Abstract
Purine nucleoside phosphorylase (PNP) deficiency in humans results in T lymphocytopenia. Forodesine, a potent inhibitor of PNP, was designed based on the transition-state structure stabilized by the enzyme. Previous studies established that forodesine in the presence of deoxyguanosine (dGuo) inhibits the proliferation of T lymphocytes. A phase 1 clinical trial of forodesine in T-cell malignancies demonstrated significant antileukemic activity with an increase in intracellular dGuo triphosphate (dGTP). High accumulation of dGTP in T cells may be dependent on the levels of deoxynucleoside kinases. Because B-cell chronic lymphocytic leukemia (B-CLL) cells have high activity of deoxycytidine kinase (dCK), we hypothesized that these lymphocytes would respond to forodesine. This postulate was tested in primary lymphocytes during in vitro investigations. Lymphocytes from 12 patients with CLL were incubated with forodesine and dGuo. These CLL cells showed a wide variation in the accumulation of intracellular dGTP without any effect on other deoxynucleotides. This was associated with DNA damage-induced p53 stabilization, phosphorylation of p53 at Ser15, and activation of p21. The dGTP accumulation was related to induction of apoptosis measured by caspase activation, changes in mitochondrial membrane potential, and PARP cleavage. Based on these data, a phase 2 clinical trial of forodesine has been initiated for CLL patients.
Introduction
The major role of mammalian purine nucleoside phosphorylase (PNP) is to catalyze the cleavage of inosine, deoxyinosine, guanosine, and deoxyguanosine (dGuo) to their corresponding base and sugar 1-phosphate by phosphorolysis.1 PNP deficiency in humans produces a relatively selective depletion of T cells.2 PNP-deficient children exhibit profound impairment in the T-cell component of their immune systems, but have normal B-cell function.3 This rare condition provided a model for the development of specific inhibitors of PNP, either to enable selective suppression of T-cell function that has been useful in the treatment of T-cell–mediated diseases or as potential T-cell–selective chemotherapeutic agents.4
The aza-C nucleosides, immucillin-H and immucillin-G, are transition-state analog inhibitors of PNP.5 Immucillin analogs modified at the 2′-, 3′-, or 5′-positions of the aza sugar moiety or at the 6-, 7-, or 8-positions of the deazapurine, as well as methylene-bridged analogs, have been synthesized and tested for their inhibition of human PNP.6 Forodesine (BCX-1777/immucillin-H) is a potent inhibitor of PNP (Figure 1). Forodesine in the presence of dGuo inhibited the proliferation of CEM-SS (T-acute lymphoblastic leukemia) cells with an IC50 of 0.015 μM. This inhibition by forodesine and dGuo was accompanied by a 154-fold and 8-fold elevation of endogenous dGuo triphosphate (dGTP) and deoxyadenosine triphosphate (dATP) pools, respectively.7 Forodesine, in the presence of dGuo (3-10 μM), inhibited human lymphocyte proliferation activated by various agents such as interleukin-2 (IL-2), mixed lymphocyte reaction, and phytohemagglutinin with IC50 values that ranged between 0.1 and 0.38 μM. Forodesine is a 10- to 100-fold more potent inhibitor of human lymphocyte proliferation than other known PNP inhibitors such as PD141955 and BCX-34.8 Previous studies demonstrated that the cytotoxic activity of forodesine in the presence of dGuo was selective to T lymphocytes.9 High kinase and low nucleotidase levels make these cells more sensitive to inhibition by forodesine and dGuo.
Figure 1.
Structure of forodesine.
Based on these observations, we conducted a phase 1 clinical trial of forodesine in patients with advanced T-cell malignancies.10 Significant antileukemic activity was correlated with an increase in plasma forodesine (median 5 μM) and dGuo (median 14 μM), and an accumulation of intracellular dGTP. As reported in cell lines, it was postulated that high accumulation of dGTP in T cells may be dependent on activity of deoxynucleoside kinases such as deoxycytidine kinase (dCK), which is a primary enzyme for the conversion of dGuo to dGMP (dGuo monophosphate), which is then converted to dGTP.
Because B-chronic lymphocytic leukemia (B-CLL) has high activity of dCK,11 we hypothesized that this disease would be sensitive to forodesine and dGuo treatment. To test our hypothesis, we conducted the present investigation using primary leukemic lymphocytes obtained from patients with CLL. We demonstrate the cytotoxic effect of forodesine with dGuo in CLL cells from 12 patients using pharmacologically achievable levels of forodesine and dGuo at different time periods. Accumulation of dGTP and effect on other deoxynucleoside triphosphates (dNTPs) were analyzed and related to induction of cell death. Molecular mechanisms such as DNA damage-induced p53 stabilization, phosphorylation of p53 at Ser15, and activation in the expression of p21 proteins were determined. Based on our current data, we have initiated a phase 2 study of forodesine in patients with fludarabine-refractory CLL.
Patients, materials, and methods
Drugs and chemicals
Forodesine for laboratory use was provided by BioCryst Pharmaceuticals (Birmingham, AL) and dGuo was purchased from Sigma (St Louis, MO). For quantitation of deoxynucleotides, dNTPs were obtained from Amersham Biosciences (Piscataway, NJ) and were used as standards. [3H]dATP and [3H]dTTP were purchased from Perkin Elmer Life Sciences (Boston, MA) and MP Biomedicals (Irvine, CA), respectively.
Patients
The present in vitro studies were carried out in leukemic lymphocytes obtained from patients with CLL (n = 12). The percentage of leukemic lymphocytes (B-cell population) in 7 patients was measured by flow cytometry method using CD19 FITC antibody obtained from BD Biosciences (San Jose, CA). Blood samples obtained from these 12 patients were used for different pharmacologic, biochemical, and molecular end points. All patients signed a written informed consent to participate in this laboratory protocol, which was approved by the institutional review board of the University of Texas MD Anderson Cancer Center.
Clinical laboratory end points
Determination of IgVH gene mutation status and ZAP-70 analysis for the patients in our study were conducted as described previously12 and provided by the Chronic Lymphocytic Leukemia Research Consortium (University of California, San Diego and the University of Texas, M. D. Anderson Cancer Center).
Fluorescent in situ hybridization (FISH) analysis data were provided by the clinical cytogenetics laboratory, Department of Hematopathology at M. D. Anderson Cancer Center. FISH technique was used to detect the chromosome 17p deletion for p53 gene in CLL cells. The detailed methodology for the assay has been described before.13
Chromosomal cytogenetics was determined using CLL lymphocytes at M. D. Anderson Cancer Center.
Isolation of lymphocytes
Whole blood was collected in heparinized tubes and diluted 1:3 with cold PBS (0.135 M NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8 mM Na2HPO4 [pH 7.4]) and layered onto Ficoll-Hypaque (specific gravity, 1.086; Life Technologies, Grand Island, NY). The blood was then centrifuged at 433g for 20 minutes, and mononuclear cells were removed from the interphase.14 Cells were washed twice with cold PBS and resuspended in 10 mL RPMI 1640, supplemented with 10% fetal bovine serum. A Coulter channelyzer (Coulter Electronics, Hialeah, FL) was used to determine cell number and the mean cell volume. The lymphocytes were then resuspended at a concentration of 1 × 107 cells/mL and fresh cells were used for all experiments.
Source of normal lymphocytes
Normal lymphocytes were isolated from buffy coat of healthy donor obtained from transfusion services at M. D. Anderson Cancer Center. B-cell and T-cell lymphocytes were isolated using EasySep B-cell enrichment cocktail and EasySep human T-cell negative selection cocktail obtained from Stemcell Technologies (Vancouver, BC). EasySep is an immunomagnetic cell-selection procedure that uses specific antibodies and tiny fluorescence-activated cell sorting (FACS)–compatible magnetic nanoparticles in a column-free magnetic system. Cells are targeted for selection or depletion using monoclonal antibodies directed against specific cell-surface antigens. These labeled cells are then cross-linked to EasySep magnetic nanoparticles provided by the manufacturer, in a standard FACS tube. The tube is then placed directly in the specially designed EasySep magnet. This handheld magnet is gently inverted to remove the cells that are not bound to the magnetic nanoparticles. The percentage of B- and T-cell populations in the isolated fractions was measured by flow cytometry using CD19 FITC antibody and the CD3 FITC antibody from BD Biosciences, respectively.
Measurement of dNTP pools
The primary CLL lymphocytes were incubated on the same day of their isolation with or without 2 μM forodesine and 10 μM dGuo for 0, 4, 8, 16, and 24 hours. These concentrations were selected based on plasma pharmacology data during phase 1 study of forodesine.10 Cultures were maintained and aliquots (1 × 107cells/mL) were removed at the end of incubation times. The nucleotides in the leukemia cells were extracted by 60% methanol as described,15 and the dNTPs were quantitated by DNA polymerase assay as modified by Sherman and Fyfe16 in these cell extracts.
Quantitation of caspases 8, 9, and 3 activity
Cells that have been induced to undergo apoptosis are collected by centrifugation and the pellet is lysed by the caspase lysis buffer. The cell lysate is then tested for protease activity by the addition of a caspase-specific peptide that is conjugated to the fluorescent reporter molecule 7-amino-4-trifluoromethyl coumarin (AFC). These peptide substrates were DEVD-AFC, IETD-AFC, and LEHD-AFC for caspases 3, 8, and 9, respectively. The cleavage of the peptide by the caspase releases the fluorochrome that, when excited by light at 400-nm wavelength, emits fluorescence at 505 nm. The level of caspase enzymatic activity in the cell lysate is directly proportional to the fluorescence signal detected with a fluorescent microplate reader according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Determination of mitochondrial membrane potential
Mitochondrial membrane potential was determined in some of these samples by flow cytometry using a lipophilic cationic Δψm-dependent fluorescent dye JC-1 (5,5′6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazol carbocyanineiodide) from Molecular Probes (Eugene, OR), which was analyzed in fluorescence detection channel 2 (FL-2). Lymphocytes with intact mitochondria excite an intense red fluorescence due to the formation of the dye aggregates, whereas the monomer dye fluoresces green in cells with a disrupted mitochondrial membrane.17 All aliquots were incubated at room temperature for 20 minutes in the dark with 2 μL of the dye diluted in PBS at a concentration of 2.5 μg/mL and were analyzed immediately with a FACSCALIBUR cytometer (Becton-Dickinson, San Jose, CA). Data, from at least 10 000 events per sample, were recorded and processed using CellQuest software (Becton-Dickinson).
Apoptotic analysis using dual annexin V–FITC/propidium iodide
The analysis of annexin V binding was carried out with a Detection Kit I (PharMingen, San Diego, CA) according to the manufacturer's instructions. Briefly, cells were washed with PBS and resuspended in 200 μL of 1 × annexin binding buffer obtained from BD Biosciences, at a concentration of 1 × 106 cells/mL. Annexin V–FITC (5 μL) was added and the cells were incubated in the dark for 15 minutes at room temperature. The labeled cells were then added to 10 μL propidium iodide (50 μg/mL) and analyzed immediately with a FACSCALIBUR cytometer (Becton-Dickinson). Data, from at least 10 000 events per sample, were recorded and processed using Cell Quest software (Becton-Dickinson). The results were expressed after subtracting endogenous level of annexin positivity in untreated timed-control samples.
Immunoblot analysis
Cells were lysed on ice for 20 minutes in lysis buffer containing 25 mM HEPES, pH 7.5, 300 mM NaCl, 1.5 mM MgCl2, 0.5% sodium deoxycholate, 20 mM glycerophosphate, 1% Triton X-100, 0.1% SDS, 0.2 mM EDTA, pH 8, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, pH 10, and protease inhibitor. Cells were centrifuged at 14 000g for 15 minutes at 4°C, and the supernatant was stored at –80°C until use. Protein content was determined using DC protein assay kit according to the manufacturer's instructions (Bio Rad Laboratories, Hercules, CA). Aliquots (30 μg) of total cell protein were boiled with Laemmli sample buffer and loaded onto 8% to 12% SDS–polyacrylamide gels and transferred to nitrocellulose membranes (GE Osmonics Labstore, Minnetonka, MN). Membranes were blocked for 1 hour in PBS Tween containing 5% nonfat dried milk and then incubated with primary antibodies for 2 hours followed by species-specific horseradish peroxidase (HRP)–conjugated secondary antibody (diluted 1:5000) for 1 hour. The blots were visualized by enhanced chemiluminescence according to the manufacturer's instructions (Pierce Biotechnology, Rockford, IL) and normalized to the actin levels (antibody to actin was obtained from Sigma) in each extract. Mouse monoclonal antibody against p53 (OP43; Oncogene Research Products, Boston, MA), mouse monoclonal anti-p21 (Sc6246; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit phospho-Ser15 of p53 (9284; Cell Signaling Technology, Beverly, MA), and mouse monoclonal antibody to PARP from BD Pharmingen International (San Diego, CA) were used to detect these proteins in each sample.
Statistical analysis
The relationship between different parameters such as caspase activation and PARP cleavage with that of increase in dGTP accumulation (given as rectangular hyperbola) was determined using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Accumulation of dGTP with forodesine and dGuo
A total of 12 patients with CLL were studied; the median white blood cell (WBC) count was 125 × 109/L (range, 35-360 × 109/L). Most of these patients (n = 10) were previously untreated. Their Rai stage, ZAP-70 status, and IgVH mutational status and cytogenetics information are provided in Table 1. Only 1 patient (no. 11) had deletion of chromosome 17. The endogenous dGTP level was low and varied significantly between 0.3 and 4.4 μM, with a median of 2.7 μM (Table 1). In order to test if CLL cells do accumulate dGTP after PNP inhibition, leukemic lymphocytes obtained from 11 patients were incubated with forodesine and dGuo, and the accumulation of dNTP pool was measured by DNA polymerase assay. Similar to T-cell leukemic lymphocytes,10 primary CLL lymphocytes also accumulated dGTP with 2 μM forodesine in presence of 10 μM dGuo but showed heterogeneity. In 10 of 11 patient samples, during the drug incubation there was a gradual accumulation of dGTP. At 4 hours, the triphosphate accumulation was a median of 2.5 μM (range, 3-50 μM), and at 8 hours it further increased to a median of 14 μM (range, 6-118 μM; Figure 2A). Lymphocytes from one patient (no. 2) had no significant increase in dGTP. Compared with dGTP levels, there was no increase in other dNTPs such as dATP, deoxycytidine triphosphate (dCTP), and deoxythymidine triphosphate (dTTP). Data from one representative patient (no. 1) are provided in Figure 2B for illustrative purposes. At start, CLL lymphocytes from this patient had 3.8-μM intracellular dGTP level, which increased to 30 μM (8-fold) at 24 hours (Figure 2B). The starting levels of dATP, dCTP, and dTTP were 1, 4, and 2 μM, respectively, and these values remained almost the same at the end of 24 hours. This was true in all samples (n = 11) analyzed for all 4 dNTPs (data not shown).
Table 1.
Patient characteristics
| Patient no. | Prior Tx | Rai stage | ZAP-70 | IgVh status | Cytogenetics | WBC, × 109/L | Endogenous dGTP, μM |
|---|---|---|---|---|---|---|---|
| 1 | T | 2 | + | - | ND | 45 | 3.8 |
| 2 | UNT | 1 | - | ND | 46,XX{20} | 159 | 4.4 |
| 3 | UNT | 3 | - | ND | 46,XY{11] | 114 | 3.7 |
| 4 | UNT | 3 | + | - | 47,XX,+12[4]/46,XX[11] | 280 | 2.5 |
| 5 | UNT | 4 | - | + | 46,XY {20} | 360 | 0.3 |
| 6 | UNT | 0 | ND | ND | 46,XY,del{13} {q12q14} {7}/46,XY{13} | 43 | 0.9 |
| 7 | UNT | 1 | ND | ND | Random numeral and structural changes {2}/46,XY{18} | 78 | 0.9 |
| 8 | UNT | 1 | + | - | 46,XY {20} | 52 | 0.8 |
| 9 | UNT | 1 | ND | ND | 46,XX{12} | 243 | 3.1 |
| 10 | UNT | 2 | - | + | 46,XX {20} | 127 | 2.7 |
| 11 | UNT | 1 | + | ND | ND | 35 | ND |
| 12 | T | 3 | - | ND | 46,XY {15} | 228 | 1.0 |
Tx indicates therapy; T, Treated; UNT, untreated; and ND, not done.
Figure 2.
Effect of forodesine on dNTP pools. Accumulation of dGTP (A) and all 4 dNTPs (B) with the incubation of forodesine in primary CLL cells. The primary CLL cells from 11 patients were incubated with 2 μM forodesine and 10 μM dGuo at 4 and 8 hours, and the nucleotides in the leukemia cells were extracted by 60% methanol and dNTPs were measured by DNA polymerase assay. The data of accumulation of dGTP for all patients (A) and the 4 dNTPs (▪, dGTP; ▴, dATP; ▾, dCTP; and ♦, dTTP) for one patient (B) are plotted.
Stabilization of p53 and phosphorylation at Ser15
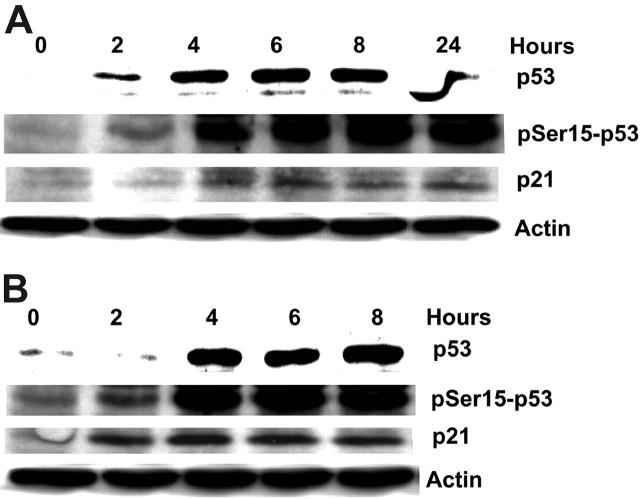
P53 regulates the expression of genes involved in DNA repair, cell-cycle arrest, and apoptosis. It is up-regulated during cell stress via a phosphorylation-dependent process. When CLL lymphocytes (patient nos. 1-5) were incubated with forodesine and dGuo, 4 (nos. 1, 3, 4, and 5) of 5 patients showed an increase in p53 protein level along with an up-regulation of downstream protein p21 (Figure 3A-B). However, one patient (no. 2) showed neither the stabilization of p53 nor the up-regulation of p21 (data not shown). Stabilization of p53 protein may be due to its phosphorylation on Ser15 (Figure 3A-B). These results suggest that DNA damage induced by forodesine up-regulates p53, via phosphorylation-dependent protein stabilization.
Figure 3.
Forodesine increases p53 stabilization and induces p53 phosphorylation on Ser15, with the activation of p21. CLL primary cells were incubated with 2 μM forodesine and 10 μM dGuo at different time points, and the expression of p53, the phosphorylation of p53 at Ser15, and the expression of p21 were measured by Western blot and normalized to actin. Western blot data of 2 patients (no. 1 in A; no. 4 in B) are presented.
Induction of apoptosis in CLL lymphocytes
When CLL lymphocytes were incubated with forodesine and dGuo there was induction of apoptosis that was measured by different experimental approaches. First, the PARP cleavage was measured at different time points in CLL cells for 5 patients (nos. 1-5). In all samples, the PARP cleavage was detected starting at 4 hours. Data from 2 representative patients, no. 1 and no. 4 (Figure 4A and B, respectively), are provided. Collective data from these 5 patient samples at 4, 6, and 8 hours after treatment were plotted to seek a relationship between fold increase in PARP cleavage and increase in dGTP (Figure 4C). There was a strong and proportional relationship (r = 0.92) between these parameters reaching a plateau after 50-fold increase in dGTP. Furthermore, to identify if PARP cleavage was associated with activation of caspases 9, 8, and 3, fluorometric assays by the addition of a caspase-specific peptides were done.
Figure 4.
Induction of apoptosis in primary CLL cells with forodesine. When CLL cells were incubated with forodesine at different time points, there was induction of apoptosis that was measured by PARP cleavage, and the Western blots were normalized to actin. Data from 2 patients (no. 1 in A; no. 4 in B) are provided. The immunoblots of PARP for 5 patients were quantitated using densitometer and the cleaved PARP was correlated with the dGTP accumulation, and the relationship between accumulation of dGTP and the PARP cleavage for 5 patients at different time periods (4, 6, and 8 hours) are plotted (C).
Data from 2 patients for caspase 8 and 9 (no. 2 and no. 3, Figure 5A-B) demonstrate that cell lysate from one patient (no. 2) did not show much activation in caspases 8 and 9 (Figure 5A). The intracellular dGTP values for this individual were 4, 6, 8, and 5 μM at 0, 2, 4, and 8 hours, respectively. In contrast to these data, cell lysates from another patient (no. 3) showed 2- to 4-fold increase in caspases 8 and 9 activation (Figure 5B). The dGTP values for this patient were 3, 40, 80, and 118 μM at 0, 2, 4, and 8 hours, respectively. The increase in caspases 8 and 9 was observed in 7 of 12 patients.
Figure 5.
Relationship between intracellular dGTP and activation of caspases in primary CLL cells with forodesine. CLL cells were incubated with 2 μM forodesine and 10 μM dGuo at different time points, and the activation of caspases 8, 9, and 3 were measured by fluorometric assay. Data from 2 patients for caspases 8 and 9 (A-B) and the relationship between accumulation of dGTP and the activation of caspase 3 (C) for 11 patients are plotted.
Nine of 11 patient samples showed a 6- to 31-fold increase in the activation of caspase 3. The increase in caspase 3 was correlated with the accumulation of dGTP, and there was a proportional relationship between these 2 parameters (r = 0.932 and P = .005; Figure 5C). As another measure of apoptosis, primary CLL cells from 14 patients (nos. 6-12, and an additional 7 patient samples) were incubated with forodesine and dGuo, and the induction of apoptosis was measured by annexin V binding using flow cytometry. Overall from 14 patients' samples tested, 7 showed annexin V positivity at 8 hours that ranged from 5% to 40%, and 11 patients' samples showed annexin V positivity at 24 hours (range, 8%-41%), confirming that these cells undergo apoptosis with forodesine (data not shown).
In limited patient samples, the disruption in the MMP was measured by flow cytometry using JC-1 dye. For MMP change, there was heterogeneity among patients in response to forodesine (data not shown).
Effect of forodesine, dGuo, or combination on CLL lymphocytes
To test if forodesine alone has any toxicity to CLL lymphocytes or if both forodesine and dGuo are needed for a cytotoxic effect, CLL lymphocytes from 3 additional patients were incubated with these drugs individually or in combination. Leukemic lymphocytes from none of these patients showed an increase in dGTP with forodesine alone; however, 2 of 3 patients accumulated and increased dGTP with dGuo alone. Samples from all 3 patients resulted in augmentation of intracellular dGTP when forodesine and dGuo were combined.
When caspase 3 was measured in these lysates, none of these samples showed activation of caspase 3 with forodesine alone; however, 2 of 3 patients showed increase in caspase 3 with dGuo alone, and all samples showed activation with the combination of both drugs (Table 2).
Table 2.
Comparison of dGTP accumulation either with forodesine, dGuo, or both
| Paramater | Untreated, 8 h | Forodesine, 8 h | dGuo, 8 h | Forodesine + dGuo, 8 h |
|---|---|---|---|---|
| Patient no. 1 | ||||
| dGTP, μM | 6.0 | 7.6 | 8.2 | 29.6 |
| Caspase 3, % activation | 74 | 93 | 50 | 122 |
| Patient no. 2 | ||||
| dGTP, μM | 7.1 | 5.0 | 17.0 | 18.7 |
| Caspase 3, % activation | 1616 | 1578 | 1999 | 1962 |
| Patient no. 3 | ||||
| dGTP, μM | 6.0 | 6.1 | 51.7 | 54.4 |
| Caspase 3, % activation | 111 | 98 | 140 | 135 |
Effect of forodesine on normal T- and B-cell lymphocytes
Normal T and B lymphocytes were obtained from 3 healthy donors (Table 3) and incubated with forodesine and dGuo, and the accumulation of dGTP and induction of apoptosis were measured by annexin V. In this limited sample analysis compared with T-cell lymphocytes, B-cell lymphocytes appear to accumulate higher levels of dGTP. Neither T nor B lymphocytes underwent apoptosis after 8 hours of treatment (Table 3).
Table 3.
Accumulation of dGTP in B and T ymphocytes isolated from healthy donors
|
dGTP, μM
|
Annexin V, %
|
JC-1, %
|
||||
|---|---|---|---|---|---|---|
| Cell type | 8 h untreated | 8 h treated | 8 h untreated | 8 h treated | 8 h untreated | 8 h treated |
| Donor no. 1 | ||||||
| T cell | 0.4 | 4.6 | 18 | 19 | 21 | 24 |
| Donor no. 2 | ||||||
| B cell | 1.1 | 37.4 | 19 | 14 | 24 | 35 |
| T cell | 0.3 | 10.9 | 36 | 21 | 13 | 9 |
| Donor no. 3 | ||||||
| B cell | 1.4 | 70 | 29 | 16 | 23 | 34 |
| T cell | 1.4 | 6.8 | 37 | 42 | 20 | 21 |
Discussion
We initiated this work based on 2 premises; first, purine nucleoside analogs have been the most effective agents for treatment of CLL, and minor modifications in the structure of these analogs have resulted in diverse activity. Second, enzymatic (adenosine deaminase, ADA) inhibitor that alters the deoxynucleotide pool in the leukemic lymphocytes has been successful for treatment of this indolent disease. The prime examples of these strategies are fludarabine, cladribine, and nelarabine for the former and pentostatin for the latter.18
Availability of forodesine, a potent PNP inhibitor, its clinical activity in T-cell diseases,4,7 demonstration of PNP inhibition, accumulation of dGuo in plasma, and dGTP in circulating leukemia cells7 prompted us to test this novel enzymatic inhibitor for CLL cells to perturb cellular milieu for dNTPs. In the present study, we demonstrate that physiologic concentration of forodesine in presence of clinically achievable level of dGuo results in accumulation of dGTP albeit at varied levels in primary CLL cells (Figure 2A).
In order to be biologically active, dGuo needs to be phosphorylated intracellularly, and its cytotoxicity depends on accumulation of the dGTP in the cells.19 When dGuo enters the cell through nucleoside transporters, it is phosphorylated to monophosphate by cytosolic dCK or mitochondrial dGuo kinase and dephosphorylated by 5′-nucleotidase. The variability in the accumulation of dGTP in these CLL lymphocytes may be due to differential activities of these enzymes. The increase in dGTP level was exclusive as there was no significant accumulation observed for the other dNTPs such as dATP, dCTP, and dTTP (Figure 2B).
Perturbance of dNTP pool leading to a p53 response has been observed with agents such as hydroxyurea, a ribonucleotide reductase inhibitor that lowers dNTP pool,20 and pentostatin, an ADA inhibitor that will increase dATP pool,21 in both cycling and indolent leukemia cells. In addition, previous studies showed that in quiescent lymphocytes, dATP accumulation interferes with proper repair of DNA, accumulation of DNA strand breaks, and p53 expression.22 The p53 network, which is normally “off,” is activated only when cells are stressed or damaged. Wild-type p53 protein is an important regulator of cell fate by its ability to accumulate after DNA damage and other types of stress and to cause growth arrest or apoptosis. Because the majority of the CLL patients in the present investigation were previously untreated, it would be expected that they harbor WT p53.23,24 Hence, we examined whether dGTP accumulation in the CLL cells leads to changes in p53 content (Figure 3A-B). Forodesine and dGuo treatment stabilized p53 protein level in all except for one patient. In concordance with our hypothesis, leukemia cells from this patient did not show an augmentation in dGTP accumulation. DNA damage-induced phosphorylation of p53 at Ser15, a residue that lies adjacent to the MDM2-binding site, has been reported.25-27 This posttranslational modification is required for p53 stabilization and activation following cell stress and is a determinant of p53-mediated apoptosis.28-32 As shown in Figure 3A-B, p53 stabilization after forodesine and dGuo treatment was associated with Ser15 phosphorylation and activation of downstream p21. Taken together, these molecular events suggest that forodesine treatment results in posttranslational modification of p53, its stability, and p53-dependent p21 activation. These data are consistent with other anticancer drugs that induce DNA damage, and p53-mediated downstream signaling leading to cell-growth arrest, DNA damage repair, or apoptosis.33
To determine if dGTP-mediated p53 response would lead to apoptosis, cell death measurements were done in these lymphocytes. The enzymatic activity of caspase 8 and 9 using a fluorometric biochemical assay showed activation of these extrinsic and intrinsic cell-death pathways only in those cells that showed an accumulation of dGTP (Figure 5B). In contrast, no effect or minor increase in dGTP pool resulted in no caspase activation (Figure 5A). This differential activation of caspases based on dGTP accumulation was also seen for caspase 3. In fact, the increase in caspase 3 was directly related to the accumulation of dGTP in these 11 patients (r = 0.932 and P = .005; Figure 5C), suggesting that the induction of apoptosis in these leukemic lymphocytes is in response to the accumulation of dGTP. Consistent with the activation of caspase 3 and as a downstream substrate of this caspase, there was PARP cleavage in most patient samples studied (Figure 4A-B), which was strongly associated with increase in intracellular dGTP (Figure 4C). Apoptosis induced by chemotherapeutic agent can be mediated either via DNA damage and p53 protein expression as shown in Figure 3 for forodesine, or directly via perturbation of mitochondrial function and change in mitochondrial permeability transition pore (mtPTP).34 The CLL lymphocytes in our study also showed change in mitochondrial membrane potential, suggesting that the mitochondria may be directly or indirectly involved in dGTP-induced cell death.
The mechanism by which intracellular dGTP would initiate apoptosis of these quiescent lymphocytes is not fully understood. However our data do suggest that fold increase in dGTP serves as a primary determinant of apoptotic response (Figures 4C,5C). In that respect, our data would mimic what was observed with ADA inhibitor pentostatin. Pentostatin, a highly effective agent for CLL,35,36 is a potent inhibitor of ADA (Ki = 2.5 pM) and induces accumulation of dATP.37 Elevated dATP levels would cause an imbalance in the cellular levels of dNTPs, resulting in the inhibition of DNA synthesis and repair. In addition, dATP is a required element of apoptosome, an assembly of Apaf-1, cytochrome c, and procaspase 9, that activates the caspase cascade leading to apoptosis.38 Moreover, increased levels of Ado or dAdo derivatives in nondividing cells can lead to an unbalanced ratio of S-adenylmethionine and S-adenylhomocysteine, thus impairing the synthesis of methylated nucleosides.39 Similar to these actions of pentostatin, forodesine inhibits PNP and induces the accumulation of another dNTP, dGTP, and causes an imbalance in the cellular levels of dNTPs, resulting in the induction of apoptosis.
These data provide rationale for use of forodesine for the treatment of CLL. First, in vitro studies on CLL suggested accumulation of intracellular dGTP. Second, there was DNA damage response as measured with p53 stabilization and phosphorylation and activation of downstream p21. Third, the measure of PARP cleavage, caspase activation, and changes in mitochondrial membrane potential demonstrated that CLL cells undergo apoptosis with forodesine and dGuo. Finally, there seems to be a therapeutic index as normal T and B lymphocytes isolated from healthy donors did not undergo apoptosis with this combination.
Based on these observations, we have initiated a phase 2 study of forodesine in patients with fludarabine-refractory CLL. Laboratory end points during this ongoing trial would provide information on the efficacy of oral forodesine for this indolent leukemia.
Acknowledgments
The authors are grateful to Min Du for obtaining blood samples and to Susan Lerner for providing information on patient characteristics and clinical laboratory evaluations. The authors are members of CLL Research Consortium. Dr Laura Rassenti, director of the tissue bank core facility of the CLL Research Consortium (CA81534), provided information regarding ZAP-70 expression and IgVH mutational status in the samples.
Prepublished online as Blood First Edition Paper, June 15, 2006; DOI 10.1182/blood-2006-03-007468.
Supported in part by grants CA57629 and CA81534, and P30-16672 cancer center support grant from the the National Cancer Institute, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Parks RE Jr, Agarwal RP. In: Boyer PD, ed. The Enzymes. New York, NY: Academic Press; 1972: 483-514.
- 2.Giblett ER, Anderson JE, Cohen F, Pollara B, Meuwissen HJ. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2: 1067-1069. [DOI] [PubMed] [Google Scholar]
- 3.Hershfield MS, Mitchell BS. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In: Scriber CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic Basis of Inherited Disease. 7th ed. New York, NY: McGraw-Hill. 1995; 1725-1768.
- 4.Markert ML. Purine nucleoside phosphorylase deficiency. Immunodefic Rev. 1991;3: 45-81. [PubMed] [Google Scholar]
- 5.Miles RW, Tyler PC, Furneaux RH, Bagdassarian CK, Schramm VL. One-third-the-sites transition-state inhibitors for purine nucleoside phosphorylase. Biochemistry. 1998;37: 8615-8621. [DOI] [PubMed] [Google Scholar]
- 6.Evans GB, Furneaux RH, Lewandowicz A, Schramm VL, Tyler PC. Exploring structure-activity relationships of transition state analogues of human purine nucleoside phosphorylase. J Med Chem. 2003;46: 3412-3423. [DOI] [PubMed] [Google Scholar]
- 7.Bantia S, Ananth SL, Parker CD, Horn LL, Upshaw R. Mechanism of inhibition of T-acute lymphoblastic leukemia cells by PNP inhibitor: BCX-1777. Int Immunopharmacol. 2003;3: 879-887. [DOI] [PubMed] [Google Scholar]
- 8.Bantia S, Miller PJ, Parker CD, et al. Purine nucleoside phosphorylase inhibitor BCX-1777 (Immucillin-H): a novel potent and orally active immunosuppressive agent. Int Immunopharmacol. 2001;1: 1199-1210. [DOI] [PubMed] [Google Scholar]
- 9.Kicska GA, Long L, Horig H, et al. Immucillin H, a powerful transition-state analog inhibitor of purine nucleoside phosphorylase, selectively inhibits human T lymphocytes. Proc Natl Acad Sci U S A. 2001;98: 4593-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi V, Kilpatrick JM, Plunkett W, et al. A proof-of-principle pharmacokinetic, pharmacodynamic, and clinical study with purine nucleoside phosphorylase inhibitor immucillin-H (BCX-1777, forodesine). Blood. 2005;106: 4253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobsson B, Albertioni F, Eriksson S. Deoxynucleoside anabolic enzyme levels in acute myelocytic leukemia and chronic lymphocytic leukemia cells. Cancer Lett. 2001;165: 195-200. [DOI] [PubMed] [Google Scholar]
- 12.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351: 893-901. [DOI] [PubMed] [Google Scholar]
- 13.Glassman AB, Hayes KJ. The value of fluorescence in situ hybridization in the diagnosis and prognosis of chronic lymphocytic leukemia. Cancer Genet Cytogenet. 2005;158: 88-91. [DOI] [PubMed] [Google Scholar]
- 14.Plunkett W, Hug V, Keating MJ, Chubb S. Quantitation of 1-beta-D-arabinofuranosylcytosine 5′-triphosphate in the leukemic cells from bone marrow and peripheral blood of patients receiving 1-beta-D-arabinofuranosylcytosine therapy. Cancer Res. 1980;40: 588-591. [PubMed] [Google Scholar]
- 15.Gandhi V, Plunkett W, Kantarjian H, Talpaz M, Robertson LE, O'Brien S. Cellular pharmacodynamics and plasma pharmacokinetics of parenterally infused hydroxyurea during a phase I clinical trial in chronic myelogenous leukemia. J Clin Oncol. 1998;16: 2321-2331. [DOI] [PubMed] [Google Scholar]
- 16.Sherman PA, Fyfe JA. Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal Biochem. 1989;180: 222-226. [DOI] [PubMed] [Google Scholar]
- 17.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411: 77-82. [DOI] [PubMed] [Google Scholar]
- 18.Parker WB, Secrist JA III, Waud WR. Purine nucleoside antimetabolites in development for the treatment of cancer. Curr Opin Investig Drugs. 2004;5: 592-596. [PubMed] [Google Scholar]
- 19.Gandhi V, Plunkett W. Combination strategies for purine nucleoside analogs. In: Cheson BD, ed. Chronic Lymphoid Leukemias. New York, NY: Marcel Dekker. 2001; 195-208.
- 20.Kumar S, Dodson GE, Trinh A, Puchalski JR, Tibbetts RS. ATR activation necessary but not sufficient for p53 induction and apoptosis in hydroxyurea-hypersensitive myeloid leukemia cells. Cell Cycle. 2005;4: 1667-1674. [DOI] [PubMed] [Google Scholar]
- 21.Dohner H, Fischer K, Bentz M, et al. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995;85: 1580-1589. [PubMed] [Google Scholar]
- 22.Carson DA, Carrera CJ, Wasson DB, Yamanaka H. Programmed cell death and adenine deoxynucleotide metabolism in human lymphocytes. Adv Enzyme Regul. 1988;27: 395-404. [DOI] [PubMed] [Google Scholar]
- 23.Fenaux P, Preudhomme C, Lai JL, et al. Mutations of the p53 gene in B-cell chronic lymphocytic leukemia: a report on 39 cases with cytogenetic analysis. Leukemia. 1992;6: 246-250. [PubMed] [Google Scholar]
- 24.Gaidano G, Ballerini P, Gong JZ, et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991;88: 5413-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banin S, Moyal L, Shieh S, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281: 1674-1677. [DOI] [PubMed] [Google Scholar]
- 26.Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281: 1677-1679. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi K, Herrera JE, Saito S, et al. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12: 2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91: 325-334. [DOI] [PubMed] [Google Scholar]
- 29.Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11: 3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashcroft M, Kubbutat MH, Vousden KH. Regulation of p53 function and stability by phosphorylation. Mol Cell Biol. 1999;19: 1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unger T, Sionov RV, Moallem E, et al. Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene. 1999;18: 3205-3212. [DOI] [PubMed] [Google Scholar]
- 32.Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin. J Biol Chem. 2000;275: 35778-35785. [DOI] [PubMed] [Google Scholar]
- 33.Meek DW. Multisite phosphorylation and the integration of stress signals at p53. Cell Signal. 1998;10: 159-166. [DOI] [PubMed] [Google Scholar]
- 34.Genini D, Adachi S, Chao Q, et al. Deoxyadenosine analogs induce programmed cell death in chronic lymphocytic leukemia cells by damaging the DNA and by directly affecting the mitochondria. Blood. 2000;96: 3537-3543. [PubMed] [Google Scholar]
- 35.Dearden CE, Matutes E, Hilditch BL, Swansbury GJ, Catovsky D. Long-term follow-up of patients with hairy cell leukaemia after treatment with pentostatin or cladribine. Br J Haematol. 1999;106: 515-519. [DOI] [PubMed] [Google Scholar]
- 36.Grever MR, Siaw MF, Jacob WF, et al. The biochemical and clinical consequences of 2′-deoxycoformycin in refractory lymphoproliferative malignancy. Blood. 1981;57: 406-417. [PubMed] [Google Scholar]
- 37.Agarwal RP, Spector T, Parks RE Jr. Tight-binding inhibitors-IV: inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977;26: 359-367. [DOI] [PubMed] [Google Scholar]
- 38.Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91: 479-489. [DOI] [PubMed] [Google Scholar]
- 39.Niitsu N, Yamaguchi Y, Umeda M, Honma Y. Human monocytoid leukemia cells are highly sensitive to apoptosis induced by 2′-deoxycoformycin and 2′-deoxyadenosine: association with dATP-dependent activation of caspase-3. Blood. 1998;92: 3368-3375. [PubMed] [Google Scholar]