Abstract
Multiple myeloma is a highly radiosensitive skeletal malignancy, but bone-seeking radionuclides have not yet found their place in disease management. We previously reported that the proteasome inhibitor PS-341 selectively sensitizes myeloma cells to the lethal effects of ionizing radiation. To extend these observations to an in vivo model, we combined PS-341 with the bone-seeking radionuclide 153-Sm-EDTMP. In vitro clonogenic assays demonstrated synergistic killing of myeloma cells exposed to both PS-341 and 153-Sm-EDTMP. Using the orthotopic, syngeneic 5TGM1 myeloma model, the median survivals of mice treated with saline, 2 doses of PS-341 (0.5 mg/kg), or a single nonmyeloablative dose of 153-Sm-EDTMP (22.5 MBq) were 21, 22, and 28 days, respectively. In contrast, mice treated with combination therapy comprising 2 doses of PS-341 (0.5 mg/kg), 1 day prior to and 1 day following 153-Sm-EDTMP (22.5 MBq) showed a significantly prolonged median survival of 49 days (P < .001). In addition to prolonged survival, this treatment combination yielded reduced clonogenicity of bone marrow–resident 5TGM1 cells, reduced serum myeloma–associated paraprotein levels, and better preservation of bone mineral density. Myelosuppression, determined by peripheral blood cell counts and clonogenicity assays of hematopoietic progenitors, did not differ between animals treated with 153-Sm-EDTMP alone versus those treated with the combination of PS-341 plus 153-Sm-EDTMP. PS-341 is a potent, selective in vivo radiosensitizer that may substantially affect the efficacy of skeletal-targeted radiotherapy in multiple myeloma.
Introduction
Multiple myeloma is a neoplasm of antibody-secreting plasma cells that infiltrate the bone marrow and form osteolytic tumors.1 It is an incurable malignancy causing more than 10 000 deaths each year in the United States.2 Standard treatment is with corticosteroids, thalidomide, alkylating agents, and stem-cell transplantation, but median survival remains only 4 years.1 Multiple myeloma is highly radiosensitive and is locally radiocurable.3,4 Novel systemic radionuclide-based therapies may therefore provide a significant advance in clinical therapy of myeloma.
Both samarium-153 ethylenediaminetetramethylene phosphonate (Sm-153-EDTMP)5-7 and Holmium-166 phosphonate chelate, 1,4,7,10 tetraazacyclododecane-1,4,7,10-tetra-methylene phosphonic acid (Ho-166-DOTMP)8 have been used as a basis for skeletal-targeted radiotherapy in multiple myeloma. 153-Sm-EDTMP emits β-particles with a range of 0.5 mm to 1.01 mm and has a half-life of 48.6 hours. It can therefore deliver effective doses of radiation to neoplastic plasma cells in bone marrow with limited toxicity to normal nonhematopoietic tissues.9 153[Sm] also emits γ-rays and is therefore amenable to noninvasive photon-based imaging studies to reveal the biodistribution of isotope in the body, facilitating complex dosimetry calculations.9,10
Besides targeting delivery to sites of tumor growth, another approach to improve the therapeutic index of radiation is to use radiation modifiers that alter the cellular response to radiation.11 The proteasome inhibitor PS-341 (pyrazylcarbonyl-Phe-Leu-boronate, bortezomib, or Velcade; Millennium Pharmaceuticals, Cambridge, MA) has been approved for patients with relapsed or refractory multiple myeloma.12 This drug has pleiotropic antimyeloma effects leading to tumor-cell killing in the bone marrow microenvironment. We recently demonstrated that PS-341 synergistically enhances the ionizing radiation-induced killing of human myeloma cell lines and primary human myeloma cells.13 However, our previous studies used a 137[Cs] source to deliver ionizing X-irradiation to myeloma cells, and there is no published data to indicate whether PS-341 can sensitize myeloma cells to killing by the β-emissions of a targeted radionuclide. In the present study we have therefore evaluated the use of a bone-seeking radiopharmaceutical, 153-Sm-EDTMP in combination with PS-341, both in cultured myeloma cells and in an orthotopic in vivo model using a nonmyeloablative regimen.
Several murine models of human multiple myeloma (MM) have been described.14 Here, we have used the 5TGM1 murine myeloma cell line, a variant of 5T33MM which originated in aging C57BL/KaLwRij mice that spontaneously develop monoclonal gammopathy.15 This is a valuable in vivo murine model for preclinical evaluation of investigational therapies because it closely mimics human myeloma disease in the following ways: (1) the circulating monoclonal paraprotein reflects tumor burden, (2) severe bone destruction is detected, and (3) rapid tumor growth occurs in an immunocompetent host enabling the evaluation of therapeutic effects on survival and growth of the myeloma cells in normal bone marrow microenvironment.16,17
The goal of the present study was to test the hypothesis that concurrent administration of the bone-seeking radiopharmaceutical (153-Sm-EDTMP) and PS-341, a known tumor-selective radiosensitizer with proven activity against multiple myeloma, will result in synergistic antimyeloma activity without synergistic toxicity to normal bone marrow. Our results show that this combination approach is highly effective in a relevant syngeneic orthotopic animal model of multiple myeloma and should therefore immediately be evaluated in the clinic.
Materials and methods
Cells
Human myeloma cell lines were either obtained from the American Type Culture Collection (ATCC, Rockville, MD; ARH-77, CRL-1621) or were a kind gift from Dr Rafael Fonseca (JJN-3, MM1) or Dr Diane Jelinek (RPMI 8226) at Mayo Clinic Foundation. These were grown in RPMI 1640 supplemented with heat-inactivated 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin. The 5TGM1 murine myeloma cell line (gift from Dr Babatunde O. Oyajobi, University of Texas Health Science Center at San Antonio) was grown in Iscoves modified Dulbecco medium (IMDM) with 10% FBS and penicillin-streptomycin antibiotics. All tissue-culture reagents were from Gibco BRL (Rockville, MD, USA).
Measurement of apoptosis
Apoptosis was determined by fluorescence-activated cell sorting (FACS) analysis after annexin V and propidium iodide (PI) staining (Santa Cruz Biotechnology, Santa Cruz, CA, USA) as described before.13 Briefly, 5 × 105 cells were incubated with PS-341 (10 nM) without or with 153-Sm-EDTMP (74 MBq/mL) for 24 hours, washed twice with PBS containing 2% bovine serum albumin (BSA), and incubated in 0.1 mL HEPES-buffered saline (with 2.5 mM CaCl2 and 2% BSA) containing annexin V-FITC (1 μg) and PI (0.05 μg) for 15 minutes at room temperature (RT). Samples were analyzed on a FACScan Plus cytometer (Becton Dickinson, Franklin Lakes, NJ), and subsequent analysis was done with CellQuest software (Becton Dickinson) where intact cells, apoptotic cells, and necrotic cells were annexin–/PI–, annexin+/PI–, and annexin+/PI+, respectively.
Western blotting
Immunoblottings were performed as described before.13 Briefly, whole-cell lysate was prepared by resuspending cell pellet in lysis buffer (2.5 mM Tris-HCl [pH 8.0], 12.5 mM NaCl, 12.5 mM NaF, 7.5 mM Na4P2O7, 0.25% Triton X-100 with 2 μg/mL aprotinin, 1 μg/mL pepstatin A, 1 mM PMSF, and 0.2 mM DTT). Protein concentrations were measured using the protein assay kit (Bio-Rad, Hercules, CA). Samples were loaded onto sodium dodecylsulfate–polyacrylamide gel (SDS-PAGE) for electrophoresis and then transferred to a PVDF membrane. The membranes were incubated with primary antibodies for 1 to 2 hours at RT against procaspase 3 (PharMingen, San Diego, CA), anti-PARP (Promega, Madison, WI), or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). After secondary antibody incubations, the signals were visualized using enhanced chemiluminescence (ECL) plus Western blotting system (Pierce, Rockford, IL).
3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
Cell viability was measured using MTT proliferation assay kit (ATCC). Cells (5 × 105) were treated with vehicle, z-VAD-fmk (100 μM; Promega), z-IETD-fmk (50 μM; Calbiochem, La Jolla, CA), or NF-κB inhibitory peptide SN-50 (20 μM; BIOMOL, Plymouth Meeting, PA) for 2 hours. Cells were then exposed to PS-341 (10 nM) and/or 153-Sm-EDTMP (74 MBq/mL) for 12 hours. After 2 washes with PBS, 0.1 mL cells were seeded/well in quadruplicate in 96-well plates, cultured for 24 hours; cell viability was examined as described previously.13 For this, 10 μL MTT-labeling reagent was added, and the cells were incubated for 3 hours at 37°C. Then, 100 μL dissolving reagent was added, and the plate was further incubated overnight at RT to dissolve the formazan crystals. The optical density was measured at 570 nm on a microplate reader (Molecular Devices, Sunnyvale, CA). Each data point represented the mean and standard deviation (SD).
In vivo experiments
Female C57BL/KaLwRij mice (4-6 weeks old) were purchased from Harlan CPB (Horst, The Netherlands) and housed in the animal facilities at the Mayo Clinic. The animal studies were performed in compliance with guidelines set by the Mayo Clinic's Institutional Animal Care and Use Committee. 5TGM1 cells (5 × 106) were injected intravenously on day 0, and mice were randomly stratified into experimental groups (n = 5/group [Figure 3] and n = 9/group [Figure 6]). Treatment was started on day 14 after tumor inoculation and consisted of intraperitoneal injections of PS-341 (Millennium Pharmaceuticals) and/or 153-Sm-EDTMP (Cytogen, Princeton, NJ) in 0.2 mL phosphate buffer saline (PBS; pH 7.2). In survival study 2, 6 mice per group were bled at baseline (day 12) and weekly thereafter for complete blood cell (CBC) counts (VetScan; Abaxis, Union City, CA) and serum preparation. Blood samples were collected by retro-orbital puncture under isoflurane-inhaled anesthesia. Mice were followed for disease-induced bone destruction by bone mineral density (BMD) scanning weekly. Body weights were taken at baseline and weekly thereafter. Five mice received no 5TGM1 cells (normal mice) and were used as controls for body weight and bone mineral density. On day 17, one mouse from each group was killed, and tibias and fibulas were removed. Bone marrow cells were isolated and (1) intracellular IgG2b FACS was done to determine tumor burden in the bone marrow, and (2) clonogenic assays were used to determine therapy-induced toxicity to the bone marrow–derived 5TGM1 cells. The rest of the mice were killed at the terminal stage of the disease showing hind limb paraplegia and IgG2b levels higher than 8 g/L.
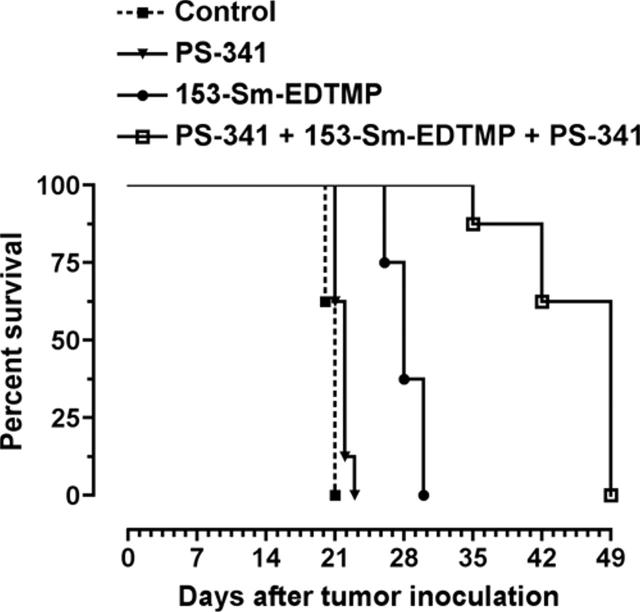
Figure 3.
Combined PS-341 and 153-Sm-EDTMP therapy prolongs survival in C57BL/KaLwRij mice. 5TGM1 cells (5 × 106) were inoculated via the tail vein on day 0. Therapy was initiated intraperitoneally on day 14, and mice were followed for disease progression. Kaplan-Meier survival plot of mice (n = 5 per group) in the pilot study is shown.
Figure 6.
Coadministration of PS-341 and 153-Sm-EDTMP prolongs the median survival time of 5TGM1-bearing mice. Kaplan-Meier survival plot of mice (n = 8 per group) with established murine multiple myeloma after intraperitoneal administration of PBS (▪; day 14 and 16), PS-341 (▾; day 14 and 16), 153-Sm-EDTMP (•; day 15), or PS-341/153-Sm-EDTMP/PS-341 (□; day 14/15/16). Data end for each group once mice succumb to disease and meet criteria for killing.
Clonogenic assays
Colony-forming ability following various treatments was evaluated as described before.13,18 Briefly, for myeloma cell lines, 1 × 106 cells were incubated with PS-341 (10 nM), 153-Sm-EDTMP (74 MBq/mL), or PS-341 and 153-Sm-EDTMP for 24 hours in the presence of RPMI 1640 complete media. Cells were washed with IMEM (StemCell Technologies, Vancouver, BC, Canada), and 0.5 mL cells (at 3 different log concentrations) in RPMI 1640 complete medium was added to 2.5 mL 1.2% basic methylcellulose medium (H4236 or M3232; StemCell Technologies). The mixtures were plated onto 35-mm Petri dishes and then maintained in a 37°C 5% CO2, fully humidified incubator. After 14 days of incubation, colonies consisting of more than 50 cells were directly scored using an inverted microscope, and colony formation for each condition was calculated in relation to values obtained for untreated control cells.
For clonogenicity of bone marrow–derived 5TGM1 cells, 5 mice (n = 1/group and 1 normal mouse) were killed on day 17, and marrow was flushed from femurs and tibias. Cells were collected, washed in IMEM (StemCell Technologies), counted, and plated in basic methylcellulose medium (M3232; StemCell Technologies). For assessing treatment-related toxicity to hematopoietic progenitor cells, marrow was taken from normal mice on day 2 that were treated with PBS or PS-341 (day –1 and +1), 153-Sm-EDTMP (day 0), or PS-341/153-Sm-EDTMP/PS-341 (day –1/0/+1, respectively). Hematopoietic progenitor (Lin–Sca+Kit+) cells were enriched using the EasySep kit (StemCell Technologies) and plated in methylcellulose medium supporting the growth of mouse progenitor cells (M3434; StemCell Technologies). Colonies were scored as described earlier in this section.
Measurement of serum IgG2b levels
IgG2b concentration in the sera was measured using a 2-site enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Bethyl Laboratories, Montgomery, TX). Briefly, goat anti–mouse IgG2b polyclonal antibody was coated onto plates overnight at 4°C. Plates were blocked with 5% bovine serum albumin for 3 hours at RT. Mouse reference serum or serum test samples were added and incubated for 1 hour at 37°C. Plates were washed and incubated with secondary peroxide–conjugated goat anti–mouse IgG2b antibody for 1 hour at 37°C. ABTS (2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) chromogen substrate solution (Zymed Laboratories, San Francisco, CA) was used as reaction substrate according to the manufacturer's instructions. The reaction product was quantified spectrophotometrically at 405 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). All assays were performed in quadruplicate, and the mean ± SD was determined.
Radiographic analysis
BMD was determined using a murine bone densitometer (PIXImus; Lunar, Madison WI). The DEXA (dual energy X-ray absorptiometry) scans generated were used to assess the BMD at femur and lumbar spine by adjusting the region of interest window. At each time point, data from 5 mice per group was evaluated to determine the mean BMD values.
Statistical considerations
Data from each of the experiments and evaluations described for clonogenic assays and survival analyses were quantitatively summarized for each treatment group separately. Specifically, for each continuous measure, means and medians were calculated as well as the SD and range. These data were also evaluated graphically for potential differences and relations in the efficacy and toxic effect measures within and among the 4 treatment groups. Differences in these continuous measures between treatment groups were also compared using nonparametric approaches (Wilcoxon rank sum test for pairwise comparisons; Kruskal-Wallis for comparisons of all 4 groups) where feasible and appropriate. The in vivo studies were conducted in mice; thus, there were no censored observations. However, overall survival was graphically evaluated using the methods of Kaplan and Meier. In addition, survival distributions between groups were compared as well as pairwise comparisons between specific groups of interest (eg, 153-Sm-EDTMP alone versus PS-341/153-Sm-EDTMP/PS-341) using log-rank statistics as well as 2-sample tests, given that there were no censored observations in these analyses. Significance for all tests was declared at P values less than .05, where no corrections were made for multiple comparisons. Because of the relatively small sample sizes, the corresponding statistical power was limited to detecting very large differences between groups; however, very large differences were scientifically of interest here. On the basis of the data of survival curve 1 (Figure 3), a 2-sided, 2-sample comparison between treatment groups had at least 80% power to detect a difference of 4 days (significance level of .05). This was assuming the most conservative comparison where the SD for one group was approximately 2 days and 3 days for another group. Many of the overall evaluations across treatment groups were graphically evaluated and explored further, where appropriate, with quantitative comparisons. For the in vitro studies, initial analyses of data across cell lines indicated that these cell lines were heterogeneous and therefore were subsequently evaluated individually.
Potential synergistic effects of 153-Sm-EDTMP and PS-341 on clonogenicity and survival were evaluated using 2 model-based approaches. A significant interaction would indicate nonadditivity and thus a synergistic relationship between the 2 treatments in their effect on these outcomes. The first approach treated the outcomes as count data, and Poisson regression models were used to assess the significance of interactions between 153-Sm-EDTMP and PS-341 administration. The second approach treated these outcomes as continuous measures and ANOVA models were used to test for interaction effects.
Results
153-Sm-EDTMP and PS-341 show synergistic cytotoxicity against multiple myeloma cell lines
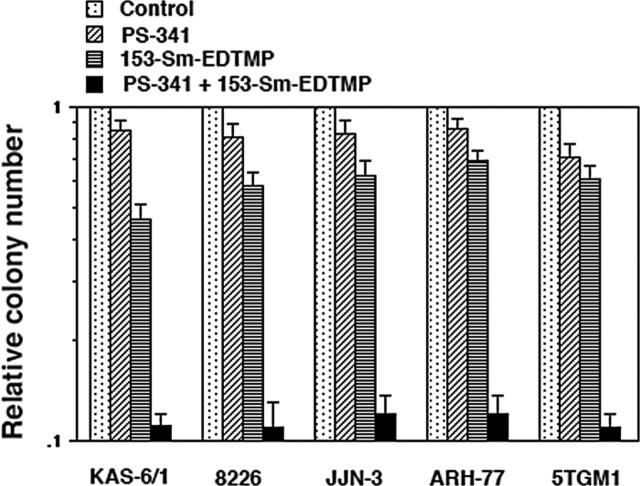
We first sought to determine whether the 153-Sm-EDTMP plus PS-341 combination would be more cytotoxic to myeloma cell lines in vitro than either agent alone. In our previous study we showed that exposing myeloma cell lines to 10 nM PS-341 for 16 hours leads to an approximate 25% cytotoxicity but renders them highly sensitive to killing by external beam ionizing radiation.13 To determine whether a similar radiosensitization of myeloma cells can be achieved by combining PS-341 and 153-Sm-EDTMP, 5 different myeloma lines were exposed to different concentrations of 153-Sm-EDTMP for 24 hours without or with PS-341, and their clonogenic ability was evaluated as described before.13 PS-341 (10 nM) marginally decreased the clonogenicity of the myeloma cells. 153-Sm-EDTMP (74 MBq/mL) alone reduced the colony number by 40% to 50%. In contrast, when cells were cotreated with PS-341 (10 nM) and 153-Sm-EDTMP (74 MBq/mL), there was a significant increase in the cell killing compared with the effect of either agent alone (Figure 1). For each of the cell lines, there was a significant interaction (nonadditive) effect in the Poisson regression models between PS-341 and 153-Sm-EDTMP (P < .001 for each cell line). Significant interactions for each of the cell lines were also observed using the ANOVA modeling approach. Overall, these data show that under in vitro conditions, PS-341 can enhance the efficacy of 153-Sm-EDTMP in a synergistic manner against myeloma cells.
Figure 1.
Clonogenicity data showing synergistic cytotoxicity with PS-341 and 153-Sm-EDTMP against myeloma cell lines. Cells were treated with PS-341 (10 nM), 153-Sm-EDTMP (74 MBq/mL), or PS-341 and 153-Sm-EDTMP for 24 hours. Clonogenic assays were read on day 14; data are expressed relative to the colony number in the untreated sample. Error bars indicate SD.
PS-341 sensitizes myeloma cells to 153-Sm-EDTMP–mediated apoptosis
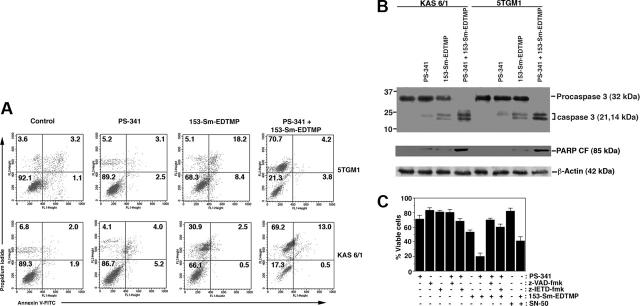
In our previous study we delineated the mechanism of PS-341–mediated sensitization of myeloma cells to ionizing radiation; PS-341 inhibited the antiapoptotic property of NF-κB and up-regulated CD95/CD95L interaction.13 We therefore wanted to ascertain whether PS-341 was increasing sensitization of myeloma cells by activation of apoptotic pathways initiated by 153-Sm-EDTMP. The induction of apoptosis of 5TGM1 and KAS 6/1 myeloma cell lines was evaluated following incubation with PS-341 with or without 153-Sm-EDTMP. The combination of annexin-V and PI staining was used to simultaneously quantify live, apoptotic, and necrotic cells. For both cell lines, a significant reduction in viable cells was detected (Figure 2A).
Figure 2.
PS-341 sensitizes myeloma cells to 153-Sm-EDTMP–mediated apoptosis. (A) Flow cytometric analysis of myeloma cell lines treated with PS-341 and/or 153-Sm-EDTMP for 24 hours. (B) Western blotting was performed on lysates from 5TGM1 and KAS 6/1 cells untreated or treated with PS-341 and/or 153-Sm-EDTMP. Blots were probed with anti–procaspase 3, anti-PARP (p85), or antiactin antibodies. (C) 5TGM1 cells were preincubated with the broad-spectrum caspase inhibitors z-VAD-fmk (100 μM), the caspase-8 inhibitor z-IETD-fmk (50 μM), or NF-κB inhibitory peptide SN-50 (20 μM) for 2 hours before the addition of PS-341 (10 nM) and/or 153-Sm-EDTMP (74 MBq/mL) for 12 hours. Cells were washed, and MTT assay was performed at 24 hours after treatment. Results are mean ± SD of 3 independent experiments.
The mechanism of induction of cell death following PS-341 and/or 153-Sm-EDTMP was examined using 5TGM1 and KAS 6/1 myeloma cell lines. Lysates were prepared from cells that were treated with PS-341 (10 nM) and/or 153-Sm-EDTMP (74 MBq/mL) for 12 hours, and immunoblotting was performed with antibodies to caspase 3 (Figure 2B). For both cell lines, treatment with 153-Sm-EDTMP resulted in cleavage of procaspase 3 that was much greater in cells cotreated with PS-341 and 153-Sm-EDTMP, demonstrating that apoptotic pathways participated in cell death. Immunoblottings with anti–poly (ADP-ribose) polymerase (PARP) antibody confirmed the increased caspase 3–mediated cleavage of 116-kDa PARP protein to an 85-kDa polypeptide. Anti-β actin antibody confirmed equal loading of total protein in each lane of the Western blot (Figure 2B).
Mechanistically, various pharmacologic inhibitors were used to assess the contribution of the mitochondrial and receptor-related apoptotic pathways in PS-341/153-Sm-EDTMP–mediated lethality (Figure 2C). Exposure of cells to PS-341 alone reduced viability by 20% to 30%; the lethality could be enhanced to approximately 80% by combining PS-341 with 153-Sm-EDTMP. The pan-caspase inhibitor z-VAD-fmk prevented apoptosis induced by PS-341/153-Sm-EDTMP combination, showing participation of caspases in cell death. When caspase-8 activity was inhibited by IETD-fmk, there was a restoration of apoptosis but not to the extent achieved with z-VAD-fmk, indicating the involvement of both casapase-9 and -8 pathways in PS-341/153-Sm-EDTMP–induced apoptosis. When NF-κB was specifically blocked using SN-50 peptide, the PS-341/153-Sm-EDTMP–induced apoptosis was significantly abolished, substantiating the finding that one of the major mechanisms of PS-341 action in myeloma is its interference with the NF-κB pathway by blocking degradation of IκB.13,19 Nevertheless, other NF-κB–independent pathways were also instrumental in apoptosis by PS-341/153-Sm-EDTMP. Overall, these data suggest that at subtoxic doses, PS-341 enhances 153-Sm-EDTMP–induced apoptosis in myeloma cells by both caspase 8– and caspase 9–dependent pathways.
153-Sm-EDTMP and PS-341 show synergistic activity in the 5TGM1 murine myeloma model
To determine whether PS-341 could improve the efficacy of 153-Sm-EDTMP in multiple myeloma in vivo we have used a well-characterized orthotopic, syngeneic murine multiple myeloma model (5TGM1).20,21 For induction of myeloma, C57BL/KaLwRij mice were injected intravenously with 5 × 106 5TGM1 cells (day 0). Therapy was initiated on day 14 when mice have both diffuse bone marrow infiltration and scattered nodular plasmacytomas,22 thus closely resembling the advanced stages of human multiple myeloma. On the basis of earlier literature, doses of PS-341 (0.5 mg/kg)23 and 153-Sm-EDTMP (22.5 MBq)24 that were known to be reasonably tolerated in mice were chosen. The radionuclide 153[Sm] has a physical half-life of 46.3 hours, and EDTMP, the diphosphonate chelator to which the 153[Sm] is complexed selectively and stably, accumulates in skeletal tissue by binding to hydroxyapatite, with a rapid clearance from the blood (< 1% of the dose remaining in the circulation 1 hour after administration). The appropriate timing of therapy with PS-341 in relation to 153-Sm-EDTMP was determined by administering PS-341 on day 14 or day 16; 153-Sm-EDTMP was given on day 15. Also to ensure continuous proteasome inhibition during the time period of maximum radiation exposure in the bone marrow, another group received PS-341 on days 14 and 16; 153-Sm-EDTMP was given on day 15. The control and the PS-341 group received PBS, pH 7.2, or PS-341 on day 14, respectively.
As shown in Figure 3, the group receiving PS-341 did not exhibit any survival advantage over the control group, with a median survival of 20 days versus 18 days, respectively. Treatment with Sm-153-EDTMP did prolong the median survival from day 18 (control group) to day 25 (P = .001). In contrast, administration of PS-341 and Sm-153-EDTMP resulted in increased median survival (P < .001) that was independent of sequence of administration of the therapeutic agents (medians, Sm-153-EDTMP/PS-341 = 38 days, PS-341/Sm-153-EDTMP = 36 days; P = .286). A further enhancement in median survival was achieved by using 2 doses of PS-341 given as PS-341/Sm-153-EDTMP/PS-341 regimen (median = 42 days; P = .001). The results from this study showed that (1) PS-341 could enhance the efficacy of 153-Sm-EDTMP in multiple myeloma in vivo, and (2) 2 doses of PS-341, given at day –1 and day +1 in relation to Sm-153-EDTMP injection (day 0), were more effective than a single dose of PS-341.
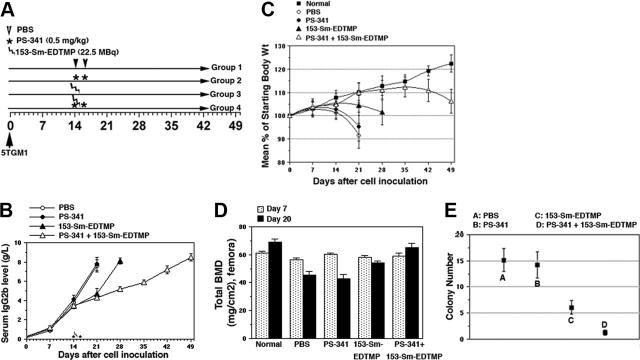
Another study was designed in which (1) control group (PBS, day 14 and 16), (2) PS-341 group (0.5 mg/kg PS-341, day 14 and 16), (3) 153-Sm-EDTMP group (22.5 MBq 153-Sm-EDTMP, day 15), and (4) PS-341 and 153-Sm-EDTMP group (PS-341/samarium/PS-341, day 14/15/16) were included. To determine whether myeloma progression was slower in mice receiving combined treatment with PS-341 and 153-Sm-EDTMP, we monitored their serum IgG2b levels and bone mineral densities. Serum level of IgG2b is an established indicator of 5TGM1 tumor burden in syngeneic mice.20 As shown in Figure 4A, on day 14 (the first day of treatment) all groups showed a paraprotein level of approximately 4 g/L with no significant difference between control and treatment groups. By day 21, the paraprotein concentrations of control and PS-341–treated mice had increased to 8 g/L, and the animals were moribund. By contrast, day-21 paraprotein levels remained low in mice treated with 153-Sm-EDTMP plus or minus PS-341. The paraprotein concentration in mice receiving 153-Sm-EDTMP monotherapy then increased rapidly to 8 g/L by day 28, whereas mice treated with PS-341 and 153-Sm-EDTMP combination did not reach 8 g/L until day 49 at which point these animals had to be killed.
Figure 4.
Combined PS-341 and 153-Sm-EDTMP therapy delays myeloma progression and bone destruction in C57BL / KaLwRij mice. (A) Schematic representation of the in vivo protocol. (B) Effect of therapy on serum IgG2b levels in 5TGM1 myeloma-bearing mice. IgG2b levels were measured by ELISA as described in “Materials and methods.” Data are means ± SD of 6 mice. (C) Percentage of change in total body weight in treatment groups receiving 153-Sm-EDTMP without or with PS-341. Tumor-bearing or normal mice were measured weekly and are presented as percentage of initial body weight ± SD. (D) Total BMD in femurs of normal mice or mice injected with 5TGM1 were measured by DEXA on days 7 and 20, n = 6 per group. (E) On day 17, one mouse per treatment group was killed, and bone marrow was used to enumerate the posttreatment clonogenicity of bone marrow–resident 5TGM1 cells. Each point represents the mean colony number per 10 fields ± SD.
As a surrogate measure of treatment-induced toxicity to 153-Sm-EDTMP and/or PS-341, body weights were monitored (Figure 4B). Mice were weighed prior to treatment and weekly thereafter to ensure that their body weight remained greater than 80% of starting weight. Both control and PS-341 groups showed a maximum weight loss of 10% to 12% on day 21. The group receiving 153-Sm-EDTMP showed no weight loss until day 28. No worsening of toxicity of 153-Sm-EDTMP was observed by coadministration of PS-341. Maximum weight loss after combined administrations of 153-Sm-EDTMP and PS-341 was seen on day 49; when compared with the normal mice that had gained 20% of their original weight, these were still at their starting weight. However, there were no treatment-related deaths or myelosuppression as discussed in Figure 5.
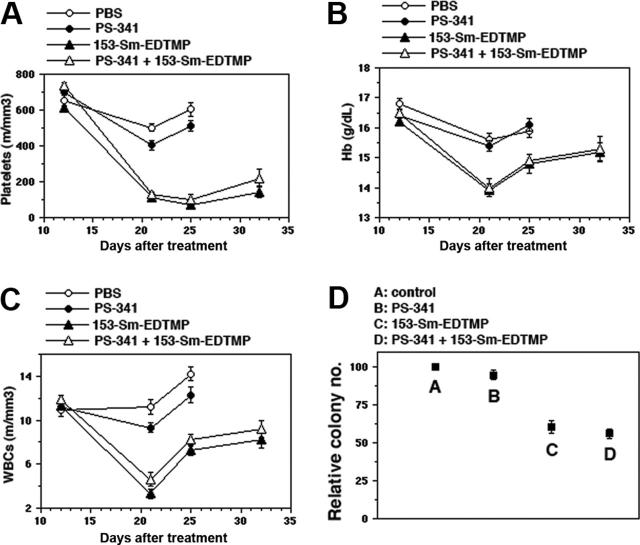
Figure 5.
PS-341 does not worsen the myelosuppressive effect of 153-Sm-EDTMP. Blood cell counts in treatment groups bearing 5TGM1-myeloma platelets (A), hemoglobin (B), and white blood cells (C) were monitored on days 12, 21, 25, and 32. Error bars represent the mean ± SD from the counts of 6 mice in each group. (D) Clonogenic assay with enriched hematopoietic progenitor cells from normal mice receiving PS-341 and/or 153-Sm-EDTMP. Data represent relative colony number (CFU-GEMM, CFU-GM, BFU-E, BFU-MK) expressed as a percentage of colony counts in control non–tumor-bearing mice. Bars indicate SD.
In the 5TGM1 model, extensive myeloma-associated bone destruction develops in mice following intravenous inoculation of the cells.20,21 BMD analyses were therefore performed by DEXA imaging on days 7 and 20 after tumor-cell administration (Figure 4C). The lumbar spine and femur regions were used for assessment because these sites contain a high proportion of trabecular bone. On day 7 the BMDs of all the treatment groups were similar and comparable to the BMDs of the control group receiving no myeloma cells. However, on day 20 the PBS control and PS-341 and 153-Sm-EDTMP monotherapy groups showed a significant decrease in total BMD in femora (Figure 4C) and lumbar vertebrae (data not shown). Mice in the PS-341 plus 153-Sm-EDTMP group showed no loss of BMD and were comparable to the normal non–tumor-bearing mice on day 20.
To evaluate the effect of each treatment on bone marrow–resident myeloma cells, we killed mice from each treatment group on day 17 and measured the viability of 5TGM1 cells extracted from their femurs by clonogenic assay. For this, methylcellulose medium devoid of growth factors was used to support exclusive growth of the myeloma cells. For each treatment, the total number of 5TGM1 colonies in 10 fields was scored, and the mean value per low power field was determined (Figure 4D). For control and PS-341 groups, the values were similar, 15.1 ± 2.2 and 14.2 ± 2.5, suggesting little effect of PS-341 on the in vivo myeloma burden. Treatment with 153-Sm-EDTMP decreased the clonogenicity of 5TGM1 by approximately 60% (6.1 ± 1.3 colonies per field), whereas cotreatment of PS-341 and 153-Sm-EDTMP resulted in a 90% (1.3 ± 0.5 colonies per field) reduction of the clonogenicity of myeloma cells. In addition, 153-Sm-EDTMP and PS-341 had a significant interaction, indicating a synergistic relationship in terms of clonogenicity measures (P < .001). Taken together, these results indicate that combining 153-Sm-EDTMP with PS-341 treatment results in a significant and rapid synergistic killing of bone marrow–resident myeloma cells in vivo.
PS-341 does not increase the myelosuppressive effect of 153-Sm-EDTMP
153[Sm] is a short-range, β-emitting radionuclide, and, when conjugated to the biphosphonate EDTMP, it localizes primarily to bone. Therefore, the major potential toxic effect of 153-Sm-EDTMP is the suppression of normal bone marrow function. In the present study we used nonmyeloablative doses of 153-Sm-EDTMP. To determine whether PS-341 cotreatment exacerbates the toxicity of 153-Sm-EDTMP to normal marrow, peripheral blood cell counts were monitored in all treatment groups.
The baseline complete blood count (CBC) was obtained on day 12 and subsequently on days 21, 25, and 32 (Figure 5A-C). In all treatment groups, an obvious decline in the mean platelet count (Figure 5A), hemoglobin (Hb) level (Figure 5B), and white blood cell (WBC) counts (Figure 5C) were observed after the first week of treatment. The decline was considerably more pronounced in groups receiving 153-Sm-EDTMP. For all treatments groups, the nadir values for Hb levels and WBC counts were observed on day 21 (8 days after initiation of therapy). Platelet counts continued to fall in the 153-Sm-EDTMP–treated groups until day 25. Importantly, cotreatment with PS-341 and 153-Sm-EDTMP did not cause a further decline in the cell counts compared with treatment with 153-Sm-EDTMP alone, nor did it lead to any incremental delay in the recovery of blood counts after therapy.
As a direct measure of toxicity to bone marrow progenitor cells, Lin–Sca+Kit+ cells25 were enriched from bone marrow on day 1 after therapy, and their in vitro colony-forming ability was determined in methylcellulose medium (Figure 5D). The data for granulocyte, erythroid, macrophage, megakaryocyte colony-forming units (CFU-GEMMs), granulocyte monocytic colony-forming units (CFU-GMs), erythroid burst-forming units (BFU-Es), and megakaryocytic burst-forming units (BFU-MK) were pooled, and the average number per 10 fields was determined. The colony number for PBS control was set to 100%, and the data were represented as relative colony number compared with the control. PS-341 monotherapy was associated with a very small reduction in colony number, whereas 153-Sm-EDTMP reduced the colony counts to 50% of control. The toxicity of 153-Sm-EDTMP was not exacerbated by the coadministration of PS-341.
153-Sm-EDTMP and PS-341 prolong median survival of 5TGM1-bearing mice
The survival curves for this experiment are shown in Figure 6. Median survival of the untreated control animals was 21 days from tumor-cell infusion. By day 20, all control mice showed signs of advanced multiple myeloma, including weight loss, scruffy coat, and development of hind-limb paralysis. At the time of this humane end point, mice were expected to die within 1 or 2 days. PS-341 monotherapy did not significantly prolong the median survival (22 days) over control mice. 153-Sm-EDTMP monotherapy resulted in a significant prolongation of median survival to 28 days (P < .001). Median survival in animals receiving the combination of 153-Sm-EDTMP and PS-341 was dramatically increased to 49 days (P < 0.001). In addition, observed significant interaction effects in Poisson regression (P = .006) as well as ANOVA (P < .001) models indicate that PS-341 and 153-Sm-EDTMP are synergistic.
Discussion
In the current manuscript, we have shown, using a relevant animal model of multiple myeloma, that short-term exposure to the proteosome inhibitor PS-341 dramatically enhances the therapeutic index of low-dose β-radiation delivered to the bone marrow via Sm-153-EDTMP. Several published studies have pointed to the possibility of using PS-341 as a radiosensitizer,26-29 but until recently this concept had not been tested with respect to multiple myeloma. We previously demonstrated that PS-341 potently sensitizes human myeloma cell lines and primary human myeloma cells to the lethal effects of ionizing radiation.13 Biochemical studies suggested at least 3 possible mechanisms underlying PS-341–mediated radiosensitization, including the inhibition of NF-κB survival signaling, cell-cycle arrest at the exquisitely radiosensitive G2/M phase of the cell cycle, and the enhancement of extrinsic apoptosis by induction of the Fas death receptor.13 For our previous studies, the myeloma cells were exposed to PS-341 for 16 hours and then received a dose of 2 or 6 Gy (dose rate 0.5 Gy/min) of γ-radiation from a 137[Cs] unit. This rapid delivery of radiation bears little resemblance to the dose-rate when using targeted radionucleotides, whereby the tissue-absorbed dose of radiation is delivered mainly in the form of β-emissions, and the rate of deposition of radiation energy in the tissues is much slower than with external beam γ-radiation.30 Considering this extreme difference in radiation dose rate, as well as published studies showing that cells respond differently to β versus γ radiation, it is significant that we have now demonstrated that PS-341 can also sensitize myeloma cells to the lethal effects of a β-emitting isotope.
The synergistic activity of PS-341 and 153-Sm-EDTMP was strikingly demonstrated in the syngeneic orthotopic 5TGM1 myeloma model. The survival times of these mice were significantly longer after treatment with 2 doses of PS-341 given 1 day before and 1 day after 153-Sm-EDTMP, versus mice receiving 153-Sm-EDTMP or PS-341 only (median survival of 49 versus 28 or 22 days, respectively; P < .001). Observed significant interactions further demonstrated that this increased activity was indeed synergistic and nonadditive. In multiple myeloma, it is well established that the 3-dimensional interactions of myeloma cells with the host bone marrow microenvironment regulate tumor-cell growth, survival, and drug resistance.31 It is therefore essential that novel therapies targeting myeloma cells should be evaluated in the context of a normal bone marrow microenvironment.32 Many experimental models for multiple myeloma are based on the implantation of primary human myeloma cells or myeloma cell lines in severe combined immunodeficient (SCID) mice,33 which are known to have a defect in their double-strand break repair, making them highly sensitive to ionizing radiation and therefore unsuitable for the preclinical evaluation of radionuclide-based therapies.34 The 5TGM1 cell line is derived from the 5T33MM model that originated as a spontaneously developing myeloma in elderly C75BL/KaLwRij mice and is propagated in the same syngeneic strain.20 5TGM1 cells, administered intravenously to C75BL/KaLwRij mice, seed predominantly to the bone marrow where they grow rapidly, resulting in hind limb paralysis after approximately 4 to 5 weeks.35,36 This model was considered to be ideal for the current study because, in contrast to SCID mice, the C75BL/KaLwRij mice have no known defect in their ability to repair DNA damage.
At the current time, the mechanisms underlying the observed in vivo synergy between PS-341 and 153-Sm-EDTMP have not been fully defined, and this is the subject of ongoing investigations. Whereas multiple myeloma is known to be a highly radiosensitive malignancy, isolated myeloma plasma cells are relatively radioresistant when compared with normal hematopoietic progenitors and other neoplastic T or B cells.37 Thus, although our in vitro studies demonstrate clearly that PS-341 has a direct radiosensitizing effect on myeloma plasma cells (through G2/M arrest, NF-κB suppression, and Fas induction), we are actively investigating the possibility that additional mechanisms are operating in vivo. In this regard, it is now well established that bone marrow–resident myeloma cells are bombarded with a variety of growth and survival signals in the form of cytokines, growth factors, and cell-cell interactions with vascular endothelial cells, osteoclasts, and other bone marrow stromal cells.17 We are therefore investigating the possibility that PS-341 and Sm-153-EDTMP act synergistically to interrupt these critical supportive stromal interactions.
The dose of 153-Sm-EDTMP (22.5 MBq per mouse) was selected based on published work showing that this dose is moderately myelosuppressive but not myeloablative in C75BL/KaLwRij mice bearing 5T33 murine myeloma.4 Similarly, the dose of PS-341 (0.5 mg/kg) was based on a published study showing that this dose was well tolerated and therapeutically effective in a human myeloma xenograft model when administered twice weekly for 4 weeks and at weekly intervals thereafter.23 In addition, we conducted preliminary studies in C57BL/6 mice to determine that this drug combination could be administered without untoward toxicities (data not shown). Additional preliminary studies were conducted to determine whether timing of PS-341 administration had a significant effect on the observed synergy between PS-341 and 153-Sm-EDTMP (data not shown). In all cases, PS-341 was administered within 24 hours of 153-Sm-EDTMP, and no schedule-dependent differences in survival were identified. However, animals receiving 2 doses of PS-341 did show statistically significant improvement in median survival time compared with animals receiving a single dose. Within a few hours of its administration, approximately 50% of an administered dose of Sm-153-EDTMP is bound irreversibly to the skeleton, and the remainder is rapidly excreted via the kidneys.38 The isotope then decays with a half-life of 48.6 hours such that significant delivery of ionizing radiation to the bone marrow space continues for approximately 10 days.9 Thus, PS-341 administered within 24 hours of the Sm-153-EDTMP would be expected to inhibit proteasome function during the critical period when the rate of β-emission in the bone marrow is maximal. However, because this is a complex relationship, further studies will be required to determine whether PS-341 administration at earlier or later times or for longer periods of time might give superior results.
The enhanced tumor efficacy that was achieved in this study, by combining PS-341 with Sm-153-EDTMP, did not come at the expense of increased bone marrow toxicity. In one sense, this was a surprising result because reversible thrombocytopenia is one of the major toxicities associated with PS-341 therapy39,40 and might therefore be expected to exacerbate the myelosuppressive activities of 153-Sm-EDTMP. However, analysis of peripheral blood cell counts and clonogenicity assays of hematopoietic progenitors isolated from the bone marrow of tumor-bearing or non–tumor-bearing mice showed no evidence for additive or synergistic myelotoxicity. In fact, there was a trend in favor of more rapid recovery of blood counts after combination therapy. There are a number of possible explanations for the relative resistance of normal hematopoietic progenitors to the radiosensitizing effects of PS-341, including a limited dependence on NF-κB pathway activation as part of their survival response to ionizing radiation, resistance to PS-341–mediated G2/M cell-cycle arrest, and distinct mechanisms underlying the myelosuppressive effects of PS-341 versus ionizing radiation. Radiation causes myelosuppression by inducing both apoptosis of hematopoietic cells and regression of the bone marrow sinusoidal neovessels.41,42 In contrast, the mechanism by which PS-341 suppresses platelet production has not been elucidated. However, it was recently shown that changes in the activity of the Aurora-B kinase, regulated by proteasomal degradation, are critical to the process of megakaryocyte polyploidization,43 suggesting that PS-341–induced thrombocytopenia may be a consequence of interference with this process.
With the currently used treatment, multiple myeloma remains incurable and causes more than 10 000 deaths each year in the United States.2 High-dose, myeloablative melphalan therapy followed by autologous stem-cell transplantation increases the number of complete remissions but does not greatly prolong the median survival, which currently stands at approximately 4 years.1 Because the majority of patients with myeloma are not candidates for stem-cell transplantation, and those who do receive high-dose therapy will eventually relapse, we chose to focus our efforts on the development of a nonmyeloablative radionuclide-based regimen. On the basis of the encouraging data reported in this manuscript, we now propose to conduct a phase 1 clinical trial combining a single, nonmyeloablative dose of Sm-153-EDTMP with increasing doses of PS-341 in patients with advanced multiple myeloma.
Acknowledgments
We thank the flow cytometry core at Mayo Clinic, Rochester, MN. PS-341 was a gift from Millennium Pharmaceuticals (Cambridge, MA). 153-Sm-EDTMP was supplied by Cytogen Corporation (Princeton, NJ).
Prepublished online as Blood First Edition Paper, January 19, 2006; DOI 10.1182/blood-2005-09-3870.
Supported by JARI Research Foundation and a grant from the National Institutes of Health (NIH; CA100634-02).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Sirohi B, Powles R. Multiple myeloma. Lancet. 2004;363: 875-887. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55: 10-30. [DOI] [PubMed] [Google Scholar]
- 3.Mill WB. Radiation therapy in multiple myeloma. Radiology. 1975;115: 175-178. [DOI] [PubMed] [Google Scholar]
- 4.Turner JH, Claringbold PG, Berger JD, Martindale AA, Glancy JR. 153Sm-EDTMP and melphalan chemoradiotherapy regimen for bone marrow ablation prior to marrow transplantation: an experimental model in the rat. Nucl Med Commun. 1992;13: 321-329. [DOI] [PubMed] [Google Scholar]
- 5.Hogan WJ, Lacy MQ, Wiseman GA, Fealey RD, Dispenzieri A, Gertz MA. Successful treatment of POEMS syndrome with autologous hematopoietic progenitor cell transplantation. Bone Marrow Transplant. 2001;28: 305-309. [DOI] [PubMed] [Google Scholar]
- 6.Knop S, Dohmen BM, Kanz L, Bares R, Einsele H. 153 Samarium-EDTMP in myeloablative dosage followed by a second autotransplantation in patients with relapsed multiple myeloma. Haematologica. 2004;89: ECR36. [PubMed] [Google Scholar]
- 7.Dispenzieri A, Wiseman GA, Lacy MQ, et al. A phase I study of 153Sm-EDTMP with fixed high-dose melphalan as a peripheral blood stem cell conditioning regimen in patients with multiple myeloma. Leukemia. 2005;19: 118-125. [DOI] [PubMed] [Google Scholar]
- 8.Giralt S, Bensinger W, Goodman M, et al. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102: 2684-2691. [DOI] [PubMed] [Google Scholar]
- 9.Goeckeler WF, Troutner DE, Volkert WA, Edwards B, Simon J, Wilson D. 153Sm radiotherapeutic bone agents. Int J Rad Appl Instrum B. 1986;13: 479-482. [DOI] [PubMed] [Google Scholar]
- 10.Bayouth JE, Macey DJ, Kasi LP, Fossella FV. Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J Nucl Med. 1994;35: 63-69. [PubMed] [Google Scholar]
- 11.Colevas AD, Brown JM, Hahn S, Mitchell J, Camphausen K, Coleman CN. Development of investigational radiation modifiers. J Natl Cancer Inst. 2003;95: 646-651. [DOI] [PubMed] [Google Scholar]
- 12.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348: 2609-2617. [DOI] [PubMed] [Google Scholar]
- 13.Goel A, Dispenzieri A, Greipp PR, Witzig TE, Mesa RA, Russell SJ. PS-341-mediated selective targeting of multiple myeloma cells by synergistic increase in ionizing radiation-induced apoptosis. Exp Hematol. 2005;33: 784-795. [DOI] [PubMed] [Google Scholar]
- 14.Gado K, Silva S, Paloczi K, Domjan G, Falus A. Mouse plasmacytoma: an experimental model of human multiple myeloma. Haematologica. 2001; 86: 227-236. [PubMed] [Google Scholar]
- 15.Radl J. Idiopathic paraproteinemia—a consequence of an age-related deficiency in the T immune system. Three-stage development—a hypothesis. Clin Immunol Immunopathol. 1979;14: 251-255. [DOI] [PubMed] [Google Scholar]
- 16.Yaccoby S, Pearse RN, Johnson CL, Barlogie B, Choi Y, Epstein J. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol. 2002;116: 278-290. [DOI] [PubMed] [Google Scholar]
- 17.Roodman GD. Role of the bone marrow microenvironment in multiple myeloma. J Bone Miner Res. 2002;17: 1921-1925. [DOI] [PubMed] [Google Scholar]
- 18.Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103: 2332-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277: 16639-16647. [DOI] [PubMed] [Google Scholar]
- 20.Garrett IR, Dallas S, Radl J, Mundy GR. A murine model of human myeloma bone disease. Bone. 1997;20: 515-520. [DOI] [PubMed] [Google Scholar]
- 21.Dallas SL, Garrett IR, Oyajobi BO, et al. Ibandronate reduces osteolytic lesions but not tumor burden in a murine model of myeloma bone disease. Blood. 1999;93: 1697-1706. [PubMed] [Google Scholar]
- 22.Olson DL, Burkly LC, Leone DR, Dolinski BM, Lobb RR. Anti-alpha4 integrin monoclonal antibody inhibits multiple myeloma growth in a murine model. Mol Cancer Ther. 2005;4: 91-99. [PubMed] [Google Scholar]
- 23.LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62: 4996-5000. [PubMed] [Google Scholar]
- 24.Turner JH, Claringbold PG, Manning LS, O'Donoghue HL, Berger JD, Glancy RJ. Radiopharmaceutical therapy of 5T33 murine myeloma by sequential treatment with samarium-153 ethylenediaminetetramethylene phosphonate, melphalan, and bone marrow transplantation. J Natl Cancer Inst. 1993;85: 1508-1513. [DOI] [PubMed] [Google Scholar]
- 25.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241: 58-62. [DOI] [PubMed] [Google Scholar]
- 26.Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5: 2638-2645. [PubMed] [Google Scholar]
- 27.Pajonk F, Pajonk K, McBride WH. Apoptosis and radiosensitization of Hodgkin cells by proteasome inhibition. Int J Radiat Oncol Biol Phys. 2000;47: 1025-1032. [DOI] [PubMed] [Google Scholar]
- 28.Pervan M, Pajonk F, Sun JR, Withers HR, McBride WH. Molecular pathways that modify tumor radiation response. Am J Clin Oncol. 2001; 24: 481-485. [DOI] [PubMed] [Google Scholar]
- 29.Russo SM, Tepper JE, Baldwin AS Jr, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys. 2001;50: 183-193. [DOI] [PubMed] [Google Scholar]
- 30.Marko NF, Dieffenbach PB, Yan G, et al. Does metabolic radiolabeling stimulate the stress response? Gene expression profiling reveals differential cellular responses to internal beta vs. external gamma radiation. FASEB J. 2003;17: 1470-1486. [DOI] [PubMed] [Google Scholar]
- 31.Richardson PG, Mitsiades CS, Hideshima T, Anderson KC. Novel biological therapies for the treatment of multiple myeloma. Best Pract Res Clin Haematol. 2005;18: 619-634. [DOI] [PubMed] [Google Scholar]
- 32.Pagnucco G, Cardinale G, Gervasi F. Targeting multiple myeloma cells and their bone marrow microenvironment. Ann N Y Acad Sci. 2004;1028: 390-399. [DOI] [PubMed] [Google Scholar]
- 33.Mundy G. Preclinical models of bone metastases. Semin Oncol. 2001;28: 2-8. [DOI] [PubMed] [Google Scholar]
- 34.Fulop GM, Phillips RA. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347: 479-482. [DOI] [PubMed] [Google Scholar]
- 35.Vanderkerken K, De Raeve H, Goes E, et al. Organ involvement and phenotypic adhesion profile of 5T2 and 5T33 myeloma cells in the C57BL / KaLwRij mouse. Br J Cancer. 1997;76: 451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menu E, Asosingh K, Van Riet I, Croucher P, Van Camp B, Vanderkerken K. Myeloma cells (5TMM) and their interactions with the marrow microenvironment. Blood Cells Mol Dis. 2004;33: 111-119. [DOI] [PubMed] [Google Scholar]
- 37.Neta R, Woloschak GE. Radiation, effects on immune system. In: Roitt IM, Delves PJ, eds. Encyclopedia of Immunology, Vol 2. London: Academic Press, 1992: 2050-2053. [Google Scholar]
- 38.Eary J, Stabin M. Samarium-153-EDTMP dosimetry. J Nucl Med. 1994;35: 191-192. [PubMed] [Google Scholar]
- 39.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20: 4420-4427. [DOI] [PubMed] [Google Scholar]
- 40.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127: 165-172. [DOI] [PubMed] [Google Scholar]
- 41.Avecilla ST, Hattori K, Heissig B, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10: 64-71. [DOI] [PubMed] [Google Scholar]
- 42.Kopp HG, Avecilla ST, Hooper AT, et al. Tie2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106: 505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Nagata Y, Yu G, et al. Aberrant quantity and localization of Aurora-B/AIM-1 and survivin during megakaryocyte polyploidization and the consequences of Aurora-B/AIM-1-deregulated expression. Blood. 2004;103: 3717-3726. [DOI] [PubMed] [Google Scholar]