Abstract
Megakaryocytes generate platelets by remodeling their cytoplasm into long proplatelet extensions, which serve as assembly lines for platelet production. Platelet packaging and release concludes at the tips of each proplatelet. Essential in this process is the distribution of organelles and platelet-specific granules into the nascent platelets. To investigate the mechanism of delivery of organelles into putative platelets, the distribution and dynamics of organelles/granules was monitored. Individual organelles are sent from the cell body to the proplatelets where they move bidirectionally until they are captured at proplatelet ends. Movement occurs at approximately 0.2 μm/min, but pauses and changes in direction are frequent. At any given time, approximately 30% of organelles/granules are in motion. Actin poisons do not diminish organelle motion, and vesicular structures are intimately associated with the microtubules. Therefore, movement appears to involve microtubule-based forces. Bidirectional organelle movement is conveyed by the bipolar organization of microtubules within the proplatelet, as kinesin-coated beads move bidirectionally on the microtubule arrays of permeabilized proplatelets. Movement of organelles along proplatelets involves 2 mechanisms: organelles travel along microtubules, and the linked microtubules move relative to each other. These studies demonstrate that the components that form platelets are delivered to and assembled de novo along proplatelets.
Introduction
Platelets release from megakaryocytes as small subcellular discs with high fidelity into blood where they function to preserve the integrity of the vascular system. In response to injury, in which the continuity of the blood vessel is compromised, platelets bind, change shape, release their granule contents, and aggregate to seal off the damaged section of the vessel. Despite the importance of blood platelets and the knowledge for 100 years that they are derived from megakaryocytes,1 there is still no consensus for how platelets are assembled by megakaryocytes.2
A megakaryocyte's brief lifetime follows a maturation process involving the destruction and removal of the residual cell body and the nuclear material and culminates in the release of 100 to 1000 platelets. Megakaryocytes must not only produce and replicate the assembly of the specialized cytoskeleton of each platelet but also load every platelet with the appropriate allotment of organelles and granules essential for their hemostatic function. Identifying the mechanisms of organelle delivery during platelet production is central to our understanding of platelet biogenesis. Two major models of platelet biogenesis have been proposed. According to the cytoplasmic fragmentation model, an extensive system of internal membranes within the megakaryocyte cytoplasm demarcates fields or territories of prepackaged platelets, and fragmentation of the cytoplasm along these fracture lines releases platelets.3,4 In contrast, the proplatelet model predicts that platelets are released from megakaryocytes in a process that remodels the entire megakaryocyte cytoplasm into long, beaded extensions termed proplatelets.5-8 The models differ dramatically in the proposed mechanisms by which organelles and granules are loaded into platelets. Therefore, establishing the mechanisms by which organelles are transported into putative platelets places constraints on global mechanisms of platelet production. The cytoplasmic fragmentation model predicts packaging of organelles within the body of the megakaryocyte.9 In contrast, the proplatelet model stipulates that platelets assemble and package their organelles de novo within proplatelets and requires that the intracellular components of platelets be sent from their sites of synthesis in the megakaryocyte cell body to the proplatelets. One major distinction between these 2 models is that, although long-range organelle transport is not essential for cytoplasmic fragmentation, it is crucial for platelet production via proplatelets.
With the aid of various culture systems using megakaryocytes isolated from humans,10,11 guinea pigs,12,13 and mice,14-17 many investigators have documented in vitro proplatelet formation and provided insights into their formation. Proplatelet intermediate structures are essential for murine platelet formation in vitro.18,19 These distinctive cellular extensions are built by scaffolds of motile microtubules. Platelet formation begins as microtubules assemble and move to the cortex of megakaryocytes. These microtubules then coalesce into bundles that fill the shafts of proplatelets and provide the force for proplatelet elongation.20 Similar to axons and dendrites of neurons, the microtubule bundles form parallel arrays that run from the cell body to the ends of the proplatelets. However, the microtubule bundles within proplatelets do not terminate at the tips as they do in the neuronal extensions, but instead make U-turns inside the platelet ends and reenter the shafts of the proplatelets.21 The finding that microtubule coils, similar to those observed in blood platelets, are detected predominantly at the bulbous ends of proplatelets and not within the platelet-sized swellings along the length has lead us to hypothesize that the formation of platelets completes at the ends of proplatelets. To increase platelet production, proplatelet ends are amplified by an actin-based mechanism that bifurcates the shafts of proplatelets.21
The cytoskeletal mechanisms that power and regulate organelle distribution during platelet production are still unknown. The high degree of spatial and temporal organization of molecules and organelles within cells is made possible by protein machines that transport components to various destinations within the cytoplasm.22 The most widely used mechanism for intracellular transport involves molecular motor proteins that carry cargo directionally along a cytoskeletal track. In general, myosin motors, particularly class V myosins, transport cargo along actin filaments, whereas kinesin and cytoplasmic dynein motors move cargo along microtubules.23 Proplatelet-producing megakaryocytes contain high concentrations of both actin filaments and microtubules. Is directed organelle motion during platelet production driven over actin filaments, microtubules, or do both polymer systems contribute? The need to transport organelles over extremely long distances along proplatelets presents an unusual challenge to developing megakaryocytes. A complete understanding of the regulation of organelle transport during platelet production requires determination of the organization of microtubules along the proplatelets and identification of the motors associated with organelle transport.
These issues motivated the present studies in which we sought to determine the mechanisms by which organelles are transported and targeted to putative platelets. If platelet production occurs predominantly along proplatelets, the intracellular components of platelets must be sent from their sites of synthesis in the megakaryocyte cell body to the proplatelets. The microtubule bundles running from the cell body to the nascent platelets are well-positioned to provide lines of transport for this long-range assembly process. To define the details of organelle transport to assembling platelets, organelle movement along proplatelets in living megakaryocytes was examined using time-lapse microscopy of cells labeled with organelle-specific fluorescent dyes. A detailed characterization of organelle dynamics in proplatelets reveals that organelles and granules translocate individually and bidirectionally along the proplatelet shaft and are captured once they enter the nascent platelet at the proplatelet tip. To investigate the cytoskeletal mechanics of this organelle motility, the motion of organelles in megakaryocytes that had been pharmacologically manipulated by cytoskeletal poisons was analyzed. Our results suggest that the organelle movements along proplatelets are microtubule based and involve the following 2 mechanisms: (1) organelles move over microtubules and (2) organelles ride “piggyback” along the surface of sliding microtubules. The force for the former is the microtubule motor protein kinesin that localizes on organelles along the proplatelets. The force for the latter is provided by cytoplasmic dynein. These findings clearly demonstrate platelets are not preassembled in the body of the megakaryocytes, and “platelet bodies” are not transported down the shafts of proplatelets as preformed units.
Materials and methods
Megakaryocyte cultures
Megakaryocytes were cultured and isolated using methods described previously.24 Mouse megakaryocyte and platelet studies complied with institutional guidelines approved by the Children's Hospital animal care and use committee.
Thin-section electron microscopy
Megakaryocytes were centrifuged onto 22-mm coverslips coated with poly-L-lysine, fixed with 1.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4 for 8 hours, dehydrated through a series of alcohols, infiltrated with propylene oxide, and embedded in Epoxy resin. Embedded cells were separated from the coverslip by immersion in liquid nitrogen. Ultrathin sections were stained and examined with a JEOL electron microscope (JEOL, Tokyo, Japan).
Visualization of the dynamics and distribution of fluorescently labeled organelles in living megakaryocytes
To visualize mitochondria, megakaryocytes were incubated with 100 nm MitoTracker Green FM in the dark for 1 hour and then washed with phosphate-buffered saline (PBS). To visualize α-granules, megakaryocytes were incubated overnight with 150 μg/mL Oregon Green 488 human fibrinogen conjugate (Molecular Probes, Eugene, OR) and 100 U/mL Hirudin (Sigma, St Louis, MO). Megakaryocytes were then washed by albumin gradient sedimentation, and the resuspended pellet was placed in a video chamber. To visualize dense granules, megakaryocytes were incubated with 50 μm mepacrine (Sigma) for 60 minutes at 37°C in the dark. Video dishes were filled with 65% Leibowitz L-15 medium (Gibco BRL, Grand Island, NY) and 35% Dulbecco modified Eagle medium (DMEM) complete with no phenol red. A 1:100 dilution of Oxyfluor (Oxyrase, Mansfield, OH) was added to quench free radicals. Megakaryocytes were analyzed on a Nikon (Garden City, NJ) microscope equipped with a 100 × objective (NA 1.4). Images were acquired with an Orca IIER CCD camera (Hamamatsu, Hamamatsu City, Japan). Electronic shutters and image acquisition were under the control of Metamorph software (Molecular Devices, Downington, PA). Images were acquired every 1 to 5 minutes with a capture time of 200 to 500 milliseconds. The percentage of organelles moving was calculated by selecting 10 organelles per process from the first frame (if there were less than 10 organelles in the process, all were counted), determining the approximate center of each of these organelles, and examining the rest of the time lapse to establish if they have moved. An organelle was considered to have moved if the center was displaced at least 1.5 μm from the initial position in 1 minute.
Immunofluorescence microscopy
Megakaryocytes were stained using previously described methods.21 Fluorescein isothiocyanate (FITC) obtained from Pierce (Rockford, IL) was conjugated to rabbit anti-human von Willebrand factor (Diagnostica Stago, Parsippany, NJ) and was used at a dilution of 1:100. Rat monoclonal antiserotonin antibody (Abcam, Cambridge, MA) was used at a 1:50 dilution. Anti-dynactin p50 and anti-dynein intermediate chain (DIC) were a gift from E. Holzbauer (University of Pennsylvania, Philadelphia, PA). Mouse monoclonal anti-kinesin heavy chain antibody was obtained from Chemicon (Temecula, CA). Rabbit anti-β1-tubulin antiserum was a gift from Nick Cowan (New York University Medical School). Alexa-conjugated secondary antibodies were purchased from Jackson Immuno Research Laboratories (West Grove, PA). Actin filament integrity was assayed by fluorescence microscopy of fixed specimens stained with 1 μM phalloidin-Alexa 488 (Molecular Probes) for 30 minutes and washed with blocking buffer.
Permeabilization of megakaryocytes
Megakaryocytes attached to coverslips were briefly treated with 30 μm taxol to stabilize microtubules, washed with PMEG buffer, permeabilized with 0.1% Triton X-100 in PMEG (100 mM PIPES, pH 6.8, 1mM EGTA, 2mM MgSO4, 0.1 mM GTP) buffer supplemented with 1 mM dithiothreitol (DTT) and protease inhibitors, and then resuspended in PMEG buffer. To study the movement of particles linked to the moving microtubules, 0.4 μm carboxylated latex beads were added to the permeabilized proplatelets, and 1 mM adenosine triphosphate (ATP) was added.
Determination of microtubule polarity with kinesin-coated latex beads
Permeabilized proplatelets were treated with 0.5 M KCl for 10 minutes (to block sliding of microtubules) and resuspended in PMEG buffer supplemented with 1 mM ATP and 1 mM DTT. Carboxylated latex beads (0.4 μm; 2.5% solution; Sigma) were diluted 300-fold into PMEG buffer supplemented with 1 mM ATP and 1 mM DTT. To attach the kinesin motor protein to the latex beads, 3 μL diluted latex beads were added to 12 μL of 0.3 mg/mL recombinant kinesin motor protein (The Cytoskeleton, Denver, CO) and incubated for 5 minutes on ice.25 The bead sample was applied to the coverslip chamber. Observations of bead movement were made by differential-interference-contrast microscopy.
Isolation of megakaryocyte organelles and in vitro translocation
Organelles were isolated from proplatelet-producing megakaryocytes using discontinuous sucrose gradient centrifugation as described previously.26 Megakaryocytes were lysed by homogenization and centrifuged (500g, 4°C, 5 minutes) to remove debris and partially disrupted cells, prior to loading onto sucrose gradients. Bands 4 (mitochondria and lysosomes), 7 (α-granules), and 9 (dense granules) had to be pooled together to obtain a sufficient amount of organelles for the assay. The organelle translocation assay was adapted from Gilbert et al.27
Preparation of photomicrographs
The digital images produced in Metamorph were assembled into composite images by using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Results
Organelles move individually along proplatelets to assembling platelets
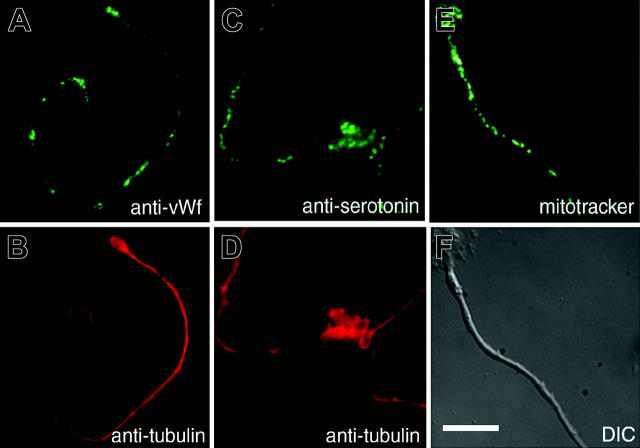
Mature platelets contain a mixture of organelles and granules essential to their function. Because platelets are assembled primarily at the ends of proplatelets, organelles must be delivered from the cell body to fill them. Organelles could translocate individually along proplatelets to nascent platelets or travel as packets prepackaged in the megakaryocyte body and then transported out to the developing platelets. To discriminate between these transport mechanisms, megakaryocytes were stained with organelle-specific probes to visualize the distribution of organelles and granules along proplatelets. The appearance of aggregates of organelles and granules along proplatelets would indicate transport as packets, whereas a relatively uniform distribution of organelles and granules would indicate delivery as single particles. Figure 1 shows the distribution of α-granules in proplatelets labeled with anti-von Willebrand factor antibody (Figure 1A-B), dense granules labeled with antiserotonin antibody (Figure 1C-D), and mitochondria stained with the fluorescent dye MitoTracker (Figure 1E-F). All 3 organelles disperse along the proplatelet shaft as individual particles. Collectively, these observations indicate that organelles translocate one by one along proplatelets and are not delivered as preformed groups.
Figure 1.
Megakaryocyte organelles and granules distribute individually in proplatelets to assembling platelets. Localization of organelles in the proplatelets of mouse megakaryocytes. Proplatelets were stained with anti-von Willebrand factor56 (A) to label α-granules, antiserotonin (C) to label dense granules, and MitoTracker (E) to label mitochondria. Corresponding antitubulin immunofluorescence (B,D) and differential-interference-contrast (F) micrographs highlight the proplatelet morphology. Scale bar, 10 μm. Granules and mitochondria are dispersed along the proplatelet shaft.
Organelles translocate bidirectionally along the length of proplatelets
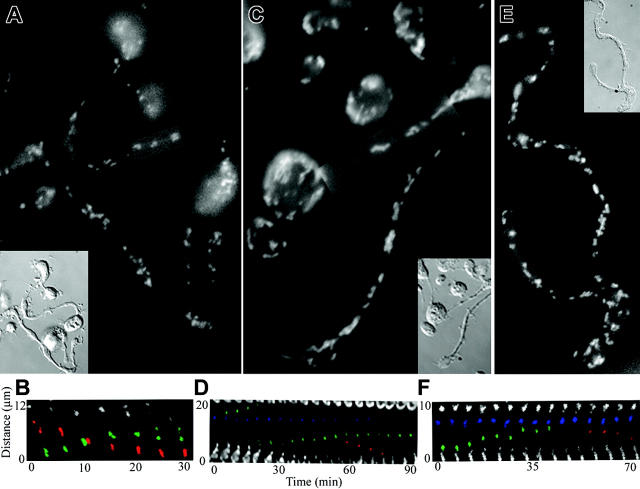
Having determined the basic nature of the transport mechanism, we next assessed the dynamics of organelle movement. The transport of organelles and granules along the proplatelets could occur unidirectionally or bidirectionally along proplatelets. To discriminate between these possibilities, mitochondria, α-granules, and dense granules in living megakaryocytes were labeled, and their delivery to nascent platelets was followed by fluorescence time-lapse microscopy. Our analysis demonstrated that all 3 organelles moved individually and in a bidirectional (back and forth) manner along the proplatelet. Organelles moved slowly (∼ 0.13-0.26 μm/min) and approximately one third of the organelles were moving at any given time. Forty percent of the organelles moved one by one, whereas 60% moved in tandem, as though they were mechanically linked (n = 110). Figure 2A-B shows the movement of mitochondria labeled by loading megakaryocytes with the fluorescent dye MitoTracker Green. In the megakaryocyte body, the density of mitochondria was too high to discern individual organelles. However, individual mitochondria are well resolved in proplatelets and are dispersed periodically throughout the length of the proplatelets as in the fixed specimen (Figure 2A). Mitochondria had the expected elongated shape and oriented almost exclusively with their long axes parallel with the microtubule bundles of the proplatelets. Mitochondria often appeared to move in tandem (59%, n = 32). Figure 2B (Movie S1, available at the Blood website; click on the Supplemental Movies link at the top of the online article) shows an example of mitochondrial movements in proplatelets with time. Movements in both directions are observed. Mitochondria moving toward the right are highlighted in red and toward the left are in green. Movement is in a saltatory manner: mitochondria stop and start, slow down and accelerate, and often reverse direction. At any given time, approximately 30% of the mitochondria are in motion. When moving, mitochondria have an average speed of 0.26 μm/min (range, 0.06-0.92 μm/min). Proplatelets contain platelet-sized swellings along their length that have led some to speculate they represent preformed “platelet units.” However, mitochondria individually entered and exited swellings and transiently piled up in the platelet-sized beads. Thus, mitochondria do not move in packets, and the continuous entry and exit of mitochondria from swellings indicate they are dynamic and do not represent completely formed platelet units. Megakaryocytes were cultured in the presence of Oregon green 488-labeled fibrinogen to label α-granules (Figure 2C-D; Movie S2).28 The fluorescent conjugate colocalizes with immunolabeling for von Willebrand factor in mouse megakaryocytes, demonstrating it to be specific for α-granules (data not shown). α-Granules, like mitochondria, were clearly visible as discrete units moving throughout the length of proplatelets. One example of a time-lapse recording displaying α-granule movement in a proplatelet is shown in Figure 2D and Movie S2. Similar to the mitochondria, α-granules move in both directions in the proplatelet and at times (59%, n = 34) in small tandem groups. α-Granules moving tipward are highlighted in green, those moving in the opposing direction in red, and stationary granules are in blue (Figure 3C). Approximately 28% of α-granules were moving at any given time. α-Granules moved at an average rate of 0.20 μm/min (range, 0.05-0.54 μm/min). To explore the mechanism of dense granule transport, megakaryocytes were labeled with mepacrine (Figure 2E; Movie S3), a reagent rapidly and specifically concentrated in dense granules.29-31 Mepacrine fluorescence colocalizes with immunolabeling for serotonin in mouse megakaryocytes, further demonstrating the specificity of this probe for dense granules (data not shown). Mepacrine, like FITC-fibrinogen for α-granules, concentrates in discrete granules in mouse megakaryocytes. An example of a time-lapse recording tracking dense granule movements in a proplatelet is shown in Figure 2F and Movie S3. Approximately 33% of the dense granules were in motion at any one time. Dense granules moved equally in both directions along the proplatelet, individually (38.5%) and in tandem groups (61.5%, n = 12) and traveled at an average rate of 0.139 μm/min (range, 0.07-0.19 μm/min). A dense granule moving upward toward the proplatelet tip has been highlighted in green, and a dense granule moving in the opposite direction has been highlighted in red (Figure 2F). Collectively, these studies validate the hypothesis that organelles move individually and reveal that movements are not restricted to one direction.
Figure 2.
Organelles move bidirectionally in proplatelets. (A-B) Mitochondria move bidirectionally in proplatelets. Mitochondria in megakaryocytes were labeled with MitoTracker, and organelle movements in proplatelets were recorded with fluorescence time-lapse microscopy (Movie S1). Micrographs (A, fluorescence; inset, DIC) show the distribution of mitochondria in a field of proplatelets after labeling with MitoTracker. (B) Kymograph of the boxed region in panel A. Fluorescent images of the labeled mitochondria were taken every 5 minutes. Two mitochondria, highlighted in green, move up in tandem and are observed to separate at 25 minutes. Three mitochondria, highlighted in red, move down during the recording period. The mitochondria pass each other at the 10-minute time point. (C-D) α-granules in proplatelets translocate bidirectionally (Movie S2). The α-granules were labeled by incubating megakaryocytes with Oregon Green 488 fibrinogen conjugate, which is taken up and stored in α-granules. The distribution and dynamics of the labeled α-granules were followed by time-lapse fluorescence microscopy. (C) Micrograph of a proplatelet field labeled with Oregon Green 488 fibrinogen conjugate. (Inset) Imaged differential-interference-contrast micrograph. (D) Kymograph showing time-lapse from the boxed region in panel C. Fluorescent images of the labeled α-granules were taken every 5 minutes. Two α-granules, highlighted in green, come together and move up until one separates (60 minutes) and then moves down (60-75 minutes). An α-granule highlighted in blue remains stationary during the recording period. (E-F) Dense granules move bidirectionally in proplatelets (Movie S3). Megakaryocytes were incubated with mepacrine and washed, and the distribution and dynamics of the dense granules were followed by fluorescence time-lapse microscopy. (E) Fluorescence micrograph of proplatelet field labeled with mepacrine. (Inset) A differential-interference-contrast image of the proplatelet in panel E. (F) Kymograph of the boxed region in panel E. Images of the fluorescently labeled dense granules are every 5 minutes. A group of dense granules, highlighted in blue, remain stationary throughout the recording period. A dense granule, highlighted in green, moves to the left (toward the proplatelet tip) and enters the stationary group. A dense granule, highlighted in red, exits the stationary group and moves to the right toward the base of the proplatelet (55-70 minutes).
Figure 3.
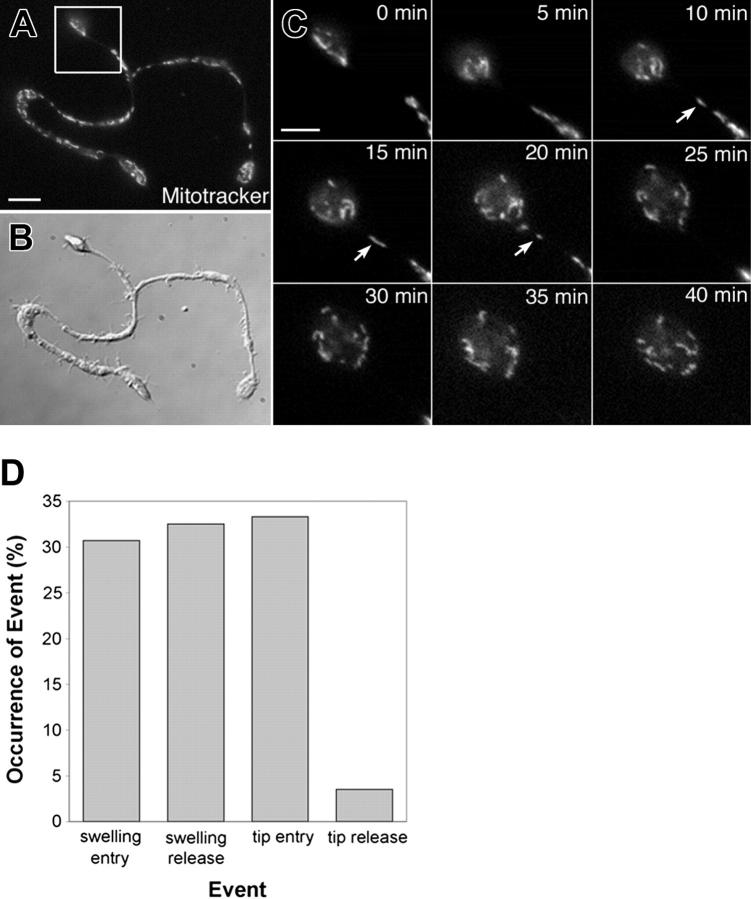
Organelles are captured at the proplatelet tip. (A-C) A representative example of mitochondria capture at the proplatelet ends. The mitochondria were labeled with MitoTracker Green, and movements were recorded using fluorescence time-lapse microscopy (See Movie 4). (A) Fluorescence and (B) differential-interference-contrast micrographs. Scale bar, 5 μm. (C) Images of the proplatelet tip (from boxed region in panel A) taken every 5 minutes are shown (See Movie 4). The arrows mark mitochondria that translocate into the proplatelet tip but do not exit. Scale bar, 3 μm. (D) Bar graph comparing the entry and exit of organelles/granules (an equal percentage of mitochondria, α-granules, and dense granules) into and out of proplatelet tips and swellings of proplatelets.
Organelles are captured at the proplatelet tip
To determine whether spatial differences existed in organelle/granule traffic throughout proplatelets and to understand how they are packaged into the nascent platelets, we examined the details of transport into swellings along the shaft and into the nascent platelets forming at the proplatelet ends (Figure 3). The behavior of organelles/granules were tracked (an equal number of mitochondria, α-granules, and dense granules), and we quantified 4 distinct classes of motility: swelling entry, swelling exit, tip entry, and tip exit (Figure 3D). Swellings were examined because some believe they represent nascent platelet bodies. An equal percentage of organelles were observed to individually enter and exit the swellings along the length of proplatelets and an equal percentage of organelles were also observed to enter proplatelet tips. However, only a very small fraction (3.5%) of organelles were observed to exit proplatelet tips, suggesting organelles may be partially confined or restricted to the ends of proplatelets. Figure 3C and Movie S4 show a representative example of MitoTracker-labeled mitochondria entering a proplatelet tip but not exiting.
Megakaryocyte organelles translocate in the absence of F-actin
The cytoskeleton of the proplatelet is constructed of both microtubules and actin filaments, and so the transport of organelles along proplatelets en route to developing platelets could occur over microtubules, actin filaments, or a combination of both. To assess the role of actin filaments in the movements of organelles, we treated megakaryocytes with actin-disrupting agents and stained the cells with organelle-specific probes to see whether the organelles continued to move out along the proplatelet (Figure 4). Megakaryocytes treated with cytochalasin D and latrunculin A still maintain the capacity to extend long slender processes. To assess the efficacy of these toxins in disrupting the actin cytoskeleton, we examined their effect on filamentous actin in treated proplatelets (Figure 4A-F). In control megakaryocytes, phalloidin stains a dense filamentous network (Figure 4A-B). Cytochalasin D treatment disrupted this network, leaving focal aggregates of actin along the proplatelets (Figure 4B-C). Following latrunculin A treatment, diffuse phalloidin staining occurred in the cell body, but F-actin was almost absent from proplatelets (Figure 4E-F). Despite the greatly reduced levels of F-actin, α-granules (Figure 4I-J, M-N), dense granules (Figure 4K-L, O-P), and mitochondria (Figure 4Q) moved into proplatelets and were dispersed normally along their length. We next examined the rate of organelle movements in the presence of latrunculin A. Figures 4Q-R and Movie S5 show a representative time-lapse recording of mitochondria movements along a proplatelet tip. Latrunculin A treatment did not appear to inhibit any aspect of organelle movement. In fact, a slight increase (∼ 10%) in the number of organelles moving at any one time was observed. The average velocity of organelle (combined mitochondria, α-granule, and dense granule) movement in latrunculin A-treated megakaryocytes of 0.21 μm/min was similar to that seen in untreated cells, and organelles continued to move equally in both the anterograde and retrograde directions along proplatelets. Nor was F-actin required to trap organelles or granules at the ends of proplatelets, as the percentage of organelles (an equal number of α-granules, dense granules, and mitochondria) that enter and exit proplatelet tips was unaffected by latrunculin A (Figure 4S, compare with Figure 3D). Collectively, these results suggest that actin is not essential for long-distance organelle transport along proplatelets or required to trap organelles at the proplatelet tips.
Figure 4.
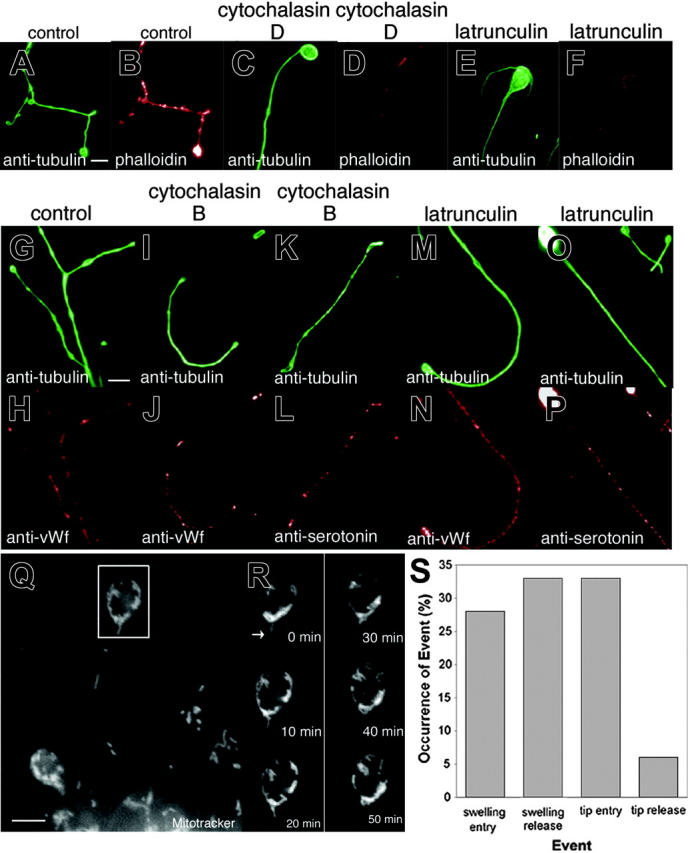
Organelles and granules continue to translocate in the presence of actin toxins. (A-F) Cytochalasin and latrunculin treatment significantly reduce phalloidin staining in proplatelets. Comparison of (A,C,E) antitubulin immunofluorescence in red to corresponding (B,D,F) phalloidin staining in green of control, cytochalasin D-, and latrunculin A-treated cells. Note that the actin-disrupting agents decreased the level of F-actin in proplatelets as visualized by phalloidin staining. (G-P) Mitochondria/granule distribution in megakaryocytes treated with actin toxins. Comparison of (G,I,K,M,O) antitubulin fluorescence (green) versus (H,J,N) anti-von Willebrand factor or (L,P) antiserotonin fluorescence (red) of proplatelets in (G-H) control, (I-L) cytochalasin B-treated, or (M-P) latrunculin A-treated megakaryocytes. Organelles/granules continue to disperse along the length of the proplatelets. Scale bars, 5 μm. (Q-S) Organelles translocate in proplatelets and are trapped at the tips following latrunculin A treatment (Movie S5). (Q) Fluorescence micrograph of a living megakaryocyte labeled with MitoTracker Green after treatment with 2 μM latrunculin A for 2 hours. Scale bar, 5 μm. (R) Images of the proplatelet tip (in the boxed region) taken every 10 minutes are shown. The arrows indicate mitochondria that translocate and enter the proplatelet tip but do not exit at the same frequency. A large amount of mitochondria are observed to cumulate at the bud node of the bulbous tip. (S) Comparison of the percentage of organelles/granules (an equal number of mitochondria, α-granules, and dense granules were followed) that enter and exit proplatelet swelling and tips.
Megakaryocyte organelles are frequently aligned along microtubules
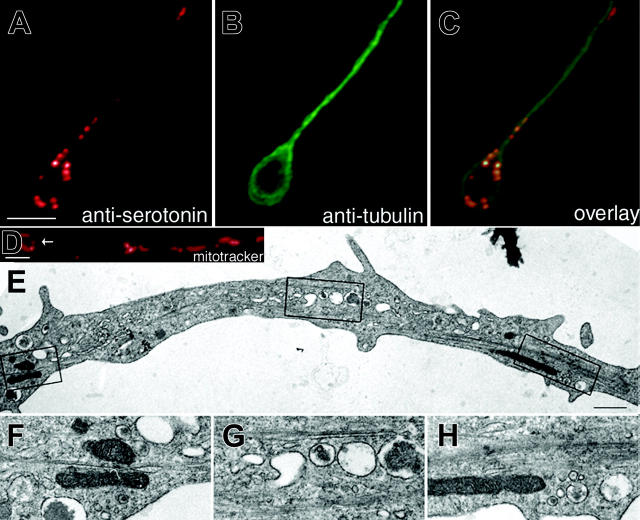
Because the actin cytoskeleton is not required for large-scale organelle/granule transport along proplatelets, the microtubules that line the proplatelet shaft and provide the power for elongation are likely to provide the force for organelle movements. If microtubules provide the tracks for transport of organelles along proplatelets, then we would expect organelles to be most abundant in regions where microtubules are highly concentrated. Support for the hypothesis that megakaryocyte organelles move along microtubules comes from the observation that organelles appear to be most abundant in regions where microtubules are maximally concentrated (Figure 5). Superimposition of tubulin and organelle patterns by double label immunofluorescence microscopy in proplatelet-producing megakaryocytes reveals that organelles frequently reside in close proximity to microtubules (Figure 5A-C). Mitochondria visualized by MitoTracker staining of living megakaryocytes are frequently aligned in a ring or tear-drop pattern at the tips of proplatelets (Figure 5D), localizing with microtubule coils. Additionally, electron microscopy of proplatelets also reveals that many organelles are situated along the length of microtubules (Figure 5E-H).
Figure 5.
Megakaryocyte organelles associate with microtubules. Double immunofluorescence microscopy of a proplatelet using antibodies against (A) serotonin to stain dense granules and (B) tubulin to highlight microtubules and (C) an overlay. Note the teardrop-shaped pattern of dense granules at the tip of the proplatelet. Scale bar, 5 μm. (D) Proplatelet of a living megakaryocyte stained with the fluorescent probe MitoTracker Red to highlight the distribution of mitochondria. Note the circular pattern of mitochondria at the proplatelet tip (arrow in D). (E) Thin-section electron micrograph showing the ultrastructure of a maturing proplatelet. (F-H) High magnification electron micrographs of the boxed regions in panel E show organelles/granules in close proximity to microtubules. Scale bar, 500 nm.
Origin of bipolar movements along proplatelet microtubules
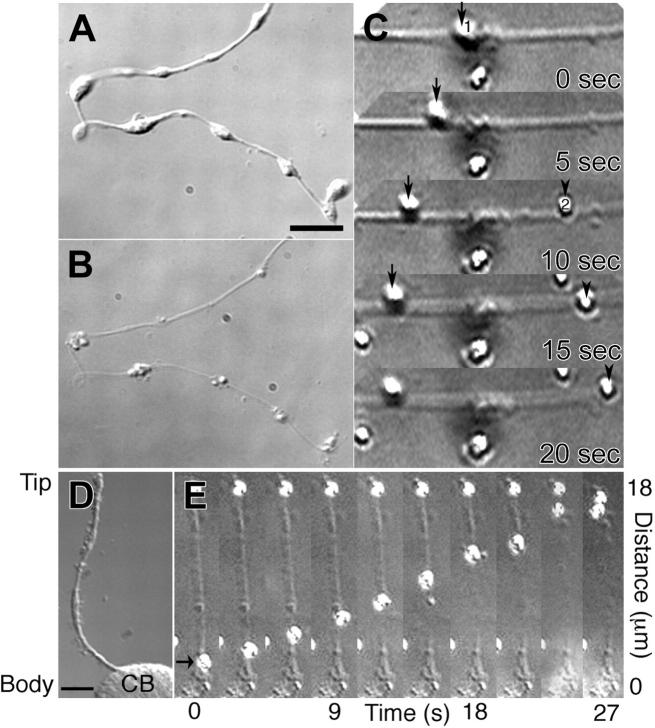
Microtubules are polarized, with chemically and morphologically distinct plus- and minus-ends.22,23 Because the polarity of microtubules within proplatelets dictates the direction motors can move, bidirectionality likely plays an essential role in regulating the distribution of organelles along these extended processes and ultimately establishing the final content of the released platelet. The microtubules that run longitudinally in proplatelets could be organized as unipolar arrays, because they are organized in neuronal axons or sperm flagella, or in bipolar arrays as they are organized in neuronal dendrites or the midzone of mitotic spindles. To determine the polarity of microtubules along proplatelets, we permeabilized megakaryocytes in a buffer that preserves microtubules (Figure 6A-B) and then treated with 0.5M KCl to release associated motor proteins. Plus-end directed kinesin-coated latex beads were added, and bead movement was monitored using video differential interference contrast microscopy25 (Figure 6A-B). Movement of beads along the permeabilized proplatelets was observed in both directions (Figure 6C; Movie S6). Approximately equal numbers of beads moved in each direction, suggesting proplatelets are filled with equal numbers of microtubules having plus-ends and minus-ends directed toward their tips. Beads bound to and moved along permeabilized proplatelets in a continuous manner, often moving more that 10 μm before dissociating and never reversed their direction of movement. Despite the presence of a turn-around zone generated when microtubules loop at the ends of proplatelet, the majority of kinesin-coated beads entering the tips of proplatelets, do not exit (37 of 41 beads) (Figure 6D-E; Movie S7).
Figure 6.
Kinesin-coated beads move in both directions on proplatelet microtubules. (A) Differential-interference-contrast micrographs of a proplatelet before and after permeabilization with Triton X-100 (B) in a microtubule stabilizing buffer containing taxol. Scale bar, 5 μm. (C) Sequence of movement by 2 kinesin-coated latex beads on the permeabilized proplatelet (Movie S6). Kinesin-coated bead 1 (arrow at 0 sec) is attached to the microtubules and moves left with time. Bead 2 attached to the same proplatelet at 10 sec (arrowhead) and moves in the opposite direction, right. (D-E) Kinesin-coated beads that move into proplatelet tips do not exit. (D) Differential-interference-contrast micrograph of a proplatelet-producing megakaryocyte before permeabilization. A single proplatelet extends from the cell body (CB). Scale bar, 10 μm. (E) High magnification video sequence of a kinesin-coated latex bead (arrow) moving tip-ward on the microtubule cytoskeleton of a permeabilized proplatelet (Movie S7). The kinesin-coated bead (arrow at 0 sec) attached to the microtubules of the permeabilized proplatelet and moved continuously toward the end of the outgrowth, where it remained attached on reaching the tip. A second bead located at the proplatelet tip remains stationary throughout the recording period. Elapsed time indicated in seconds.
Microtubule-based mechanisms of organelle movement along proplatelets
The movement of organelles along proplatelets could be due to the translocation of organelles along stationary microtubules, or the sliding activity of microtubules to which they are tethered, or a combination of both processes. To dissect apart the potential relative contribution of each of these mechanisms, we used a permeabilized proplatelet system to which we could add specific molecules and analyze their movements. To test whether megakaryocyte organelles/granules carry motor proteins on their surface and use them to translocate along proplatelet microtubules, isolated megakaryocyte organelles were added to permeabilized proplatelets treated with 0.5 M KCl to render the microtubules immotile. Figure 7A-B and Movie S8 show that in the presence of ATP organelles move along the permeabilized proplatelet shafts. Megakaryocyte organelles move in both directions at instantaneous velocities of 3.24 μm/min along permeabilized proplatelets. Surprisingly, megakaryocyte organelles moved at rates approximately 10 times faster on the microtubules of permeabilized proplatelets than in situ. Like kinesin-coated beads, organelles do not change their direction of travel. Organelle movement depended on ATP and other nucleotide triphosphates, including guanosine triphosphate (GTP), uridine triphosphate (UTP), cytidine triphosphate (CTP), or inosine triphosphate (ITP), failed to support organelle transport. These data demonstrate that a force-transducing motor is associated with some of the moving organelles along proplatelets. Although megakaryocyte organelles have the capacity to move over stationary microtubules, the sliding activity of microtubules could provide a second potential mechanism for movement if organelles tether to this motile network. To further investigate this piggyback mechanism of transport, uncoated latex beads were attached to permeabilized proplatelets. They remained stationary until sliding was initiated with the addition of ATP, to the lysed microtubule network (Figure 7C-F; Movies S9-S10). Tandem displacement of beads tethered at different points suggests that organelles could translocate in a piggyback fashion when they are linked to moving microtubules.
Figure 7.
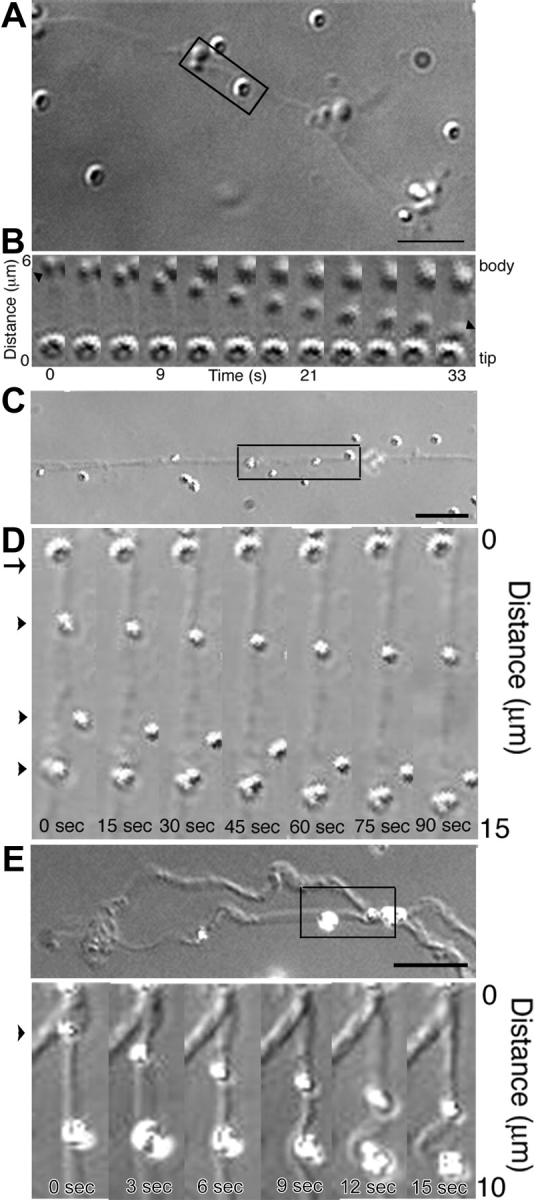
A permeabilized proplatelet system to study organelle transport. (A-B) Megakaryocyte organelles move along permeabilized proplatelet microtubules. (A) DIC micrograph of a permeabilized proplatelet. (B) DIC time-lapse sequence showing megakaryocyte organelle movement along the immotile microtubules of a permeabilized proplatelet from the boxed region in panel A (Movie S8). The sequence demonstrates an organelle attached to the microtubule cytoskeleton of a permeabilized proplatelet (arrowhead at 0 sec) and moves downward in the direction of a stationary particle attached to the immotile microtubules. Frames are every 5 seconds. Scale bar, 5 μm. (C-E) Polystyrene latex beads attached to permeabilized proplatelets move with sliding microtubules. Uncoated latex polystyrene beads were attached to Triton X-100 permeabilized proplatelets. The beads remain stationary until the addition of ATP. Microsphere transport began when the preparation was perfused with 1 mM ATP (0 seconds). Scale bars, 5 μm. All beads marked with arrowheads moved relative to the stationary marker, labeled with an arrow. (C,E) Differential-interference-contrast micrograph of proplatelets following permeabilization with Triton X-100 in a microtubule-stabilizing buffer containing taxol. (D) High magnification video sequence of 3 uncoated latex beads (arrowheads) moving on the microtubule cytoskeleton of a permeabilized proplatelet (Movie S9). Beads moved in tandem, as though they were mechanically linked. (F) High magnification video sequence of 2 beads attached to a sliding microtubule (Movie S10). Both beads move approximately 5 μm, demonstrating tandem movements along the same microtubule.
Discussion
As megakaryocytes mature, they exhibit morphologic and cytoplasmic asymmetry that become most striking when proplatelets are elaborated. Asymmetry is achieved in part by a transport system that sends platelet-specific cytoskeletal and organelle/granule components into the developing proplatelets and restricts the entry of apoptotic components to the residual cell body.32 The present studies document the dynamics of organelle movement in the proplatelets. We have found that single organelles and granules enter and move through proplatelets using forces derived from the microtubule cores of these structures. Movement is slow, characterized by frequent pauses and reversals of direction, reflecting the bipolar organization of the underlying microtubule elements. Organelles and granules appear to move both over proplatelet microtubule tracks and ride piggyback on microtubules as they slide relative to one another. Importantly, the proplatelet ends containing nascent platelets have the ability to capture and hold vesicular cargo once it arrives. These observations are incompatible with the notion that platelets are preformed as “units” in the megakaryocyte body and released via cytoplasmic fragmentation, or that proplatelets are simply chains of preassembled platelet units that have been spooled out by megakaryocytes.3,4,33 Because developed platelet cytoskeletons are not found within the cytoplasm of mature megakaryocytes, and the cytoplasmic breakup of the megakaryocyte cell body has never been observed in vitro, cytoplasmic fragmentation is not a viable model for platelet production.6-8,34,35
Our observations add further support to the idea that proplatelets are essential intermediates in platelet assembly and provide new insight into how nascent platelets are filled with their vesicular contents. As we have reported, the first recognizable change observed in cultured megakaryocytes that announces the commencement of proplatelet assembly21 is when radial microtubules release from their centrosomes and collect in the cell cortex. These microtubules rapidly reorganize into bipolar arrays that generate the forces for the initial blunt proplatelet protrusions and, as proplatelets elongate and thin, turn into the bundles that line the shafts and form the teardrop-shaped loops in the proplatelet ends. Proplatelet ends are continuously amplified by a mechanism that repeatedly bends and bifurcates the shafts. Microtubules from these loops are then rolled into coils generating the discoid signature of the mature resting blood platelet. Now we report that as proplatelets elaborate using microtubule-based sliding forces, only single organelles and granules move along the microtubule cores. Movement is not simple or continuous, but instead is intermittent with long pauses and changes of direction, a process that disperses the organelles and granules throughout the proplatelet. The organelles and granules continue to move in proplatelets unless they are captured at proplatelet ends, the sites of platelet assembly. We previously concluded that platelet assembly culminates primarily at the ends of proplatelets based on the restriction of microtubule coils to these locations. However, the process of release has never been observed and hence, the precise mechanisms by which individual platelets are released from proplatelets remain to be established. Individual platelets could release sequentially from the proplatelet tips, which would require the continuous and directional transport of cargo to the most distal proplatelet ends. Alternatively, large, relatively immature proplatelets could release from megakaryocytes and subsequently mature into platelets. Newly released proplatelets would be required to reorganize the microtubules in the fragmented ends into a loop functionally identical to that in the opposing end. Microtubule loop formation would allow it to configure nascent platelets and capture cargo, and we and others have frequently observed elongated and barbell-shaped proplatelets which have microtubule loops at both ends in megakaryocyte cultures and in mammalian blood.21,36 If this strategy of releasing long proplatelets is used in large scale, then the goal of the megakaryocyte transport system is to disperse platelet-specific cytoskeletal and vesicular components throughout the proplatelets. The organelle transport system of the megakaryocyte appears to function with this in mind.
One of the most striking observations is that the rate of vesicular traffic in proplatelets is 0.1 to 2 μm/min, which is at least 10-fold slower than organelle movement in other long-range microtubule-based transport systems.22,23 These transport rates are considerably slower than the rates at which proplatelets grow (∼ 1 μm/min) and microtubules slide within proplatelets (∼ 3-4 μm/min). One could argue that, although the rates are slow, proplatelet formation requires 2 to 10 hours to complete, leaving ample time for organelles to find their targets, the proplatelet ends.21 The tight bundling of microtubules in situ or specific microtubule-binding proteins could reduce movement. The saltatory motion of organelles, where organelles transiently move, stop, and change direction is widely observed in cells, although in most cases, it is not clear why this energy-inefficient mode of transport is used.37 In megakaryocytes, a “random walk”38 of organelles provides a simple mechanism to distribute organelles throughout proplatelets. Bidirectional transport may also be used to mix platelet organelles, ensuring that each mature platelet receives its quota. The random walk of organelles also allows cargo to explore large regions of cellular space and increases their chance of finding their target.39 This simple delivery strategy may explain the large variations in the numbers of organelles found within mature platelets.40-43 The distribution of mitochondria throughout proplatelets may also serve to supply the localized energy demands of microtubule assembly and sliding.44,45 Thus, for mitochondria, the journey through proplatelets is perhaps just as important as the ultimate destination.
The position and polarity of the microtubules within proplatelets are likely to play an essential role in governing transport of organelles to assembling platelets. Our findings suggest that roughly equal portions of microtubules are oriented with plus-ends toward the proplatelet tip and plus-ends directed toward the cell body. How does the bipolar organization of microtubules within proplatelets arise? The bipolar arrangement of microtubules likely occurs as the initial pseudopodia forms. During the formation of the pseudopodia, cortical microtubules become looped at the tip and then reenter the shaft to create an antiparallel orientation of microtubule bundles. Another mechanism that likely amplifies the bipolar organization of microtubules within proplatelets is the repeated bending and branching of proplatelets, which causes an increase in the number of “looped” and antiparallel microtubules. Our studies focused primarily on elongated proplatelets because we were unable to resolve individual organelles in short pseudopodia. Thus, alternative mechanisms of transport may exist at this initial stage. Although our results suggest that microtubule-based long-distance transport constitutes a major mechanism in establishing the dispersal of organelles along proplatelets, additional mechanisms may also exist. For example, unconventional myosin-based transport along actin filaments could still play a potential role in organelle movement, optimizing organelle positioning after microtubule-based transport.
Mechanism of cargo transport in proplatelets
There are 3 obvious mechanisms to account for the observed microtubule-associated bidirectional transport of organelles in proplatelets: (1) one or more motors bind to organelles and propel them along microtubules; (2) organelles bind microtubules but do not move along them and instead move relative to the cell as microtubules slide past one another; and (3) one or more transport motors move organelles relative to microtubules while the same, or different motors, slide adjacent microtubules past each other. Our findings suggest that organelles both move over microtubules and ride piggyback46 on them. In vitro, kinesin-coated beads bind and translocate over the microtubules of permeabilized proplatelets. Beads attach and move in either direction, although once moving they do not change direction. However, beads lacking motor protein could also attach to the microtubule bundles of proplatelets and move as the microtubules slide. In similar fashion to the kinesin beads, megakaryocyte organelles added to permeabilized proplatelets in the presence of ATP move along immotile microtubules prevented from sliding, demonstrating that motor proteins associate with some megakaryocyte organelles. Additional evidence for this idea comes from the vesicular staining of cytoplasmic structures whose properties are consistent with membrane-bounded organelles, although further studies are necessary to identify the specific cargo containing kinesin in proplatelets. If membrane-associated motors provide the major forces for cargo transport, the movements of cargos relative to each other in proplatelets would be highly variable, influenced by number and types of associated motors, the sizes of the cargo, and the linearity and polarity of the underlying tracks. However, many examples of where organelles that are separated by 5- to 10-μm distances move in tandem are apparent, indicating they may be linked to the same sliding microtubule, as suggested by our in vitro experiment. However, these organelles frequently pop off of the microtubules, indicating they are not permanently linked as a preformed group. Why might megakaryocytes use 2 mechanisms to transport organelles? Because proplatelets can grow to near-millimeter lengths, their microtubule bundles are composed of linear assemblies of shorter microtubules. This discontinuous arrangement of microtubules requires organelles to hop when they arrive at a microtubule end to another microtubule, a process that may be aided by motors associated with organelles. This may explain why organelles move by translocating over microtubules and riding piggyback on the moving polymers.
Capture of organelles at the proplatelet ends
How do the organelles get captured at proplatelet ends? Organelles may accumulate in nascent platelets as a consequence of the looped organization of microtubules at the proplatelet ends, although inactivation or delocalization of organelle-associated motors could also play a role. Studies in permeabilized proplatelets provide the strongest evidence for this conclusion whereby kinesin-coated beads enter proplatelet tips but are unable to exit, in an identical fashion to organelles and granules entering the ends of living proplatelets. Once caught in the proplatelet end, organelles/granules are not quiescent and instead continue to circle the periphery of the bud, suggesting that the ultrastructural organization of the microtubule coil within the proplatelet end may restrict exit from the tip.
Although our studies focused on the delivery of platelet-specific cargo into the proplatelets, it is likely that there is also specific exclusion of material from proplatelets. For example, evidence has accumulated, indicating that apoptotic pathways contribute to platelet biogenesis and that caspase activation is restricted to the megakaryocyte cell body.32,47,48 Because our findings suggest that molecules that enter proplatelets are destined for packing into platelets, the exclusion of these molecules during platelet biogenesis would most likely occur at the cell body-proplatelet junction. How this is achieved will presumably provide an additional reason why proplatelets have evolved as an essential intermediate in platelet production.
What is the relationship of the microtubule-based mechanism that transports organelles along proplatelets to that used by other cells with an exaggerated dependence on organelle transport?22,23 Perhaps 2 of the best-studied systems for understanding organelle transport are the neuronal processes (axons and dendrites) and the sperm flagellum, both of which are highly specialized for long-distance transport.49,50 Both neuronal extensions and sperm flagella, like proplatelets, use microtubules for the transport of organelles over long distances. In these processes, many individual organelles move in one direction in these processes and then switch direction when cargoes reach the ends of these elongated structures. One obvious exception is mitochondria, which move in an intermittent and bidirectional manner along neuronal processes.45,51,52 Unlike many other organelle systems, mitochondria have the ability to move in both directions at any time in an axon or a localized region of the cytoplasm. Thus, mitochondria can switch overall direction or transport at seemingly any time. This pattern of movement, in which organelles move in an intermittent and bidirectional manner, is very similar to that observed by the organelles along proplatelets. The bipolar distribution of microtubules along proplatelets may play an essential role in establishing the organelle content of proplatelets. In contrast to proplatelets, axons and flagella have unidirectional microtubule highways. Dendritic projections, on the other hand, are more structurally similar to proplatelets because their microtubule arrays have a mixed polarity, are highly branched, and also taper at the end.53 It is well established that axons and dendrites, which have different microtubule organizations, differ in their complements of cytoplasmic organelles. Most notable in this regard, ribosomes and Golgi elements are present in dendrites but excluded from axons. The differences between the organelle composition of the axons and dendrites are produced by differences in the organization of the microtubules.54,55 Thus, establishment of nonuniform microtubule arrays within proplatelets may provide a mechanism to include organelles that are normally excluded from extensions with unipolar arrays. In addition to the similarities observed with mammalian cells, we also note that striking similarities were observed between the mechanisms by which organelles move in proplatelets and that of the free-living formanifera, Reticulomyxa filosa.56 We conclude that the creation of a high degree of spatial/temporal organization by using protein motors to transport molecules and organelles along cytoskeletal tracks is a theme used across cell types and that megakaryocytes capitalize on this theme to transport and target organelles during platelet biogenesis.
Supplementary Material
Acknowledgments
We thank Dr T.P. Stossel and R.I. Handin for their supportive environment and Jagesh Shah for valuable suggestions. We thank Dr Chloe Bulinski, Dr Niels Galjart, Dr Rob Flaumenhaft, Dr Sunita Patel, and Dr Harald Schulze for helpful discussions.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-06-2206.
Supported by grants from the National Institutes of Health (HL68130) (J.E.I. Jr) and (R01 HL 063143) (R.A.S.).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Wright J. The origin and nature of blood platelets. Boston Med Surg J. 1906;154: 643-645. [Google Scholar]
- 2.Schulze H, Shivdasani RA. Molecular mechanisms of megakaryocyte differentiation. Semin Thromb Hemost. 2004;30: 389-398. [DOI] [PubMed] [Google Scholar]
- 3.Kosaki G, Inosita K, Okuma H. Human thrombocytogenesis. In: Didisheim P, ed. Platelets, Thrombosis, and Inhibitors. Vol 97. Stuttgart-New York: FK Shattauer; 1974: 97-102. [Google Scholar]
- 4.Yamada F. The fine structure of the megakaryocyte in the mouse spleen. Acta Anat. 1957;29: 267-290. [DOI] [PubMed] [Google Scholar]
- 5.Becker RP, DeBruyn PP. The transmural passage of blood cells into myeloid sinusoids and the entry of platelets into the sinusoidal circulation; a scanning electron microscopic investigation. Am J Anat. 1976;145: 1046-1052. [DOI] [PubMed] [Google Scholar]
- 6.Radley JM. Ultrastructural aspects of platelet formation. Prog Clin Biol Res. 1986;215: 387-398. [PubMed] [Google Scholar]
- 7.Radley JM, Haller CJ. The demarcation membrane system of the megakaryocyte: a misnomer? Blood. 1982;60: 213-219. [PubMed] [Google Scholar]
- 8.Radley JM, Scurfield G. The mechanism of platelet release. Blood. 1980;56: 996-999. [PubMed] [Google Scholar]
- 9.Kosaki G. In vivo platelet production from mature megakaryocytes: does platelet release occur via proplatelets? Int J Hematol. 2005;81: 208-219. [DOI] [PubMed] [Google Scholar]
- 10.Choi E. Regulation of proplatelet and platelet formation in vitro. In: Kuter D, Hunt P, Sheridan W, Zucker-Franklin D, Kuter DJ, eds. Thrombopoiesis and Thrombopoietins: Molecular, Cellular, Preclinical, and Clinical Biology. Totowa, NJ: Humana Press; 1997: 271-284.
- 11.Cramer EM, Norol F, Guichard J, et al. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997; 89: 2336-2346. [PubMed] [Google Scholar]
- 12.Leven RM, Yee MK. Megakaryocyte morphogenesis stimulated in vitro by whole or partially fractionated thrombocytopenic plasma: a model system for the study of platelet formation. Blood. 1987;69: 1046-1052. [PubMed] [Google Scholar]
- 13.Tablin F, Castro M, Leven RM. Blood platelet formation in vitro: the role of the cytoskeleton in megakaryocyte fragmentation. J Cell Sci. 1990; 97: 59-70. [DOI] [PubMed] [Google Scholar]
- 14.An E, Ogata K, Kuriya S, Nomura T. Interleukin-6 and erythropoietin act as direct potentiators and inducers of in vitro cytoplasmic process formation on purified mouse megakaryocytes. Exp Hematol. 1994;22: 149-156. [PubMed] [Google Scholar]
- 15.Radley J, Hartshorn M, Green S. The response of megakaryocytes with processes to thrombin. Thromb Haemost. 1987;58: 732-736. [PubMed] [Google Scholar]
- 16.Radley J, Rogerson J, Ellis S, Hasthorpe S. Megakaryocyte maturation in long-term marrow culture. Exp Hematol. 1991;19: 1075-1078. [PubMed] [Google Scholar]
- 17.Radley JM, Haller, CJ. Fate of senescent megakaryocytes in the bone marrow. Br J Haematol. 1983;53: 277-287. [DOI] [PubMed] [Google Scholar]
- 18.Lecine P, Villeval J, Vyas P, Swencki B, Yuhui X, Shivdasani RA. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92: 1608-1616. [PubMed] [Google Scholar]
- 19.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, et al. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81: 695-704. [DOI] [PubMed] [Google Scholar]
- 20.Tablin F, Oliver A, Walker N, Crowe L, Crowe J. Membrane phase transition of intact human platelets: correlation with cold-induced activation. J Cell Phys. 1996;168: 305-313. [DOI] [PubMed] [Google Scholar]
- 21.Italiano JEJ, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999; 147: 1299-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112: 467-480. [DOI] [PubMed] [Google Scholar]
- 23.Mallik R, Gross SP. Molecular motors: strategies to get along. Curr Biol. 2004;14: R971-982. [DOI] [PubMed] [Google Scholar]
- 24.Lecine PL, Villeval J, Vyas P, Swencki B, Xu Y, Shivdasani RA. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92: 1608-1616. [PubMed] [Google Scholar]
- 25.Sloboda RD. Methods for the purification and assay of microtubule-associated motility proteins. In: Carraway K, Carraway C, eds. The Cytoskeleton: A Practical Approach. New York, NY: Oxford University Press; 1992: 167-196.
- 26.Broekman MJ. Homogenization by nitrogen cavitation technique applied to platelet subcellular fractionation. Methods Enzymol. 1992;215: 21-32. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert SP, Allen RD, Sloboda RD. Translocation of vesicles from squid axoplasm on flagellar microtubules. Nature. 1985;315: 245-248. [DOI] [PubMed] [Google Scholar]
- 28.Harrison P, Wilbourn B, Debili N, et al. Uptake of plasma fibrinogen into the alpha granules of human megakaryocytes and platelets. J Clin Invest. 1989;84: 1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorez HP, Da Prada M, Pletscher A. Flashing phenomenon in blood platelets stained with fluorescent basic drugs. Experientia. 1975;31: 593-595. [DOI] [PubMed] [Google Scholar]
- 30.Reddington M, Novak EK, Hurley E, Medda C, McGarry MP, Swank RT. Immature dense granules in platelets from mice with platelet storage pool disease. Blood. 1987;69: 1300-1306. [PubMed] [Google Scholar]
- 31.Skaer RJ, Flemans RJ, McQuilkan S. Mepacrine stains the dense bodies of human platelets and not platelet lysosomes. Br J Haematol. 1981;49: 435-438. [DOI] [PubMed] [Google Scholar]
- 32.Kaluzhny Y, Ravid K. Role of apoptotic processes in platelet biogenesis. Acta Haematol. 2004;111: 67-77. [DOI] [PubMed] [Google Scholar]
- 33.Zucker-Franklin D, Petursson S. Thrombocytopoiesis: analysis by membrane tracer and freeze-fracture studies on fresh human and cultured mouse megakaryocytes. J Cell Biol. 1984;99: 390-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radley J, Hatshorm M. Megakaryocyte fragments and the microtubule coil. Blood Cells. 1987;12: 603-608. [PubMed] [Google Scholar]
- 35.Zucker-Franklin D. The ultrastructure of megakaryocytes and platelets. In: Gordon A, ed. Regulation of Hematopoiesis. Vol 55. New York, NY: Appleton-Century-Crofts; 1970: 1553-1586. [Google Scholar]
- 36.Behnke O, Forer A. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur J Haematol Suppl. 1998;60: 3-23. [DOI] [PubMed] [Google Scholar]
- 37.Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14: R525-R537. [DOI] [PubMed] [Google Scholar]
- 38.Maly IV, Vorobjev IA. Centrosome-dependent anisotropic random walk of cytoplasmic vesicles. Cell Biol Int. 2002;26: 791-799. [DOI] [PubMed] [Google Scholar]
- 39.Wacker I, Kaether C, Kromer A, Migala A, Almers W, Gerdes HH. Microtubule-dependent transport of secretory vesicles visualized in real time with a GFP-tagged secretory protein. J Cell Sci. 1997;110(Pt 13): 1453-1463. [DOI] [PubMed] [Google Scholar]
- 40.Corash L, Costa JL, Shafer B, Donlon JA, Murphy D. Heterogeneity of human whole blood platelet subpopulations, III: density-dependent differences in subcellular constituents. Blood. 1984;64: 185-193. [PubMed] [Google Scholar]
- 41.Corash L, Tan H, Gralnick HR. Heterogeneity of human whole blood platelet subpopulations, I: relationship between buoyant density, cell volume, and ultrastructure. Blood. 1977;49: 71-87. [PubMed] [Google Scholar]
- 42.Costa JL, Reese TS, Murphy DL. Serotonin storage in platelets: estimation of storage-packet size. Science. 1974;183: 537-538. [DOI] [PubMed] [Google Scholar]
- 43.Sixma J, van den Berg A, Jockusch B, Hartwig J. Immunoelectron microscopic localization of actin, α-actinin, actin-binding protein and myosin in resting and activated human blood platelets. Eur J Cell Biol. 1989;48: 271-281. [PubMed] [Google Scholar]
- 44.De Vos KJ, Sable J, Miller KE, Sheetz MP. Expression of phosphatidylinositol (4,5) bisphosphate-specific pleckstrin homology domains alters direction but not the level of axonal transport of mitochondria. Mol Biol Cell. 2003;14: 3636-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overly CC, Rieff HI, Hollenbeck PJ. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J Cell Sci. 1996;109(Pt 5): 971-980. [DOI] [PubMed] [Google Scholar]
- 46.Allen RD. Cytoplasmic streaming and locomotion in marine foraminifera. In: Allen RD, Kamiya N, eds. Primitive Motile Systems in Cell Biology. London, United Kingdom: Academic Press; 1964: 407-432.
- 47.Clarke MC, Savill J, Jones DB, Noble BS, Brown SB. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol. 2003; 160: 577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Botton S, Sabri S, Daugas E, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100: 1310-1317. [DOI] [PubMed] [Google Scholar]
- 49.Brown A. Axonal transport of membranous and nonmembranous cargoes: a unified perspective. J Cell Biol. 2003;160: 817-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3: 813-825. [DOI] [PubMed] [Google Scholar]
- 51.Hollenbeck PJ. The pattern and mechanism of mitochondrial transport in axons. Front Biosci. 1996;1: d91-d102. [DOI] [PubMed] [Google Scholar]
- 52.Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131: 1315-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baas P, Deitch J, Black M, Banker G. Polarity orientation of microtubules in hippocampal neurons— uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85: 8335-8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeCamilli P, Solimena M, Moretti M, Navone F. Studying the intrinsic determinants of neuronal form and function. In: Lasek R, Black M, eds. Intrinsic Determinants of Neuronal Form and Function. New York, NY: Liss; 1988: 1-60.
- 55.Peters A, Palay S, de Webster H. The Fine Structure of the Nervous System. Philadelphia, PA: Saunders; 1979.
- 56.Orokos DD, Cole RW, Travis JL. Organelles are transported on sliding microtubules in Reticulomyxa. Cell Motil Cyto. 2000;47: 296-306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.