf–/– ES cells cocultured with OP9 cells. The number of (A) day-8 hematopoietic clusters and (B) day-9 myeloid progenitors generated from 1 × 105 day-5 mesodermal colony cells in the presence or absence of TPO (50 ng/mL from day-5 replating onward) are presented. (C) The fold expansion, calculated by dividing the number of clusters and progenitors generated in the presence of TPO by those generated in the absence of TPO, is plotted. (A-C) The data represent the average ± SD for 3 independent experiments.
Abnormal in vitro differentiation of B-raf–/– myeloid progenitors
We next characterized myeloid development in the OP9 system beyond 9 days in culture to evaluate the role of B-Raf in the differentiation of myeloid progenitors into mature blood cells. When cultured in the absence of added cytokines, B-raf–/– ES cell–derived hematopoietic cells expand (Figure 4A, upper panel) and differentiate (Figure 4B upper panel) similarly to wild-type ES cells, including a low number (< 5%) of megakaryocyte-lineage cells, and a marked expansion of mast cells from day 13 onward (Figure 4B top panel). When cultured with EPO to promote erythroid development, there was also no developmental difference between B-raf–/– and B-raf+/+ ES cell cultures. For both genotypes, erythrocytes constituted more than 90% of cells on day 11 to day 15, and mast cells constituted only 10% to 20% of cells on day 17 (data not shown).
Figure 4.
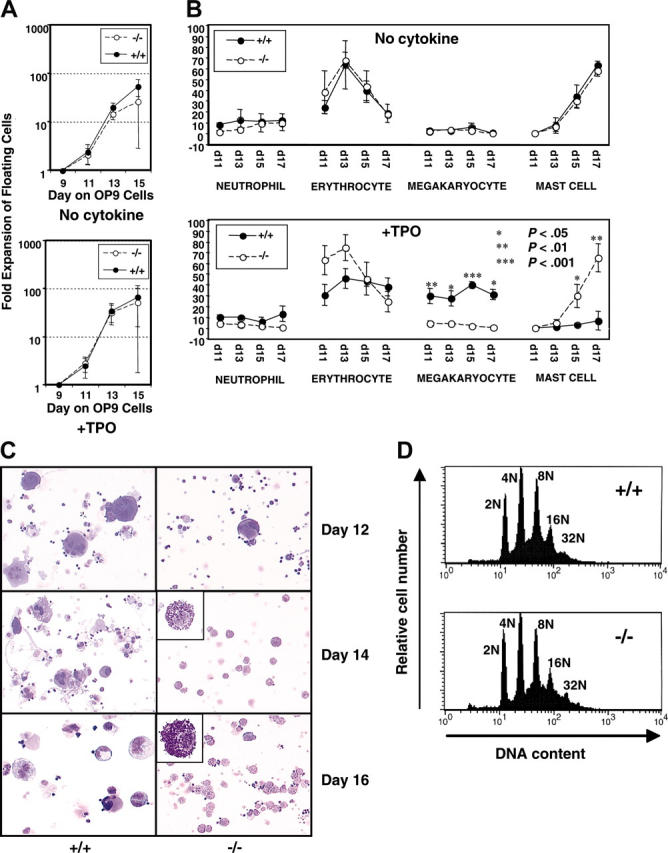
Myeloid-lineage development of B-raf–/– ES cells cocultured with OP9 cells. (A) B-raf–/– (–/–) and wild-type (+/+) hematopoietic cells from day 9 of OP9 coculture were replated on a fresh OP9 layer, and the number of viable floating and loosely adherent cells generated on days 11 to 15 in the absence (top) or presence (bottom) of 50 ng/mL TPO was determined using trypan blue dye exclusion. The fold expansion for each day is plotted on log scale. (B) Cell differentials of the floating cells generated on days 11 to 17 in the absence (top) or presence (bottom) of TPO (50 ng/mL) were determined by microscopic examination of Giemsa-stained cells. The y-axis indicates the percentage of each lineage cell type. Immature blastic cells and macrophages were also detected in floating cells on day 11 and days 13 to 17, respectively (data not shown). (A-B) The data represent the average ± SD for 3 independent experiments. (C) Cell morphology of wild-type and B-raf–/– ES-derived hematopoietic cells developed on days 12 to 16 of coculture in the presence of 50 ng/mL TPO is presented using cytospun cells stained with Diff-Quick. Original magnification, × 200;× 1000 for insets, which highlight the mast cells. Images were obtained using a Leica DMLB microscope (Leica, Bannockburn, IL) and ScionImage software (ScionImage, Frederick, MD), and were cropped with Adobe Photoshop. (D) DNA ploidy distribution for B-raf–/– (–/–) and wild-type (+/+) ES-derived CD41+ cells shows no significant difference between the 2 genotypes, with DNA ploidy ranging from 2N to 32N for each. One of 3 representative DNA ploidy experiments is shown.
Given Tpo's role in early and late hematopoiesis,35 and that it activates the classic MAP kinase pathway in megakaryocytes,12,34 we performed similar OP9 developmental experiments in the presence of added Tpo. In the presence of Tpo, B-raf–/– and B-raf+/+ ES cells expanded similarly from day 9 onward, (Figure 4A, lower panel), but there was a marked difference in their differentiation (Figure 4B lower panel). With Tpo, 35% to 45% of the B-raf+/+ cells from days 11 to 17 were of the megakaryocyte lineage, as defined by morphologic examination of Giemsa-stained cells and CD41 expression. In contrast, B-raf–/– ES cells failed to ever generate more than 8% megakaryocyte-lineage cells (Figure 4B), and the same developmental phenotype was observed for 2 independently derived B-raf–/– ES cell lines. Strikingly, the cell population generated from B-raf–/– ES cells, including the marked expansion of mast cells starting at day 13, matches that generated from wild-type or B-raf–/– ES cells grown without added Tpo (Figure 4B-C). Therefore, while B-Raf is not required for Tpo-induced expansion of hematopoietic progenitors (Figure 3C), and its absence has little effect on the total number of hematopoietic cells obtained after 9 days of culture (Figure 4A), B-Raf plays a critical role in Tpo-induced expansion of the megakaryocyte lineage (Figure 4B-C). Despite this marked quantitative defect in generating megakaryocyte-lineage cells in the absence of B-Raf, maturation along the megakaryocyte lineage, as defined by DNA ploidy distribution of ES-derived CD41+ cells, is unaffected by the absence of B-Raf (Figure 4D).
Myeloid development of B-raf–/– ES cells in vivo
To further explore the role of B-Raf in myelopoiesis in vivo, chimeric animals were generated by injecting B-raf–/– ES cells into wild-type C56BL/6 blastocysts. Four of 13 pups generated from B-raf–/– ES cell injections had agouti coat color indicating contribution from the injected B-raf–/– ES cell, as did 1 of 3 pups following blastocyst injection with B-raf+/– ES cells. PCR confirmed contribution of the ES cell genotype (B-raf–/– or B-raf+/–) in the toe DNA and peripheral blood DNA from only the agouti mice. The complete blood counts, including white cell differentials, did not differ between the 4 pups with B-raf–/– contribution in the peripheral blood and the 9 pups without.
To better understand the role of B-Raf in myeloid progenitor cell development, bone marrow harvested from the B-raf–/– chimeric animals was plated in methylcellulose, and individual colonies were picked and genotyped for B-Raf. All colonies scored either B-raf–/– or B-raf+/+, confirming clonality, and the percentage of B-raf–/– myeloid progenitors ranged from 2.1 to 86.7 (Table 1). B-Raf is therefore not required in a cell-autonomous manner for myeloid progenitor formation in vivo.
Table 1.
The relative ES cell contribution to the indicated tissues in chimeric mice
| Injected ES cells and mouse no. | % ES contribution in toe tissue | % ES contribution in myeloid CFCs (no. B-raf-/- colonies/total no. colonies assayed) | % ES contribution in megakaryocytes (% contribution relative to myeloid CFCs) |
|---|---|---|---|
| B-raf-/- | |||
| 1 | 23.1 | 25.0 (16/64) | 6.4 (25.6) |
| 2 | 25.9 | 35.8 (19/53) | NA |
| 8 | 61.1 | 86.7 (39/45) | 32.7 (37.7) |
| 9 | 31.4 | 2.1 (1/47) | 0.2 (9.5) |
| B-raf-/- | |||
| 3 | 6.4 | 13.6 (6/44) | 8.7 (64.0) |
CFCs indicates colony-forming cells; NA, not applicable.
Given the difference in the blood cell differential generated from Tpo-stimulated B-raf–/– and B-raf+/+ ES cells in vitro, including a 4- to 5-fold decrease in the percentage of megakaryocyte-lineage cells generated in the absence of B-Raf (Figure 4B), real-time PCR was used to determine the relative B-Raf genotype contribution in mature megakaryocytes from the chimeric mice. The B-raf–/– genotype contribution to megakaryocytes averaged only 24% (range, 10%-38%) of the B-raf–/– genotype contribution found in the myeloid progenitors of the same mouse (Table 1). This relative reduction in B-raf–/– contribution to megakaryocytes relative to hematopoietic progenitor cells parallels the 4- to 5-fold difference in megakaryocyte-lineage cells generated in vitro from wild-type and B-raf–/– ES cells (Figure 4B lower panel), further supporting a quantitative role for B-Raf in the development of megakaryocyte-lineage cells from myeloid progenitors.
Discussion
Using 3 complementary genetic models, we have identified a role for B-Raf in hematopoietic progenitor cell formation and in megakaryocytopoiesis. B-raf–/– embryos have a quantitative defect in fetal liver hematopoietic progenitor cell development (Figure 1), which is reproduced in vitro when B-raf–/– ES cells are cocultured with OP9 cells (Figure 2). Biochemical data suggest that this phenotype is due to the loss of a normally occurring rise in B-Raf expression and its associated rise in basal ERK1/2 activation (Figure 2). However, the presence of B-raf–/– pluripotent progenitors in adult chimeric mice (Table 1) demonstrates the lack of an obligate cell-autonomous requirement for B-Raf in definitive hematopoietic progenitor cell formation. Tpo is known to activate the classic MAP kinase pathway36 and has physiologic effects both in early (stem/progenitor cells) and late (megakaryocyte lineage) hematopoiesis.37,38 Tpo-stimulated expansion of ES cell–derived myeloid progenitor cells is not altered by the lack of B-Raf (Figure 4A), but Tpo-induced expansion of the megakaryocyte lineage, and concomitant suppression of mast cell production, is abrogated in the absence of B-Raf (Figure 4B). The quantitative defect in megakaryocyte-lineage expansion in vitro is consistent with our chimeric mice data that show reduced B-raf–/– genotype contribution in megakaryocytes relative to myeloid progenitors (Table 1).
The absence of B-Raf produces a quantitative defect in myeloid progenitor cells. However, the diminished number of progenitor cells in B-raf–/– fetal livers is not simply due to reduced replication or increased apoptotic cell death within the liver (Figure 1D-E), 2 biologic functions often associated with Raf family members.9,31 In aggregate, these data suggest that B-Raf plays a critical role in establishing the proper number of myeloid progenitors within the fetal liver. Mechanistically, this quantitative defect could be secondary to decreased production of the hematopoietic cells that populate the developing liver, or to their retention within the developing liver; the latter could reflect a defect intrinsic to the hematopoietic cells or in hepatogenesis. Long-term repopulating hematopoietic stem cells (LR-HSCs) first appear and expand in the embryo proper in the aortagonad-mesonephros (AGM) region, and then migrate into the fetal liver where development of definitive hematopoiesis progresses.39,40 B-Raf could be important for establishing the proper AGM environment for the development and expansion of HSCs, a hypothesis consistent with our chimeric animal data.
Primary endothelial cells isolated from the mouse AGM region41 and a stromal cell line derived from the murine AGM region42 can each support the expansion of murine bone marrow HSCs. Given the known endothelial defect in B-raf–/– embryos,19 defective AGM endothelium may explain the impaired definitive hematopoiesis in B-raf–/– embryos. A conditional B-Raf knock-out mouse under development will help us address these possibilities. Furthermore, a recent report of endothelial cell development within mesodermal colonies generated on OP9 cells43 suggests that murine ES/OP9 cell coculture may provide an in vitro model to study the role of endothelial cells in the early hematopoietic microenvironment.
Raf protein activity is traditionally measured by its kinase activity, or by activation of the classic MAP kinase signaling cascade that leads to ERK 1/2 phosphorylation.11,44-46 Day-5 B-raf–/– mesodermal colony cells have a marked reduction in basal ERK 1/2 phosphorylation (Figure 2F), a developmental time point that appears critical for establishing the proper number of hematopoietic progenitor cells, and where B-Raf expression is normally up-regulated (Figure 2B-D). However, the B-Raf contribution to basal ERK 1/2 phosphorylation is developmental-context dependent, being important in day-5 mesodermal colony cells but not in undifferentiated ES cells (Figure 2E-F), which correlates with the developmental increase in B-Raf expression in the day-5 differentiated derivatives (Figure 2D). Taken together, the data suggest that the loss of rising basal B-Raf activity, a reflection of increased B-Raf expression, underlies the quantitative defect in hematopoietic progenitor formation seen in the absence of B-Raf. However, a mechanistically important kinase-independent role for B-Raf is also possible. Of note, others have also reported a key role for B-Raf in basal ERK activity using a B-raf–/– avian B-cell line.47 Moreover, using cells deleted for the raf-1 and/or B-raf genes, they found that B-Raf is responsible for baseline MAP kinase signaling activity, while B-Raf and Raf-1 contribute to ERK 1/2 phosphorylation following acute stimulation.47 Our data also suggest that during hematopoiesis different Raf isoforms play different roles in ERK 1/2 activation at baseline and following stimulation (Figure 2F). Genetic studies using cells lacking both Raf-1 and B-Raf should further our understanding of the role of Raf isoforms in ERK 1/2 activation during hematopoiesis.
In addition to its effect on hematopoietic progenitor development, the absence of B-Raf also affected megakaryocytopoiesis. Wild-type and B-raf–/– ES cells cocultured with OP9 cells without added cytokines generate an identical mature cell differential after day 9 of culture (Figure 4B). However, the Tpo-induced expansion of the megakaryocyte lineage, and the suppression of mast cell development observed with wild-type ES cells are abrogated by the absence of B-Raf (Figure 4B). Interestingly, Epo-induced cell type production (ie, differential) is unaffected by the absence of B-Raf, demonstrating a distinct difference in the role of B-Raf downstream of Tpo and the Epo receptors. This not only demonstrates the importance of physiologic context when identifying the biologic role of Raf family members, but it further validates the abnormal phenotype manifested in the presence of Tpo. Moreover, combined with the recently described requirement of Raf-1 in erythropoiesis48 but not in megakaryocytopoiesis,12 it appears that Raf family members do not simply provide redundant activities during hematopoiesis.
Prevailing thought holds that hematopoietic cytokines are permissive and not instructive, and that cell fate in myeloid-lineage development is driven by stochastic events occurring within individual cells.49,50 However, if Tpo simply permits the expansion of cells already capable of maturing along the megakaryocyte lineage, one would not predict a decrease in the production of other cell lineages (ie, mast cells) as we have observed (Figure 3B). Interestingly, Raf-1 expression level was recently reported to dictate lineage choice by FDCP myeloid progenitor cells.51 As such, B-Raf expression level might influence megakaryocyte/mast cell lineage choice at an early multipotent progenitor level, an effect made apparent in conditions supporting megakaryocyte-lineage survival.
Our genetic approach to B-Raf function provides the first link between B-Raf and specific aspects of myelopoiesis. Taken together, our findings implicate at least 2 independent roles for B-Raf in myelopoiesis, one during hematopoietic progenitor cell development and the other during megakaryocytopoiesis. With the recent introduction of Raf inhibitors into clinical cancer therapy trials,24-26 these findings may help to predict possible side effects and to tailor monitoring and therapy accordingly. Our next goal is to understand the specific molecular mechanisms that underlie the genetic phenotypes described, which we will pursue using B-Raf f/f mice with an inducible recombination system.
Acknowledgments
We thank Martin McMahan (UCSF) for helpful discussions, and we are grateful to Genentech for their generous support in providing recombinant human Tpo.
Prepublished online as Blood First Edition Paper, March 22, 2005; DOI 10.1182/blood-2004-11-4458.
Supported by National Institutes of Health (NIH) grant HL54476 (Transfusion Medicine SCORE).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81: 2844-2853. [PubMed] [Google Scholar]
- 2.Wendling F, Maraskovsky E, Debili N, et al. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994;369: 571-574. [DOI] [PubMed] [Google Scholar]
- 3.Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369: 568-571. [DOI] [PubMed] [Google Scholar]
- 4.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369: 533-538. [DOI] [PubMed] [Google Scholar]
- 5.Bartley TD, Bogenberger J, Hunt P, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994;77: 1117-1124. [DOI] [PubMed] [Google Scholar]
- 6.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A. 1998;95: 1195-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaushansky K. Thrombopoietin and hematopoietic stem cell development. Ann N Y Acad Sci. 1999;872: 314-319. [DOI] [PubMed] [Google Scholar]
- 8.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22: 153-183. [DOI] [PubMed] [Google Scholar]
- 9.Troppmair J, Rapp UR. Raf and the road to cell survival: a tale of bad spells, ring bearers and detours. Biochem Pharmacol. 2003;66: 1341-1345. [DOI] [PubMed] [Google Scholar]
- 10.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(pt 2): 289-305. [PMC free article] [PubMed] [Google Scholar]
- 11.Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272: 4378-4383. [DOI] [PubMed] [Google Scholar]
- 12.Kamata T, Pritchard CA, Leavitt AD. Raf-1 is not required for megakaryocytopoiesis or TPO-induced ERK phosphorylation. Blood. 2004;103: 2568-2570. [DOI] [PubMed] [Google Scholar]
- 13.Garcia J, de Gunzburg J, Eychene A, Gisselbrecht S, Porteu F. Thrombopoietin-mediated sustained activation of extracellular signal-regulated kinase in UT7-Mpl cells requires both Ras-Raf-1- and Rap1-B-Raf-dependent pathways. Mol Cell Biol. 2001;21: 2659-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchard CA, Samuels ML, Bosch E, McMahon M. Conditionally oncogenic forms of the A-Raf and B-Raf protein kinases display different biological and biochemical properties in NIH 3T3 cells. Mol Cell Biol. 1995;15: 6430-6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta. 2003;1653: 25-40. [DOI] [PubMed] [Google Scholar]
- 16.Wiese S, Pei G, Karch C, et al. Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat Neurosci. 2001;4: 137-142. [DOI] [PubMed] [Google Scholar]
- 17.Kolch W. To be or not to be: a question of B-Raf? Trends Neurosci. 2001;24: 498-500. [DOI] [PubMed] [Google Scholar]
- 18.Wojnowski L, Stancato LF, Larner AC, Rapp UR, Zimmer A. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech Dev. 2000;91: 97-104. [DOI] [PubMed] [Google Scholar]
- 19.Wojnowski L, Zimmer AM, Beck TW, et al. Endothelial apoptosis in Braf-deficient mice. Nat Genet. 1997;16: 293-297. [DOI] [PubMed] [Google Scholar]
- 20.Radich JP, Kopecky KJ, Willman CL, et al. N-ras mutations in adult de novo acute myelogenous leukemia: prevalence and clinical significance. Blood. 1990;76: 801-807. [PubMed] [Google Scholar]
- 21.Smith ML, Snaddon J, Neat M, et al. Mutation of BRAF is uncommon in AML FAB type M1 and M2. Leukemia. 2003;17: 274-275. [DOI] [PubMed] [Google Scholar]
- 22.Lee JW, Soung YH, Park WS, et al. BRAF mutations in acute leukemias. Leukemia. 2004;18: 170-172. [DOI] [PubMed] [Google Scholar]
- 23.Lee JW, Yoo NJ, Soung YH, et al. BRAF mutations in non-Hodgkin's lymphoma. Br J Cancer. 2003;89: 1958-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64: 7099-7109. [DOI] [PubMed] [Google Scholar]
- 25.Bollag G, Freeman S, Lyons JF, Post LE. Raf pathway inhibitors in oncology. Curr Opin Investig Drugs. 2003;4: 1436-1441. [PubMed] [Google Scholar]
- 26.Ahmad T, Eisen T. Kinase inhibition with BAY 43-9006 in renal cell carcinoma. Clin Cancer Res. 2004;10: 6388S-6392S. [DOI] [PubMed] [Google Scholar]
- 27.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265: 1098-1101. [DOI] [PubMed] [Google Scholar]
- 28.Eto K, Murphy R, Kerrigan SW, et al. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A. 2002;99: 12819-12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiraga M, Ritchie A, Aidoudi S, et al. Primary megakaryocytes reveal a role for transcription factor NF-E2 in integrin alpha IIb beta 3 signaling. J Cell Biol. 1999;147: 1419-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy EL, Lee TH, Chafets D, et al. Higher human T lymphotropic virus (HTLV) provirus load is associated with HTLV-I versus HTLV-II, with HTLV-II subtype A versus B, and with male sex and a history of blood transfusion. J Infect Dis. 2004;190: 504-510. [DOI] [PubMed] [Google Scholar]
- 31.Kerkhoff E, Rapp UR. Cell cycle targets of Ras/Raf signalling. Oncogene. 1998;17: 1457-1462. [DOI] [PubMed] [Google Scholar]
- 32.Ku H, Yonemura Y, Kaushansky K, Ogawa M. Thrombopoietin, the ligand for the Mpl receptor, synergizes with steel factor and other early acting cytokines in supporting proliferation of primitive hematopoietic progenitors of mice. Blood. 1996;87: 4544-4551. [PubMed] [Google Scholar]
- 33.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87: 2162-2170. [PubMed] [Google Scholar]
- 34.Rojnuckarin P, Drachman JG, Kaushansky K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis. Blood. 1999;94: 1273-1282. [PubMed] [Google Scholar]
- 35.Kaushansky K. Thrombopoietin: from theory to reality. Int J Hematol. 2002;76(suppl 1): 343-345. [DOI] [PubMed] [Google Scholar]
- 36.Drachman JG, Rojnuckarin P, Kaushansky K. Thrombopoietin signal transduction: studies from cell lines and primary cells. Methods. 1999;17: 238-249. [DOI] [PubMed] [Google Scholar]
- 37.Kaushansky K. Thrombopoietin: a tool for understanding thrombopoiesis. J Thromb Haemost. 2003;1: 1587-1592. [DOI] [PubMed] [Google Scholar]
- 38.Kaushansky K. Thrombopoietin: accumulating evidence for an important biological effect on the hematopoietic stem cell. Ann N Y Acad Sci. 2003;996: 39-43. [DOI] [PubMed] [Google Scholar]
- 39.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1: 291-301. [DOI] [PubMed] [Google Scholar]
- 40.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86: 897-906. [DOI] [PubMed] [Google Scholar]
- 41.Li W, Johnson SA, Shelley WC, et al. Primary endothelial cells isolated from the yolk sac and para-aortic splanchnopleura support the expansion of adult marrow stem cells in vitro. Blood. 2003;102: 4345-4353. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka S, Tsuji K, Hisakawa H, et al. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood. 2001;98: 6-12. [DOI] [PubMed] [Google Scholar]
- 43.Hirashima M, Bernstein A, Stanford WL, Rossant J. Gene-trap expression screening to identify endothelial-specific genes. Blood. 2004;104: 711-718. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakis JM, App H, Zhang XF, et al. Raf-1 activates MAP kinase-kinase. Nature. 1992;358: 417-421. [DOI] [PubMed] [Google Scholar]
- 45.Howe LR, Leevers SJ, Gomez N, Nakielny S, Cohen P, Marshall CJ. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71: 335-342. [DOI] [PubMed] [Google Scholar]
- 46.Dent P, Haser W, Haystead TA, Vincent LA, Roberts TM, Sturgill TW. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992;257: 1404-1407. [DOI] [PubMed] [Google Scholar]
- 47.Brummer T, Shaw PE, Reth M, Misawa Y. Inducible gene deletion reveals different roles for B-Raf and Raf-1 in B-cell antigen receptor signalling. EMBO J. 2002;21: 5611-5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolbus A, Pilat S, Husak Z, et al. Raf-1 antagonizes erythroid differentiation by restraining caspase activation. J Exp Med. 2002;196: 1347-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa M. Stochastic model revisited. Int J Hematol. 1999;69: 2-5. [PubMed] [Google Scholar]
- 50.Goncalves F, Lacout C, Villeval JL, Wendling F, Vainchenker W, Dumenil D. Thrombopoietin does not induce lineage-restricted commitment of Mpl-R expressing pluripotent progenitors but permits their complete erythroid and megakaryocytic differentiation. Blood. 1997;89: 3544-3553. [PubMed] [Google Scholar]
- 51.Haughn L, Hawley RG, Morrison DK, von Boehmer H, Hockenbery DM. BCL-2 and BCL-XL restrict lineage choice during hematopoietic differentiation. J Biol Chem. 2003;278: 25158-25165. [DOI] [PubMed] [Google Scholar]