Abstract
Interleukin-16 (IL-16) induces the chemotaxis and activation of mast cells (MCs) and other cell types. While it has been concluded that CD4 is the primary IL-16 receptor on T cells, at least one other IL-16 receptor exists. We now show that the IL-16–responsive human MC line HMC-1 lacks CD4, and that the IL-16–mediated chemotactic and Ca2+ mobilization responses of this cell can be blocked by anti-CD9 monoclonal antibodies (mAbs) but not by mAbs directed against CD4 or other tetraspanins. Anti-CD9 mAbs also inhibited the IL-16–mediated activation of nontransformed human cord blood–derived MCs and mouse bone marrow–derived MCs by 50% to 60%. The chemotactic response of HMC-1 cells to IL-16, as well as the binding of the cytokine to the cell's plasma membrane, was inhibited by CD9-specific antisense oligonucleotides. CD9 is therefore essential for the IL-16–mediated chemotaxis and activation of the HMC-1 cell line. In support of this conclusion, IL-16 bound to CD9-expressing CHO cell transfectants. The ability of wortmannin and xestopongin C to inhibit the IL-16–mediated chemotactic response of these cells suggests that the cytokine activates a phosphatidylinositol 3-kinase (PI3K)/inositol trisphosphate–dependent signaling pathway in MCs. This is the first report of a tetraspanin that plays a prominent role in a cytokine-mediated chemotactic response of human MCs.
Introduction
Interleukin-16 (IL-16; for review, see Cruikshank et al1) is a pleiotropic proinflammatory cytokine that is a potent chemotactic factor for T cells, mast cells (MCs), eosinophils, monocytes, and dendritic cells.2-7 In addition to its chemotactic activity, IL-16 induces T cells to increase their surface expression of the IL-2 receptor and MHC class II protein, as well as their intracellular levels of Ca2+ and inositol-1,4,5-trisphosphate (IP3).8,9 IL-16 induces eosinophils to generate and release substantial amounts of RANTES, eotaxin, IL-4, and leukotriene C4.10 The cytokine promotes the differentiation and granule maturation of MCs by enhancing the kit ligand (KitL)/stem cell factor–mediated expression of tryptase and chymase.4 IL-16 also inhibits the HIV-1 infection of T cells, monocytes, dendritic cells, and MCs.4,11-15
IL-16 is translated as a 631-residue precursor protein16,17 that undergoes caspase 3–dependent processing18 inside cells to yield an N-terminal fragment that translocates to the nucleus to induce G0/G1 cell-cycle arrest in the IL-16–expressing cell.19 The resulting C-terminal 121-residue fragment that is exocytosed possesses chemotactic activity. IL-16 was the first described T-cell chemoattractant. Despite the fact that the size of its biologically active C-terminal domain is comparable with that of many chemotactic factors, IL-16 lacks the conserved Cys residues found in the CC and CXC families of chemokines. T cells are responsive to many chemokines. Nevertheless, due to its poor degree of amino acid sequence identity with varied CC and CXC chemokines, IL-16 does not exert its biologic effects via a known chemokine receptor such as CCR3, which is expressed on the surfaces of T cells and MCs.
Studies have been carried out to identify the surface proteins that participate in IL-16–dependent signaling pathways in immune cells. In this regard, it has been noted that increased IL-16 expression in tissues often is correlated with the presence of large numbers of extravasated CD4+ cells.20,21 The ability of IL-16 to induce an effective chemotactic response in monocytes often is correlated with the amount of CD4 present on the cell's surface, and the chemotactic response of CD4+ T cells and monocytes to IL-16 is diminished when both populations of cells are exposed to Fab fragments of the anti-CD4 monoclonal antibody (mAb) OKT4.6 The ability of IL-16 to activate eosinophils also is diminished if these granulocytes are pre-exposed to the OKT4 mAb or to a recombinant, soluble form of CD4.10 Despite the perceived importance of CD4 in IL-16–mediated signaling of T cells, the peripheral blood mononuclear phagocytes22 and Langerhans cells23 isolated from CD4-null mice are as responsive to IL-16 as the corresponding cells present in wild-type mice. Based on these data, IL-16 must recognize at least 2 distinct receptors on hematopoietic cells. The data also imply that cells in the myeloid lineage express one or more alternate IL-16 receptors. Human MCs, monocytes, macrophages, and dendritic cells originate from a common CD34+ myeloid progenitor in the bone marrow. We recently noted that IL-16 is a potent chemotactic factor for in vitro–differentiated human MCs, and that this chemotactic response is associated with Ca2+ mobilization.4 The observation that the IL-16–mediated chemotaxis of these nontransformed cells could be inhibited only approximately 50% with the OKT4 mAb suggested that human cord blood–derived MCs (hCB-MCs) express an alternate IL-16 receptor. In support of this conclusion, the amount of CD4 mRNA in the IL-16–responsive human MC line HMC-1 is below detection by RNA blot analysis.4
Each member of the tetraspanin superfamily of surface proteins possesses 4 transmembrane domains.24 Normal in vivo–differentiated human MCs, normal in vitro–differentiated hCB-MCs (http://www.nch.go.jp/imal/English_index.htm), and the transformed MCs in patients with aggressive/malignant mastocytosis express the tetraspanins CD9, CD63, CD81, and/or CD82.25-29 CD81 is a receptor for hepatitis C virus,30 but the extracellular ligands on the surfaces of human MCs that recognize the other tetraspanins have not been identified. The observation that some tetraspanins form multimeric complexes with CD4,24,31,32 coupled with the observation that some tetraspanins participate in cell migration,24,33 led us to hypothesize that a tetraspanin is a component of the alternate IL-16 receptor on the surfaces of MCs. We now report that CD9 participates in the IL-16–mediated chemotaxis and activation of human and mouse MCs and that this tetraspanin appears to be the primary IL-16 receptor on the surface of the HMC-1 cell line.
Materials and methods
Antibodies and cytokines
Recombinant human IL-16 and the Alexa Fluor 488–conjugated cytokine were obtained from R&D Systems (Minneapolis, MN) and Molecular Probes (Eugene, OR), respectively. Recombinant human KitL and mouse IL-3 were obtained from R&D Systems. Fluorescein isothiocyanate (FITC)–conjugated mouse mAbs that recognize human CD4, CD9, CD63, and CD81 were obtained from BD Biosciences (San Jose, CA), as were purified rat anti–mouse CD4 and CD9 mAbs. FITC-conjugated mouse anti–human CD9 mAb and FITC-conjugated rabbit anti–mouse immunoglobulin G (IgG) were from Dako (Glostrup, Denmark); mouse antitryptase mAb was from Chemicon International (Temecula, CA); and mouse anti–human CD117 IgG conjugated to phycoerythrin was from Beckman Coulter (Fullerton, CA). Sense (5′-TCGATCAAATACCTGCTG-3′) and antisense (5′-CAGCAGGTATTTGATCGA-3′) oligonucleotides corresponding to residues 76 to 94 in the human CD9 transcript noted at GenBank accession number M38690 were synthesized by Invitrogen (Auckland, New Zealand). The CD9 cDNA used in our transfection experiments has been described.34
Generation of mouse bone marrow–derived MCs (mBMMCs) and hCB-MCs
mBMMCs were obtained as previously described35 by culturing bone marrow cells from the femurs and tibias of 12-week-old BALB/c mice for at least 3 weeks in enriched medium (RPMI-1640 containing 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 50 μM 2-mercaptoethanol, 10% fetal calf serum [FCS]) supplemented with 10 ng/mL IL-3. After 3 weeks, more than 98% of nonadherent cells in the cultures were MCs as assessed by toluidine blue histochemistry. hCB-MCs were generated as previously described.36
Immunocytochemistry
Cytocentrifugation preparations of mBMMCs and hCB-MCs were air dried and placed in Carnoy fixative for 15 minutes at room temperature for antitryptase staining and in acetone fixative for anti-CD4 and CD9 mAb staining. After the slides were washed with Tris-HCl–buffered saline (TBS, pH 7.6), they were incubated with 3% hydrogen peroxide in methanol for 10 minutes and then with normal rabbit serum diluted 1:10 in TBS for 10 minutes to reduce nonspecific background staining. hCB-MCs were stained with mouse anti–human tryptase mAb, whereas mBMMCs were stained with rat anti–mouse CD9 and CD4 mAbs at room temperature for 1 hour and then incubated with alkaline phosphatase–conjugated rabbit anti–mouse Ig (6 μg/mL) for 1 hour at room temperature. Slides were developed by the addition of a freshly prepared alkaline phosphatase substrate containing 0.2 mg/mL naphthol AS-MX phosphate with 0.1 mg/mL Fast Red TR and levamisole in 0.1 M Tris-HCl (pH 8.2) for 20 minutes. Cells that express tryptase, CD4, or CD9 appear pink in these immunohistochemical assays.
Double immunocytochemistry of human MCs was used to evaluate the coexpression of tryptase and CD9. For these analyses, slides were sequentially placed in Carnoy fixative, 3% H2O2 in methanol, and rabbit serum (1:10 vol/vol) for 15, 10, and 10 minutes, respectively. The fixed and blocked slides were incubated overnight at 4°C with mouse anti–human CD9 IgG, followed by rabbit anti–mouse IgG–horseradish peroxidase (6 μg/mL) for 1 hour at room temperature, and then with freshly prepared 3,3′-diaminobenzidine (DAB) substrate solution. Finally, the treated slides were incubated with alkaline phosphatase–labeled mouse anti–human tryptase IgG (2 μg/mL) for 2 hours before development with alkaline phosphatase substrate.
Chemotaxis assay
The in vitro migrations of mBMMCs, hCB-MCs, and the HMC-1 human MC line37 were evaluated using transwells (Millipore, Bedford, MA) containing fibronectin (Sigma-Aldrich, St Louis, MO)–coated polycarbonate filters with 8- or 5-μm pores.4 Varying amounts of IL-16 were placed in the lower compartment of each chemotaxis chamber. HMC-1 cells and hCB-MCs were incubated with different concentrations and combinations of anti-CD4, anti-CD9, anti-CD63, and anti-CD81 mAbs for 1 hour at 4°C. Alternately, HMC-1 cells were exposed to various amounts of CD9-specific sense or antisense oligonucleotides for 12 hours at 37°C before the start of the chemotaxis assay. Cells were incubated with different concentrations of anti-CD9 mAb in the presence of a fixed concentration of human IL-16 or mouse IL-3 for 1 hour at 4°C. Cells (200 000) were placed in the upper compartment of a 24-well plate, and each transwell was incubated for 3 hours at 37°C and 5% CO2. The filters used in the experiments with hCB-MCs were removed, gently washed with phosphate-buffered saline (PBS), fixed with 95% ethanol and 5% acetic acid, and then stained with antitryptase mAb followed by FITC-labeled rabbit anti–mouse IgG for 1 hour at room temperature. The filters used in the chemotaxis experiments were removed, gently washed with PBS, fixed with 3% glutaraldehyde, and then stained with Mayer hematoxylin for HMC-1 cells and with DiffQuick (Fronine, Sydney, Australia) for mBMMCs. Cell migration was measured by counting the number of tryptase+, hematoxylin+, or DiffQuick+ cells that firmly attached to the lower surface of the filter in 3 high-power fields. Each concentration of the IL-16 and IL-3 solutions was tested in triplicate. Cell counts were compared with unblocked control cell migration that was normalized to 100%. Results were expressed as the mean percentage of control migration plus or minus SD.
Generation of CD9-expressing CHO cells and evaluation of IL-16 binding to the transfectants
Stable transfections were performed with SuperFect (Qiagen, Hilden, Germany) according to manufacturer's instructions. CHO cells, cultured in DMEM/F12 medium supplemented with 10% FCS, were plated at a density of 5 × 105 in 60-mm dishes 24 hours prior to transfection. On the day of the transfection, 5 μg CD9-expression cDNA construct was placed in 150 μL serum-free DMEM/F12 medium. SuperFect transfection reagent (20 μL) was added. After a 10-minute incubation at room temperature to allow formation of the transfection complexes, 1 mL DMEM/F12 medium containing serum and antibiotics was added. CHO cells were cultured for 3 hours at 37°C and 5% CO2. The transfection medium was removed, the treated cells were washed 4 times with 4 mL PBS, and then fresh medium was added containing 300 ng/mL G418. The transfectants were maintained in DMEM/F12 medium supplemented with 10% FCS and 300 ng/mL G418 until colonies appeared. The expression vector (pcDNA3) lacking the CD9 cDNA was used as a negative control in these experiments.
CHO cells (5 × 104) transfected with pcDNA3 containing or lacking the CD9 cDNA were seeded in 24-well chamber slides (NUNC, Naperville, IL). After 3 days of culture, the cells were washed with PBS containing 0.01% sodium azide. Alexa Fluor 488–conjugated IL-16 (10 μg/mL) or mouse IgG (DakoCytomation) was added to each well. The treated cells were incubated 1 hour at room temperature, and then were washed twice with PBS. The slides were mounted with glycerol and sealed with nail polish. Cellular binding of IL-16 was then evaluated using a fluorescence microscope (Leica Microsystems, Heidelberg, Germany).
CD9 immunoblotting
HMC-1 cells were washed with protein-free medium and then disrupted in lysis buffer (1% Triton X-100 [Sigma-Aldrich], 2 mM phenylmethylsulfonyl fluoride, 20 μg/mL aprotinin, 10 μg/mL leupeptin [Roche Applied Science, Mannheim, Germany], 150 mM NaCl, 5 mM MgCl2, and 20 mM HEPES, pH 7.5). The insoluble material was removed by centrifugation. The proteins in the resulting lysates were separated by nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. After blocking with 5% nonfat milk in PBS with 0.1% Tween-20, the protein blots were developed with mouse anti–human CD9 mAb. This was followed by horseradish peroxidase–labeled rabbit anti–mouse IgG, and finally chemiluminescence.
Flow cytometry analysis
HMC-1 cells (1 × 106) were washed with PBS and resuspended in 500 μL ice-cold PBS supplemented with 2% FCS and 0.1% sodium azide. After centrifugation, the supernatant was removed. For blocking experiments, 50 μg/mL anti-CD9, anti-CD63, and anti-CD81 mAbs were added to the cell pellets. The cells were incubated for 1 hour at 4°C. For the antisense/sense cDNA experiments, 100 to 300 μM of a CD9-specific oligonucleotide was added to each culture and the treated cells were incubated for 12 hours at 37°C. The cells were washed 2 times with PBS. FITC-conjugated, mouse anti–human CD9 mAb was then added to the oligonucleotide-treated cells. In other experiments, Alexa Fluor 488–conjugated IL-16 (10 μg/mL) was added to either the mAb- or oligonucleotide-treated cells. The resulting cells were incubated for an additional 1 hour at 4°C in the dark. The cell pellets were then washed and resuspended in 0.5 mL PBS. The analysis was carried out by means of a flow cytometer (FACStarplus; BD Biosciences).
Measurement of intracellular Ca2+ in HMC-1 cells and CHO cell transfectants
For Ca2+ studies, HMC-1 cells were cultured on fibronectin-coated 19-mm glass coverslips in 12-well plates for 2 to 3 days in Iscove modified Dulbecco medium. CHO cells were cultured for 7 to 10 days on the coverslips in DMEM/F-12 medium (Life Technologies, Bethesda, MD). HMC-1 cells, CHO cells, and the CD9-expressing CHO cell transfectants were incubated with 2 μM Fluo-4 (Molecular Probes) in medium for 15 minutes at 37°C. The cultures were then placed into a chamber, sealed with wax, and attached to a rapid sample perfusion system as previously described.4 In each experiment, the cells were sequentially exposed to IL-16 (100 ng/mL) and ionomycin (10 μM). To confirm the role of CD9 in the IL-16–dependent changes in intracellular levels of calcium, the CD9-expressing transfectants were incubated overnight with the CD9-specific sense or antisense oligonucleotide (300 nM). The oligonucleotide-treated cells were then exposed to IL-16 (100 ng/mL). In the PI3K inhibition studies, the CD9-expressing transfectants were incubated with wortmannin (10 nM) for 15 minutes at 37°C before the cells were exposed to IL-16 and ionomycin. For the IP3 inhibition experiments, the CD9-expressing transfectants were incubated with 5 μM xestospongin C (Sigma-Aldrich) prior to IL-16 and ionomycin exposure. Xestospongin C was applied while monitoring the changes in calcium transients. Ca2+-dependent changes in fluorescence were recorded on a TCS SP2 System (Leica Microsystems) fitted with a HC × PL APO 63 ×/1.20 W CORR objective. The chamber was continuously perfused with PBS, pH 7.4, except during the IL-16 incubation period. The Ca2+-dependent fluorescence changes were calibrated at the end of each experiment by adding ionomycin (10 μM) to obtain the Fmax. The Ca2+ transients are represented as a ratio F/Fmax fluorescence of Fluo-4. All experiments were performed at room temperature.
Results
Tetraspanin expression in HMC-1 cells, hCB-MCs, and mBMMCs
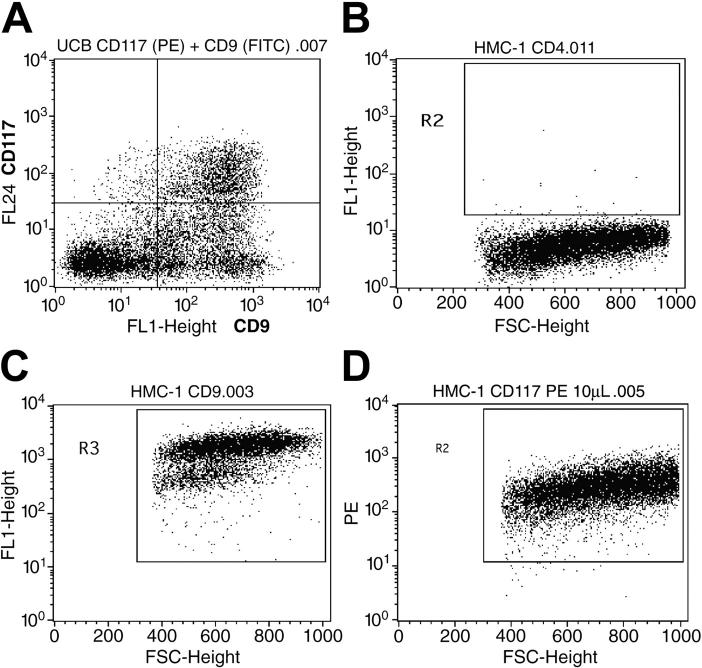
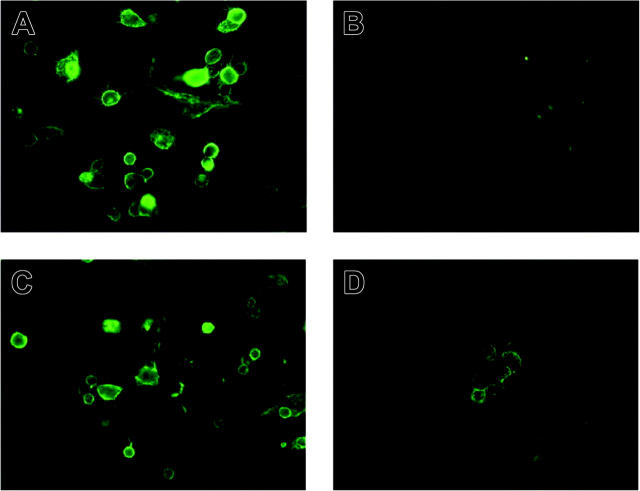
Others have reported that numerous populations of human MCs (including those generated in vitro from cord blood progenitors) express the tetraspanins CD9, CD63, and CD81. Preliminary mRNA profiling studies revealed that the HMC-1 cell line expresses the transcripts that encode the tetraspanins CD9, CD37, CD53, CD63, CD81, TM4SF7/NAG-2, and TM4SF5/L6H, but not the tetraspanins TSPAN-5/NET-4, NET-5, TM4SF4/ILTMP, or TSPAN-1/NET-1. SDS-PAGE immunoblot analysis revealed the presence of substantial amounts of the appropriately sized CD9 protein in the lysates of HMC-1 cells (Figure S1; see the Supplemental Figures link at the top of the online article, at the Blood website). Thus, the CD9 transcript is translated in HMC-1 cells. To evaluate the presence of CD9 protein on the surface of a population of nontransformed human MCs at the individual cell level, we next determined how many MCs in our cord blood cultures coexpressed CD9 and c-kit/CD117. As can be seen in Figure 1A, most of the CD117+ cells in a typical culture also expressed CD9. As assessed immunohistochemically, the level of CD4 protein on the surface of the HMC-1 cell line was below detection (Figure 1B). Nevertheless, virtually all HMC-1 cells in these cultures coexpressed CD9 (Figure 1C) and CD117 (Figure 1D). After 3 to 4 weeks of culture in IL-3–enriched medium, more than 98% of the cells in the mBMMC cultures were immature toluidine blue+ MCs (Figure S2A) that expressed CD9 (Figure S2C) but not CD4 (Figure S2B).
Figure 1.
Expression of CD4, CD9, and CD117 on the surfaces of HMC-1 cells and hCB-MCs. (A) Evaluation of the relative number of cells in a typical hCB-MC culture that coexpress CD9 and CD117 (top right quadrant). (B-D) Scattergram analysis of HMC-1 cells. The cells in the indicated windows are the ones that express significant levels of CD4 (B), CD9 (C), and CD117 (D).
IL-16 induced migration of human and mouse MCs via CD9
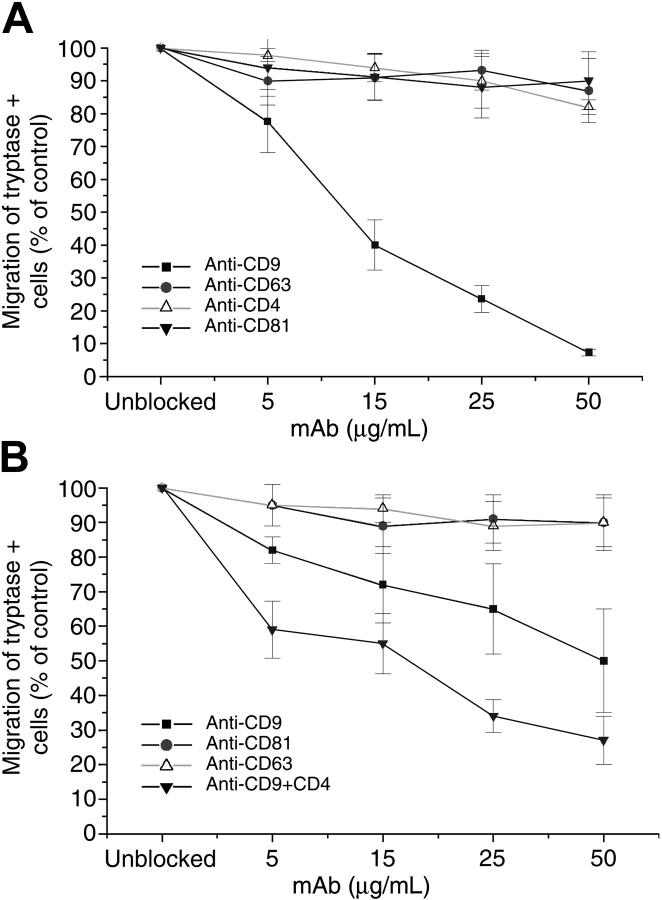
We previously noted that IL-16 is a potent chemotactic factor for the human MC line HMC-1 even though the steady-state level of the CD4 transcript in these cells is below detection by blot analysis. To determine whether the IL-16–mediated chemotactic response is dependent on a tetraspanin, we evaluated the ability of anti-CD4, anti-CD9, anti-CD63, and anti-CD81 mAbs to inhibit the IL-16–dependent migration of HMC-1 cells. At 5 μg/mL and 50 μg/mL, anti-CD9 mAb brought about a respective approximately 20% and approximately 90% inhibition in the IL-16–induced migration of HMC-1 cells (Figure 2A). No significant inhibition was detected with comparable amounts of anti-CD4, anti-CD63, or anti-CD81 mAbs.
Figure 2.
Evaluation of the potential inhibitory effects of anti-CD4 and antitetraspanin mAbs on the IL-16–mediated chemotaxis of HMC-1 cells and hCB-MCs. The IL-16–mediated migrations of HMC-1 cells (A) and hCB-MCs (B) were evaluated in the presence of varied amounts and combinations of anti-CD4, anti-CD9, anti-CD63, and anti-CD81 mAbs. Results (mean ± SD, n = 3) are expressed as a percentage of that obtained for replicate IL-16–treated cells cultured in the absence of a mAb. In each instance, 200 000 cells were added to the upper chamber in the chemotaxis assay. In the absence of a blocking mAb, IL-16 induced the migration of 223 ± 8 HMC-1 cells/3 high-power fields and 44 ± 5 hCB-MCs/3 high-power fields through the membrane in the 3-hour assay. Thus, in this assay, HMC-1 cells are more responsive to IL-16 than hCB-MCs.
While we previously noted that the anti-CD4 mAb OKT4 can inhibit the IL-16–mediated chemotaxis of hCB-MCs, the maximal inhibition was only approximately 50% of that of untreated cells.4 Because this finding suggested the presence of a second IL-16 receptor on this population of MCs, we next compared the ability of anti-CD9, anti-CD63, and anti-CD81 mAbs to inhibit the IL-16–mediated chemotaxis of the tryptase+ MCs in our cord blood cultures in the absence or presence of anti-CD4 mAb. No significant inhibition was observed with anti-CD63 or anti-CD81 mAbs (Figure 2B). In contrast, anti-CD9 mAb, by itself, inhibited the migration of these cells in a dose-dependent pattern up to approximately 50%. The IL-16–mediated chemotaxis of these human MCs was inhibited to a greater extent if they were exposed to both anti-CD4 and anti-CD9 mAbs. These data are consistent with our conclusion that hCB-MCs must contain at least 2 IL-16 receptors.
Because mBMMCs are a more homogenous population of cells than hCB-MCs, we next evaluated the ability of these nontransformed mouse CD4- MCs to respond to IL-16 in the presence and absence of anti-CD9 antibodies. mBMMCs underwent a significant chemotactic response, which was dose dependent and maximal at 50 ng/mL IL-16 (Figure S3A). The IL-16–induced migration of mBMMCs was significantly inhibited in a dose-dependent manner by anti-CD9 mAb with an approximately 60% reduction at 50 ng/mL (Figure S3B).
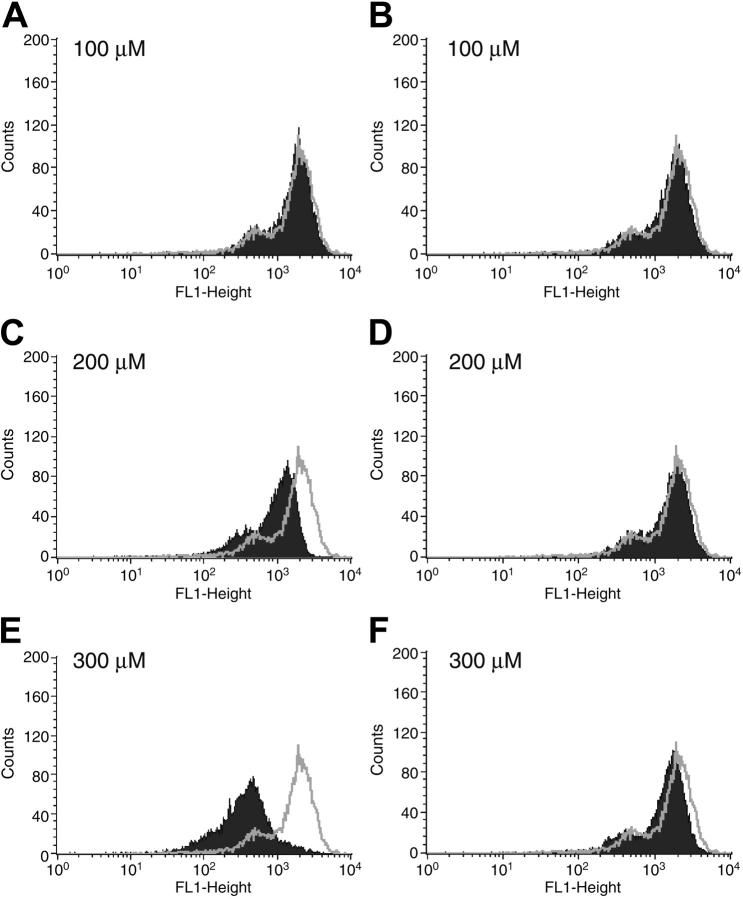
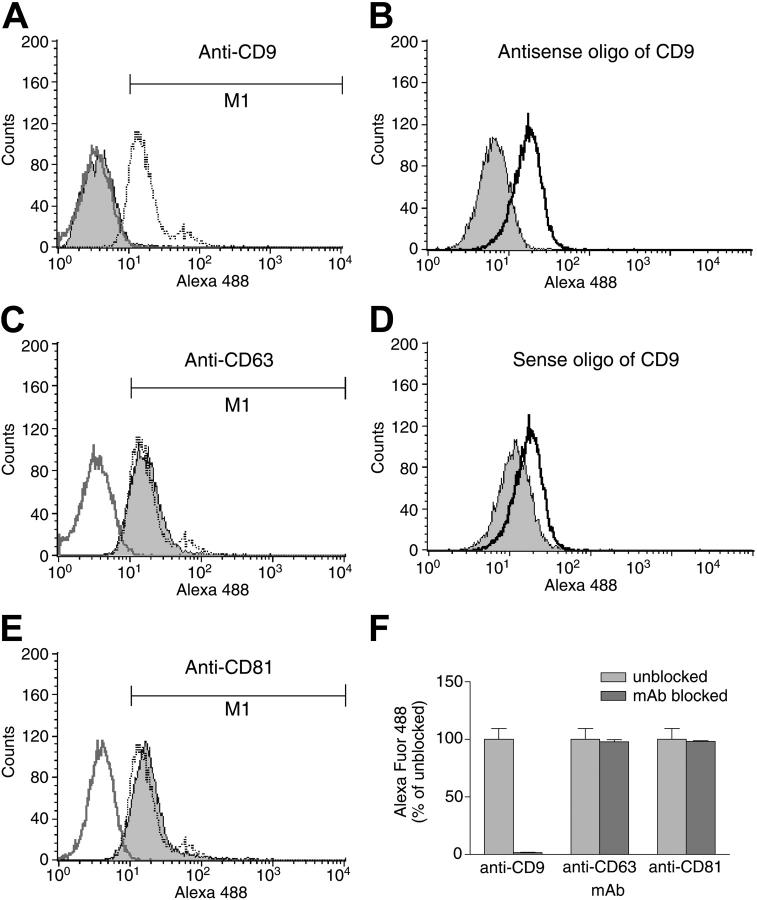
Because HMC-1 cells apparently express only one IL-16 receptor, we focused our attention on this transfectable MC line to confirm the ability of CD9 to function as an alternate IL-16 receptor. HMC-1 cells were next exposed for 12 hours to varied concentrations of sense and antisense 18-mer oligonucleotides that correspond to a region near the translation-initiation site in the CD9 transcript. As assessed by fluorescence-activated cell sorter (FACS) analysis with FITC-labeled anti–human CD9 mAb, the CD9-specific antisense oligonucleotide (Figure 3A,C,E) but not the CD9-specific sense oligonucleotide (Figure 3B,D,F) induced a dose-dependent inhibition of expression of the tetraspanin on the cell's surface. The binding of Alexa Fluor 488–labeled IL-16 to HMC-1 cells was reduced when these cells were exposed to the anti-CD9 mAb (Figure 4A,F) or the CD9-specific antisense oligonucleotide (Figure 4B), but not the anti-CD63 mAb (Figure 4C,F), the anti-CD81 mAb (Figure 4E-F), or the CD9-specific sense oligonucleotide (Figure 4D). The additional finding that the IL-16–induced migration of HMC-1 cells was inhibited substantially by the antisense oligonucleotide (Figure S4) supports the conclusion that the cytokine exerts its bioactivity primarily via CD9 in this MC line.
Figure 3.
Effects of CD9-specific sense and antisense oligonucleotides on the expression of the tetraspanin on the surfaces of HMC-1 cells. HMC-1 cells were incubated with increasing amounts of a CD9-specific antisense (A,C,E) or sense (B,D,F) oligonucleotide. Twelve hours later, FACS analysis was carried out using anti-CD9 mAb. The gray line in each panel represents the surface expression of CD9 in the untreated control cells. The data depicted in black are the FACS data obtained with the 6 populations of oligonucleotide-treated cells.
Figure 4.
IL-16 binding to HMC-1 cells. HMC-1 cells were incubated with anti-CD9 (A), anti-CD63 (C), or anti-CD81 (E) mAbs. Alternately, the cells were exposed to CD9-specific antisense (B) or sense (D) oligonucleotides. The ability of IL-16 to bind to the surfaces of the 5 populations of cells was then evaluated. The blackline in each panel represents the binding of labeled IL-16 to the untreated control cells. Shown in gray are the IL-16–binding data obtained with the mAb- or oligonucleotide-treated cells. Shown in dark gray in panels A, C, and E are the control data obtained when cells are incubated with an antitetraspanin mAb in the absence of the labeled cytokine. The bar graph in panel F summarizes the obtained data from 3 different antibody-blocking experiments. Data represent the mean ± SD of 3 experiments.
CHO cell transfectants were generated that expressed human CD9 in order to confirm that the tetraspanin can function as an alternate IL-16 receptor. In the first set of experiments, the cellular binding and surface distribution of labeled IL-16 was investigated by fluorescence microscopy. As shown in Figure 5A, the majority of the CD9-expressing transfectants bound the cytokine. In some transfectants, a more diffuse intracellular binding was noted in addition to cellular margin staining. In contrast, IL-16 did not bind to the nontransfectants (Figure 5B). To demonstrate specific binding to the transfectants, anti-CD9 mAb or matched isotype-control mAb was added followed by the labeled cytokine. No inhibition was detected when the CD9-expressing cells were incubated with an isotype control mAb (Figure 5C). However, preincubation with 50 μg/mL anti-CD9 mAb resulted in a significant reduction in binding of IL-16 to the transfectants (Figure 5D).
Figure 5.
Specific binding of IL-16 to CD9-expressing CHO cells. CHO cells transfected with pcDNA3 alone (B) or pcDNA3 containing a CD9 cDNA (A,C-D) were seeded in 24-well chamber slides. Three days later, the cultured cells were washed and preincubated with no Ab (A-B), isotype-matched mouse IgG (C), or anti-CD9 mAb (D). The binding of IL-16 to the transfectants was visualized using Alexa Fluor-488–conjugated IL-16 and was evaluated by fluorescence microscopy using a Leica Microsystems Diaplan fluorescence microscope and 25×/0.60 water immersion objective. Images were acquired using a Leica Microsystems DFC 480 digital camera and Leica Microsystems DFC Twain 6.1.1 software. Final images were processed using Adobe Photoshop 7.0.1 (Adobe Systems, San Jose, CA).
IL-16 induced Ca2+ mobilization and activation of a PI3K pathway in CD9-expressing cells
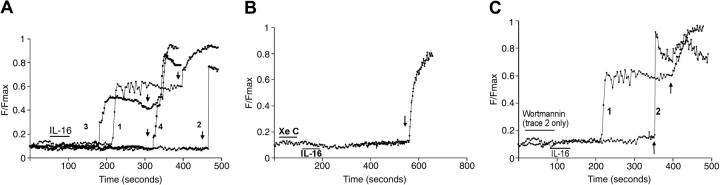
In the absence of extracellular calcium, IL-16 induced a substantial rise in the intracellular levels of Ca2+ in CD9-expressing HMC-1 cells (Figure 6A, trace 1, and Figure 6B). The maximal level of intracellular calcium was approximately half of that obtained with 10 μM ionomycin. The CD9-specific antisense oligonucleotide (Figure 6A, trace 3, and Figure 6B) inhibited the IL-16–mediated increase in intracellular levels of Ca2+ in HMC-1 cells to a much greater extent than did the CD9-specific sense oligonucleotide (Figure 6A, trace 2, and Figure 6B). In a similar manner, IL-16 induced a substantial rise in the intracellular levels of Ca2+ in CD9-expressing CHO cell transfectants (Figure 7A, trace 1). Likewise, the CD9-specific antisense oligonucleotide (Figure 7A, trace 4) but not the CD9-specific sense oligonucleotide (Figure 7A, trace 3) inhibited the IL-16–induced effect. As expected, the control cells responded to ionomycin but not IL-16 (Figure 7A, trace 2). Pretreatment of the CD9-expressing transfectants with 5 μM xestospongin C (Figure 7B) or 10 nM wortmannin (Figure 7C, trace 2) blocked the IL-16–induced rise in intracellular levels of Ca2+ in the transfectants. The inhibitory effects of xestospongin C and wortmannin were not due to a nonspecific, drug-induced toxicity because in both instances the treated cells subsequently responded to ionomycin.
Figure 6.
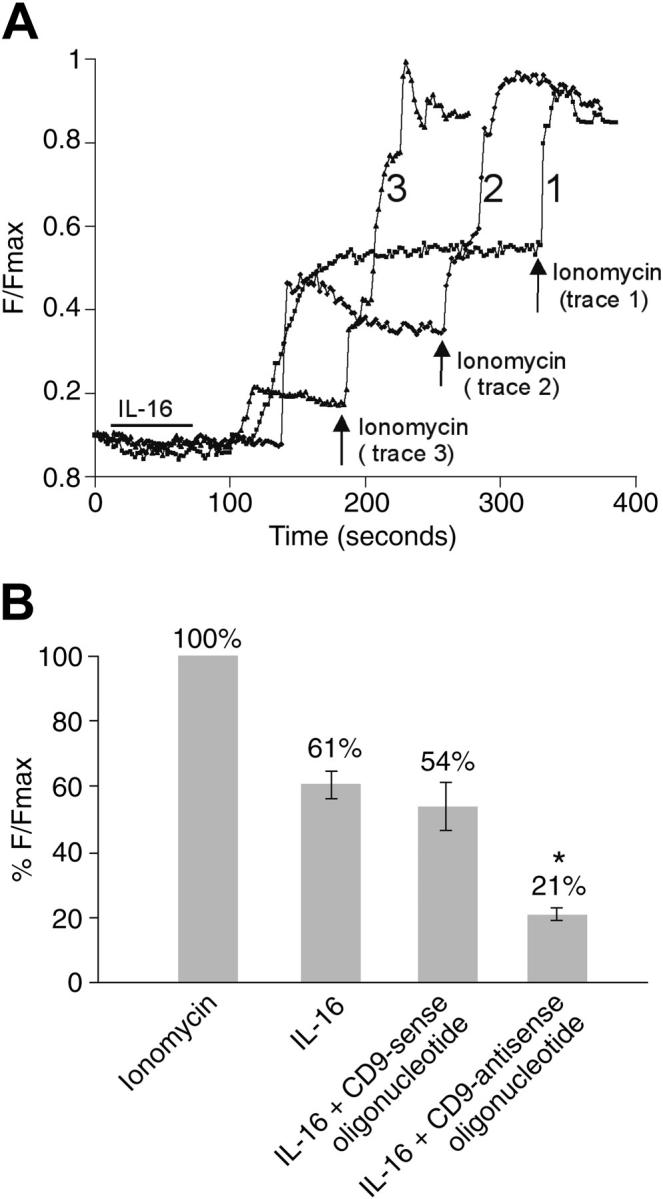
IL-16 mediated changes in the intracellular levels of calcium in HMC-1 cells. (A) Untreated HMC-1 cells (trace 1), HMC-1 cells that had been previously exposed to the CD9-specific sense oligonucleotide (trace 2), and HMC-1 cells that had been previously exposed to the CD9-specific antisense oligonucleotide (trace 3) were Fluo-4 loaded and exposed to IL-16 for 1 minute (indicated by the bar), and the time-dependent rise in calcium levels [Ca2+]i was measured in each instance. At the indicated time points (arrows), the treated cells were exposed to ionomycin to achieve a maximal calcium response. (B) Histogram representing differences in %F/Fmax fluorescence intensity changes of Fluo-4 in untreated and oligonucleotide-treated HMC-1 cells in the absence of extracellular calcium (mean ± SD, n = 3; *P < .005 [Student t test]). A scale of 0% to 100% was used, where 0% represents no change in %F/Fmax and 100% represents change in %F/Fmax due to the positive control obtained with ionomycin.
Figure 7.
[Ca2+]i transients in CD9-expressing CHO cells. (A) Control CHO cells (trace 2), CD9-expressing CHO cells (trace 1), and CD9-expressing CHO cells that were given a CD9-specific sense (trace 3) or antisense oligonucleotide (trace 4) were exposed to IL-16 (bar) followed by ionomycin (arrows), and the intracellular levels of calcium were determined. Data from Fluo-4–loaded CHO cells typical of 2 to 10 cells from at least 3 separate cultures are shown. (B) CD9-expressing CHO cells were sequentially exposed to xestospongin C (Xe C) (first bar), IL-16 (second bar), and then ionomycin (arrow). The same population of CD9-expressing CHO cells was used in the experiments that led to the data presented in panels A and B. The experiments were carried out on the same day. Thus, the noninhibitor control for the xestospongin C data presented in panel B is trace 1 of panel A. (C) CD9-expressing CHO cells were sequentially exposed to wortmannin (first bar), IL-16 (second bar), and then to ionomycin (arrows, trace 2). The positive control cells for this experiment (trace 1) were CD9-expressing cells that did not encounter wortmannin before they were exposed to IL-16 and then ionomycin.
Discussion
The chemotactic responses of MCs and their progenitors to IL-16 likely reflect a complex biologic process involving numerous discrete cellular activation events, such as adhesion, cytoskeletal reorganization, transendothelial migration, and increased mediator expression. Many chemokines recognize more than one receptor. The ability of IL-16 to induce chemotaxis of varied immune cells isolated from CD4-null mice22,23 documents that CD4 is not the only IL-16 receptor on the surface of hematopoietic cells. While the IL-16–mediated chemotaxis of hCB-MCs can be partially inhibited by anti-CD4 mAbs,4 the inability to block the IL-16 response more than 50% implies that these cells also express an alternate IL-16 receptor. The finding that the IL-16–responsive HMC-1 cell line does not express CD4 (Figure 1B) was fortuitous in that it allowed us the opportunity to identify the primary IL-16 receptor on the surface of this MC line. It is well known that tetraspanins form multimeric complexes with several endogenous surface molecules. The physical association of the tetraspanins CD9, CD81, and CD82 with CD4 on the surface of the T cell24,31,32 was the impetus for our investigation of the possible involvement of one or more of these tetraspanins in the IL-16–mediated migration of mBMMCs, hCB-MCs, and the HMC-1 cell line.
Pregnancy-specific glycoprotein 17 (Psg17)/carcino-embryonic antigen 2 has been reported to be a ligand for mouse and human CD9 based on in vitro studies.38 Because Psg17 is preferentially expressed in the mouse's placenta,39 it was not apparent how this female-restricted placenta protein could regulate the CD9+ MCs that reside in the skin, lung, and other connective tissue sites of a male mouse. The nucleotide sequence of the human genome has been deduced. BLAST searches of GenBank's nucleotide and protein databases revealed that “biliary glycoprotein 1” is the human protein that is most similar to mouse Psg17. However, the fact that its amino acid sequence is only 31% identical to that of Psg17 indicates that no human ortholog of mouse Psg17 exists. Thus, the primary physiologic receptor on the surfaces of human MCs that recognizes CD9 remained to be identified.
We now show that CD9 (but not the related tetraspanins CD63 and CD81) is essential for the IL-16–mediated chemotaxis and activation of the HMC cell line (Figure 2A). CD9 also appears to contribute to IL-16–dependent signaling in hCB-MCs (Figure 2B) and mBMMCs (Figure S3). Depending on their tissue microenvironments, MCs differ substantially as to which cytokine, lipid, and proteases they express. As assessed immunohistochemically, BALB/c mBMMCs lack CD4 (Figure S2). We also discovered that not all in vivo–differentiated human MCs express CD4 (J.C.Q. and S.A.K., unpublished observation, January 16, 2002). In contrast, all human and mouse MCs that have been evaluated to date express CD9. For example, HMC-1 cells, hCB-MCs, and mBMMCs express CD9 (Figures 1, 2, S1, and S2). IL-16 binds to these cells (Figure 4), as well as to CD9-expressing CHO cells (Figure 5). Anti-CD9 mAbs block the IL-16–mediated chemotaxis of HMC-1 cells and hCB-MCs (Figure 2), as well as mBMMCs (Figure S3). Exposure of HMC-1 cells (Figures 3, 4, 6, and S4) and CD9-expressing CHO cells (Figure 7) to a CD9-specific antisense oligonucleotide also resulted in diminished IL-16 responses.
The CHO cell transfection data (Figures 5 and 7) imply that IL-16 interacts directly with CD9. However, the fact that many cell types express CD9 raises the possibility that the tetraspanin is posttranslationally modified in MCs in a manner that creates a receptor with higher affinity for IL-16 as occurs with many posttranslationally modified CXC and CC chemokine receptors. Alternately, the IL-16 receptor on the surface of the MC could be a multimeric complex consisting of CD9 and an unidentified protein. However, if that is the case, the second protein also must be constitutively expressed on the surface of the CHO cell. Of more importance, the fact that IL-16 does not activate CHO cells unless these cells are induced to express CD9 (Figure 7) indicates that the latter tetraspanin is an essential component of the receptor complex no matter how many peptide chains it possesses. Whether CD9 functions as an alternate IL-16 receptor in other IL-16–response immune cells (eg, T cells, eosinophils, dendritic cells, and mononuclear phagocytes) remains to be determined.
The known proteins/peptides that are chemotactic for mouse and/or human MCs and their progenitors include IL-3, IL-8, IL-16, RANTES, KitL, transforming growth factor-β, and the anaphylatoxins C3a and C5a. Because these factors interact with distinct receptors on the surface of the MC, numerous signaling pathways inside an MC ultimately can induce a chemotactic response. We therefore have begun to address how IL-16 signals in the HMC-1 cell line (Figure 6) and CD9-expressing CHO cell transfectants (Figure 7) downstream of its plasma membrane receptor. Exposure of T cells to IL-16 results in phosphorylation of p56lck,40 a rise in the intracellular levels of IP3 and Ca2+,8 and translocation of protein kinase C from the cytosol to the plasma membrane.41 To investigate the specificity of the interaction of IL-16 with CD9, we evaluated the ability of IL-16 to alter Ca2+ mobilization in CD9-expressing HMC-1 cells and CHO cell transfectants. We also studied the effects of the PI3K-inhibitor wortmannin and the IP3-inhibitor xestospongin C. Activation of receptor-associated PI3K results in the generation of phosphatidylinositol 3,4,5-trisphosphate. This lipid second messenger binds to several pleckstrin homology domain–containing intracellular proteins (eg, phospholipase Cγ [PLCγ]),42,43 which results in their translocation from the cytosol to the plasma membrane. The coactivation of PI3K and PLCγ results in the generation of increased amounts of IP3. Binding of IP3 to its receptors in the endoplasmic reticulum results in the release of intracellular stores of Ca2+ into the cytoplasm.44 This mechanism of activation is well known for receptors with intrinsic tyrosine kinase activity45 and correlates with release of IP3 and calcium.46 While xestospongin C inhibits activation of IP3 receptors, wortmannin inhibits the catalytic site of PI3K, resulting in inhibition of IP3 generation downstream of PI3K activation and hence diminished calcium transients as seen in several cell types.47-50 IL-16 induced a rise in the cytosolic levels of calcium in HMC-1 cells and CD9-expressing CHO cells, even if the treated cells were cultured in the absence of extracellular calcium. Metabolic inhibitors were used in the study to affect MCs and CD9-expressing CHO cells. The observation that both xestospongin C (Figure 7B) and wortmannin (Figure 7C) inhibit the cytosolic accumulation of calcium in the latter transfectants suggests that IL-16 induces its effects on MCs by activating a PI3K/IP3 signaling pathway downstream of CD9.
Dr Kinet's group recently showed that CD61 and CD83 negatively regulate the FcεRI-dependent activation of rodent MCs.51,52 CD9 is required in our studies for an optimal chemotactic response of human and mouse MCs to IL-16. The accumulated data suggest that at least 3 tetraspanins participate in key signaling pathways that control the accumulation, development, and function of MCs in tissues.
Supplementary Material
Acknowledgments
We thank Dr Claude Boucheix (Institute André-Lwoff, Villejuif Cedex, France) for the human CD9 cDNA used in this study.
Supported by grants from the National Health and Medical Research Council of Australia and by grants HL036110 and AI054950 from the National Institutes of Health of the United States.
Prepublished online as Blood First Edition Paper, September 6, 2005; DOI 10.1182/blood-2005-03-1312.
J.C.Q. and J.W. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Cruikshank WW, Kornfeld H, Center DM. Interleukin 16. J Leukoc Biol. 2000;67: 757-766. [DOI] [PubMed] [Google Scholar]
- 2.Center DM, Cruikshank W. Modulation of lymphocyte migration by human lymphokines: I, identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol. 1982;128: 2563-2568. [PubMed] [Google Scholar]
- 3.Cruikshank W, Center DM. Modulation of lymphocyte migration by human lymphokines: II, purification of a lymphotactic factor (LCF). J Immunol. 1982;128: 2569-2574. [PubMed] [Google Scholar]
- 4.Qi JC, Stevens RL, Wadley R, et al. IL-16 regulation of human mast cells/basophils and their susceptibility to HIV-1. J Immunol. 2002;168: 4127-4134. [DOI] [PubMed] [Google Scholar]
- 5.Rand TH, Cruikshank WW, Center DM, Weller PF. CD4-mediated stimulation of human eosinophils: lymphocyte chemoattractant factor and other CD4-binding ligands elicit eosinophil migration. J Exp Med. 1991;173: 1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruikshank WW, Berman JS, Theodore AC, Bernardo J, Center DM. Lymphokine activation of T4+ T lymphocytes and monocytes. J Immunol. 1987;138: 3817-3823. [PubMed] [Google Scholar]
- 7.Kaser A, Dunzendorfer S, Offner FA, et al. A role for IL-16 in the cross-talk between dendritic cells and T cells. J Immunol. 1999;163: 3232-3238. [PubMed] [Google Scholar]
- 8.Cruikshank WW, Greenstein JL, Theodore AC, Center DM. Lymphocyte chemoattractant factor induces CD4-dependent intra-cytoplasmic signaling in lymphocytes. J Immunol. 1991;146: 2928-2934. [PubMed] [Google Scholar]
- 9.Parada NA, Center DM, Kornfeld H, et al. Synergistic activation of CD4+ T cells by IL-16 and IL-2. J Immunol. 1998;160: 2115-2120. [PubMed] [Google Scholar]
- 10.Bandeira-Melo C, Sugiyama K, Woods LJ, et al. IL-16 promotes leukotriene C4 and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J Immunol. 2002;168: 4756-4763. [DOI] [PubMed] [Google Scholar]
- 11.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins AL. Human CD4+ cells transfected with an IL-16 cDNA are resistant to HIV-1 infection: inhibition of mRNA expression. Nat Med. 1997;3: 659-664. [DOI] [PubMed] [Google Scholar]
- 12.Maciaszek JW, Parada NA, Cruikshank WW, et al. IL-16 represses HIV-1 promoter activity. J Immunol. 1997;158: 5-8. [PubMed] [Google Scholar]
- 13.Truong MJ, Darcissac EC, Hermann E, et al. Interleukin-16 inhibits human immunodeficiency virus type 1 entry and replication in macrophages and in dendritic cells. J Virol. 1999;73: 7008-7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amiel C, Darcissac E, Truong MJ, et al. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis. 1999;179: 83-91. [DOI] [PubMed] [Google Scholar]
- 15.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16 [letter]. Nature. 1995;378: 563. [DOI] [PubMed] [Google Scholar]
- 16.Cruikshank WW, Center DM, Nisar N, et al. Molecular and functional analysis of a lymphocyte chemoattractant factor: association of biologic function with CD4 expression. Proc Natl Acad Sci U S A. 1994;91: 5109-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baier M, Bannert N, Werner A, Lang K, Kurth R. Molecular cloning, sequence, expression, and processing of the interleukin 16 precursor. Proc Natl Acad Sci U S A. 1997;94: 5273-5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Center DM, Wu DM, et al. Processing and activation of pro-interleukin-16 by caspase-3. J Biol Chem. 1998;273: 1144-1149. [DOI] [PubMed] [Google Scholar]
- 19.Wilson KC, Cruikshank WW, Center DM, Zhang Y. Prointerleukin-16 contains a functional CcN motif that regulates nuclear localization. Biochemistry. 2002;41: 14306-14312. [DOI] [PubMed] [Google Scholar]
- 20.Franz JK, Kolb SA, Hummel KM, et al. Interleukin 16, produced by synovial fibroblasts, mediates chemoattraction for CD4+ T lymphocytes in rheumatoid arthritis. Eur J Immunol. 1998;28: 2661-2671. [DOI] [PubMed] [Google Scholar]
- 21.Laberge S, Ghaffar O, Boguniewicz M, et al. Association of increased CD4+ T-cell infiltration with increased IL-16 gene expression in atopic dermatitis. J Allergy Clin Immunol. 1998;102: 645-650. [DOI] [PubMed] [Google Scholar]
- 22.Mathy NL, Bannert N, Norley SG, Kurth R. CD4 is not required for the functional activity of IL-16. J Immunol. 2000;164: 4429-4432. [DOI] [PubMed] [Google Scholar]
- 23.Stoitzner P, Ratzinger G, Koch F, et al. Interleukin 16 supports the migration of Langerhans cells, partly in a CD4-independent way. J Invest Dermatol. 2001;116: 641-649. [DOI] [PubMed] [Google Scholar]
- 24.Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58: 1189-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valent P, Ashman LK, Hinterberger W, et al. Mast cell typing: demonstration of a distinct hematopoietic cell type and evidence for immunophenotypic relationship to mononuclear phagocytes. Blood. 1989;73: 1778-1785. [PubMed] [Google Scholar]
- 26.Guo CB, Kagey-Sobotka A, Lichtenstein LM, Bochner BS. Immunophenotyping and functional analysis of purified human uterine mast cells. Blood. 1992;79: 708-712. [PubMed] [Google Scholar]
- 27.Nilsson G, Forsberg K, Bodger MP, et al. Phenotypic characterization of stem cell factor-dependent human foetal liver-derived mast cells. Immunology. 1993;79: 325-330. [PMC free article] [PubMed] [Google Scholar]
- 28.Baghestanian M, Bankl H, Sillaber C, et al. A case of malignant mastocytosis with circulating mast cell precursors: biologic and phenotypic characterization of the malignant clone. Leukemia. 1996;10: 159-166. [PubMed] [Google Scholar]
- 29.Saito H, Nakajima T, Matsumoto K. Human mast cell transcriptome project. Int Arch Allergy Immunol. 2001;125: 1-8. [DOI] [PubMed] [Google Scholar]
- 30.Pileri P, Uematsu Y, Campagnoli S, et al. Binding of hepatitis C virus to CD81. Science. 1998;282: 938-941. [DOI] [PubMed] [Google Scholar]
- 31.Imai T, Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol. 1993;151: 6470-6481. [PubMed] [Google Scholar]
- 32.Toyo-Oka K, Yashiro-Ohtani Y, Park CS, et al. Association of a tetraspanin CD9 with CD5 on the T cell surface: role of particular transmembrane domains in the association. Int Immunol. 1999;11: 2043-2052. [DOI] [PubMed] [Google Scholar]
- 33.Miyake M, Koyama M, Seno M, Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med. 1991;174: 1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boucheix C, Benoit P, Frachet P, et al. Molecular cloning of the CD9 antigen: a new family of cell surface proteins. J Biol Chem. 1991;266: 117-122. [PubMed] [Google Scholar]
- 35.Razin E, Ihle JN, Seldin D, et al. Interleukin 3: a differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984;132: 1479-1486. [PubMed] [Google Scholar]
- 36.Li L, Macpherson JJ, Adelstein S, et al. Conditioned medium from a cell strain derived from a patient with mastocytosis induces preferential development of cells that possess high-affinity IgE receptors and the granule protease phenotype of mature cutaneous mast cells. J Biol Chem. 1995;270: 2258-2263. [DOI] [PubMed] [Google Scholar]
- 37.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12: 345-355. [DOI] [PubMed] [Google Scholar]
- 38.Waterhouse R, Ha C, Dveksler GS. Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J Exp Med. 2002;195: 277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudert F, Saunders AM, Rebstock S, Thompson JA, Zimmermann W. Characterization of murine carcinoembryonic antigen gene family members. Mamm Genome. 1992;3: 262-273. [DOI] [PubMed] [Google Scholar]
- 40.Ryan TC, Cruikshank WW, Kornfeld H, Collins TL, Center DM. The CD4-associated tyrosine kinase p56lck is required for lymphocyte chemoattractant factor-induced T lymphocyte migration. J Biol Chem. 1995;270: 17081-17086. [DOI] [PubMed] [Google Scholar]
- 41.Parada NA, Cruikshank WW, Danis HL, Ryan TC, Center DM. IL-16- and other CD4 ligand-induced migration is dependent upon protein kinase C. Cell Immunol. 1996;168: 100-106. [DOI] [PubMed] [Google Scholar]
- 42.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275: 665-668. [DOI] [PubMed] [Google Scholar]
- 43.Stokoe D, Stephens LR, Copeland T, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997; 277: 567-570. [DOI] [PubMed] [Google Scholar]
- 44.Rameh LE, Rhee SG, Spokes K, et al. Phosphoinositide 3-kinase regulates phospholipase C-mediated calcium signaling. J Biol Chem. 1998; 273: 23750-23757. [DOI] [PubMed] [Google Scholar]
- 45.Rhee SG, Choi KD. Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 1992;267: 12393-12396. [PubMed] [Google Scholar]
- 46.Brenner B, Koppenhoefer U, Lepple-Wienhues A, et al. The CD40 ligand directly activates T-lymphocytes via tyrosine phosphorylation dependent PKC activation. Biochem Biophys Res Commun. 1997;239: 11-17. [DOI] [PubMed] [Google Scholar]
- 47.Bonser RW, Thompson NT, Randall RW, et al. Demethoxyviridin and wortmannin block phospholipase C and D activation in the human neutrophil. Br J Pharmacol. 1991;103: 1237-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakanishi S, Catt KJ, Balla T. Inhibition of agonist-stimulated inositol 1,4,5-trisphosphate production and calcium signaling by the myosin light chain kinase inhibitor, wortmannin. J Biol Chem. 1994;269: 6528-6535. [PubMed] [Google Scholar]
- 49.Barker SA, Caldwell KK, Hall A, et al. Wortmannin blocks lipid and protein kinase activities associated with PI 3-kinase and inhibits a subset of responses induced by FcεR1 cross-linking. Mol Biol Cell. 1995;6: 1145-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vossebeld PJ, Homburg CH, Schweizer RC, et al. Tyrosine phosphorylation-dependent activation of phosphatidylinositide 3-kinase occurs upstream of Ca2+-signalling induced by Fc receptor cross-linking in human neutrophils. Biochem J. 1997;323: 87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fleming TJ, Donnadieu E, Song CH, et al. Negative regulation of FcεRI-mediated degranulation by CD81. J Exp Med. 1997;186: 1307-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraft S, Fleming T, Billingsley JM, et al. Anti-CD63 antibodies suppress IgE-dependent allergic reactions in vitro and in vivo. J Exp Med. 2005;201: 385-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.