Abstract
Surface protrusions of Plasmodium falciparum-infected erythrocytes, called knobs, display focal aggregates of P falciparum erythrocyte membrane protein 1 (PfEMP1), the adhesion ligand binding endothelial-cell receptors. The resulting sequestration of infected erythrocytes in tissues represents an important factor in the course of fatalities in patients with malaria. The main component of knobs is the knob-associated histidine-rich protein (KAHRP), and it contributes to altered mechanical properties of parasite-infected erythrocytes. The role of KAHRP domains in these processes is still elusive. We generated stable transgenic P falciparum-infected erythrocytes expressing mutant versions of KAHRP. Using atomic force and electron microscopy we show that the C-terminal repeat region is critical for the formation of functional knobs. Elasticity of the membrane differs dramatically between cells with different KAHRP mutations. We propose that the 5′ repeat region of KAHRP is important in cross-linking to the host-cell cytoskeleton and this is required for knob protrusion and efficient adhesion under physiologic flow conditions. (Blood. 2006;108:370-378)
Introduction
Plasmodium falciparum is the most lethal malaria parasite of humans.1 An important aspect in virulence of P falciparum is the ability of infected erythrocytes to sequester in and obstruct the microvasculature of different organs.2 These abnormal circulatory properties of erythrocytes involve parasite-induced alterations in their biomechanical and adhesive properties and are important for survival and pathogenicity of P falciparum.3
Cytoadhesion is mediated by the antigenically variant P falciparum erythrocyte membrane protein-1 (PfEMP1), which can bind to host receptors including CD36 and chondroitin sulfate A (CSA).4 PfEMP1 is concentrated on electron-dense elevations of the membrane referred to as knobs,5-7 providing a platform for adherence under physiologic flow conditions.8 Increased erythrocyte rigidity and adhesiveness result in dramatically augmented hemodynamic resistance observed in microvasculature perfused with P falciparum-infected erythrocytes.9 Knobs consist predominantly of the knob-associated histidine-rich protein (KAHRP),10,11 assembling on the cytoplasmic face of the membrane. KAHRP is required for knob formation.8
KAHRP has a signal sequence with a recessed hydrophobic core that directs the protein into the parasitophorous vacuole via the endomembrane system.12 A second signal, we termed the “Plasmodium export element” (PEXEL),13 is required for transfer across the parasitophorous vacuole membrane to the erythrocyte cytosol.13,14 The PEXEL is followed by a histidine-rich region containing sequences responsible for interaction with Maurer clefts,15 structures assembled in the P falciparum-infected erythrocyte cytosol important in protein sorting and trafficking.12 There are also 2 blocks of highly charged repeats, designated the 5′ and 3′ repeats according to their location.10,11
Current evidence suggests KAHRP interacts with various cytoskeletal components of the erythrocyte including spectrin, actin, and spectrin-actin band 4.1 complexes16-18 and these interactions may be responsible for changes in host-cell physical properties.18,19 Additionally, certain regions of KAHRP can self-assemble,17 whereas the His-rich and the 5′ and 3′ repeats can separately bind to the acidic terminal sequence (ATS) of PfEMP1 located on the cytoplasmic face of P falciparum-infected erythrocytes.20
The binding characteristics of KAHRP domains have been analyzed in vitro but the contribution to in vivo properties of P falciparum-infected erythrocytes is unknown. In this study, we show the C-terminal repeat regions are required for establishment of knobs beneath the erythrocyte membrane. The 5′ repeats initiate knob assembly but establishment of the full structure relies on both repeat regions. In addition, PfEMP1 trafficking to the erythrocyte surface is not hindered in the absence of knobs or KAHRP. However, membrane rigidity and cytoadherence under physiologic flow conditions are dramatically altered in parasites lacking the C-terminal repeat sequences.
Materials and methods
Plasmids, parasites, Southern blots, transfection, trypsin assay, and Western blot analysis
KAHRP (PfB0100c) was targeted using fragments amplified by polymerase chain reaction (PCR) from gDNA (P falciparum 3D7) with the oligonucleotides shown in Table S1 (available on the Blood website; see the Supplemental Table link at the top of the online article) and cloned into plasmid pHH1.21 3D7 P falciparum parasites were transfected with 100 μg plasmid (Qiagen, Valencia, CA)22,23 and cultured with WR99210 (10 nM).12,24 The KAHRP knock-out (KO) line has been described previously8 and generated by a single crossover recombination event and expresses a small truncated product (data not shown). Therefore, the mutant cell lines without repeat regions are expected to display a similar cytoadherence pattern compared with KAHRP KO parasites. gDNA extracted from trophozoites (24-36 hours) was analyzed by Southern blot hybridization using standard procedures and the KAHRP gene as probe.25
The trypsin-cleavage assay to determine surface PfEMP1 was performed as described using synchronized trophozoites (20-28 hours after invasion) harvested by magnetic cell sorting (CS columns; Mitenyi Biotec, Auburn, CA).26 The SDS-soluble fractions of the preparations were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and probed with polyclonal rabbit anti-ATS antiserum (1:1000) for PfEMP1. For detection of KAHRP, immunoblots were probed with mouse anti-K1A antiserum raised to the histidine-rich region (1:1000) followed by horseradish peroxidase-conjugated secondary antibodies (Silenius, 1:1000) and enhanced chemiluminescence (ECL) detection (Amersham, Freiburg, Germany).
Microscopy
In immunofluorescence assays, acetone/methanol (90%/10%) fixed smears of 3D7-K119- and 3D7-K(re)-infected erythrocytes were probed with either mouse anti-K1A (1:100) rabbit anti-KAHRP(repeats) (1:200), rabbit anti-ATS (1:100), or mouse anti-MAHRP1 (1:500) antiserum. Secondary antibodies were Alexa-Fluor 488-conjugated anti-rabbit IgG (Molecular Probes, Eugene, OR) or Alexa-Fluor 594-conjugated anti-mouse IgG or both (Molecular Probes). Similar results were obtained with both affinity-purified and serum for these antibodies. Z-sections were captured through relevant P falciparum-infected cells to observe rim fluorescence (data not shown). Cells were viewed with a Zeiss Plan-Apochromat 100 ×/1.4 numeric aperture oil-immersion lens on a Zeiss Axioskop 2 or Axiovert 200 MOT microscope equipped with a PCO SensiCam (12 bit) camera and Axiovision 3 or 4 software (Zeiss, Oberkochen, Germany). Captured images were processed using Photoshop and ImageJ software (http://rsb.info.nih.gov/ij).
Scanning electron microscopy (SEM) was performed with trophozoites (20-28 hours) harvested by magnetic cell sorting (CS columns; Miltenyi Biotec) followed by glutaraldehyde fixation (2% in PBS, Electron Microscopy Sciences, Hatfield, PA), for 30 minutes at room temperature. Cells were washed 3 times in PBS, transferred to polyethylenamine-coated coverslips (Sigma, St Louis, MO), immersed in 10% ethanol, and dehydrated (25%, 50%, 70%, 90%, 2 × 100%; 10 minutes each). Cells were subjected to critical point drying (CPD030; Bal-Tech, Balzers, Liechtenstein), coated with platinum in a sputter coater (S150B Sputter Coater; Edwards, Wilmington, MA), and viewed in a Philips XL30 FEG scanning electron microscope at 2 kV. Transmission electron microscopy (TEM) was performed as described27 and viewed in a Philips CM120 transmission electron microscope equipped with a Multiscan 600 CW, wide-angle CCD camera (Gatan, Pleasanton, CA).
Atomic force microscopy (AFM) used magnet-purified parasites (28-34 hours after invasion) fixed in 4% paraformaldehyde/0.1% glutaraldehyde for 30 minutes on ice and washed twice in PBS. Coverslips were coated with 0.5 mg/mL poly-l-lysine followed by 1% glutaraldehyde.28 Cells were attached to coverslips and imaged in a capillary bridge of PBS using a Dimension 3100 atomic force microscope with Nanoscope IIIa controller (Digital Instruments, Santa Barbara, CA). Samples were assembled on its motorized sample stage and images of the surface collected in Tapping-Mode at drive amplitude of approximately 6 kHz with sharpened triangular-shaped cantilevers (cantilever D of the MSCT Microlever substrate [Veeco], 220 μm long, 0.03 N m-1 nom). The size and height of elevations on the surface were measured from height profiles using the WSxM AFM analysis package (Nanotec Electronica, Madrid, Spain). Additional data from the phase, amplitude, and first-derivative images were used to define the width of the protuberances.
Flow-based cytoadherence assay and membrane mechanical properties
The number of parasitized erythrocytes binding to CD36 expressed on the surface of monolayers of adherent platelets in flat, glass microcapillary tubes under flow conditions was determined as described.29,30 Error bars represent mean values ± SEM of 3 independent experiments. The shear elastic modulus of the erythrocyte-membrane skeleton of all transfectants was determined by micropipette aspiration of individual erythrocytes as previously described.31
Results
Truncations of KAHRP
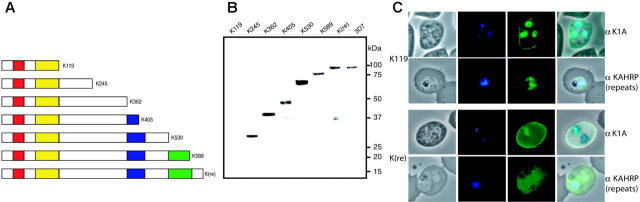
To assess function of different KAHRP domains in knob formation, membrane rigidity, and cytoadherence, we created cell lines expressing truncations of KAHRP. Gene fragments of KAHRP encoding 119, 245, 362, 405, 530, 589 amino acids and the full-length protein were cloned into pHH121 and transfected into P falciparum line 3D7 (Table S1). This strategy allowed insertion of truncated constructs into endogenous KAHRP by single crossover recombination (Figure 1A). The resulting P falciparum transgenic cell lines were: 3D7-K119, -K245, -K362, -K405, -K530, -K589, and -K(re). The 3D7-K(re) parasites would express full-length KAHRP as the gene was reconstituted. The plasmids recombined into the endogenous KAHRP locus on chromosome 2 as shown by pulsed-field gel electrophoresis (PFGE; data not shown) and Southern blot analysis (Figure 1B). Southern blot hybridization confirmed a single homologous crossover into the endogenous gene resulting in insertion of the plasmid and reconstitution of KAHRP with the expected truncation (Figure 1B).
Figure 1.
Targeted gene truncation of KAHRP. (A) Schematic representations of the plasmid vector pHH1 used for integration, the endogenous KAHRP gene, and the expected integration event. Promoter (arrow) and terminator (T) regions are depicted as shaded, the resistance marker human DHFR (hDHFR) in white, the endogenous KAHRP gene in gray, and the truncated KAHRP gene in black boxes. Relevant restriction enzyme sites are shown and sizes of each expected fragment are shown below in kilobases. (B) Southern blot analysis of Afl II-HindIII-digested gDNA from 1 to 8 of: 3D7-K119, -K245, -K362, -K405, -K530, -K589, -K(re), and 3D7 parental parasite lines. Note that for 3D7-K119, the construct does not have the second AflII site in the truncated KAHRP gene. Therefore, there is no 1.45-kb band present on the blot but a band representing the distance between the first Afl II site and the HindIII site. For 3D7-K245 the band happens to be a very similar size to the 4.088-kb band and therefore only one double band is visible for this mutant cell line.
Transgenic P falciparum express truncated KAHRP
To verify expression of truncated KAHRP, immunoblots were probed with anti-K1A antibody (Figure 2A). 3D7-K245, -K362, and -K405 displayed proteins of 28 kDa, 41 kDa, and 47 kDa, respectively, corresponding to the expected molecular mass of the truncated protein (Figure 2B). KAHRP in 3D7-K530, -K589, and -K(re) ran differently than predicted (70 kDa, 75 kDa, and 92 kDa instead of predicted 60 kDa, 65 kDa, and 71 kDa, respectively) most likely due to charged residues in the C-terminus (Figure 2B). Anomalous migration of proteins containing repeats is well known in P falciparum. The protein from parasites with reconstituted KAHRP (3D7-K(re)) corresponded in migration with full-length KAHRP. Furthermore, the mutant lines did not show an additional endogenous full-length KAHRP protein, confirming disruption of the gene and replacement with the desired truncation.
Figure 2.
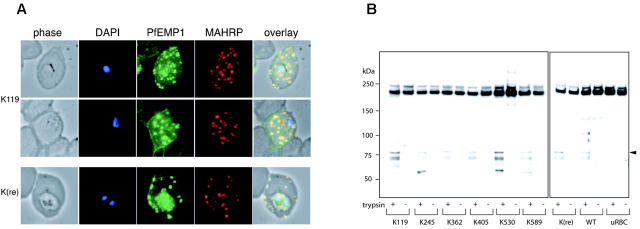
Expression of truncated KAHRP. (A) Schematic representation of truncated proteins (K119, etc) expressed in the transfected lines indicating the number of amino acids of each protein. Red boxes represent the signal peptide, yellow boxes the His-rich repeats, blue boxes the 5′, and green boxes the 3′ repeat regions (not drawn to scale). (B) Western blot analysis of all mutant cell lines compared to 3D7 parasite line detected with anti-K1A antisera. (C) Immunofluorescence assay of K119 compared to K(re) probed with 2 antibodies against different regions of KAHRP. The anti-K1A antibody recognizes the His-rich repeat region, whereas the anti-KAHRP (repeats) antibody was raised against the 5′ and 3′ repeat regions. The first panel of each row shows a phase-contrast image of an infected erythrocyte, the second panel shows nuclear DNA of the parasite stained with DAPI, the third panel shows the reaction with the KAHRP-specific antibody, and the fourth panel represents an overlay of the 3 previous panels. The anti-K1A antibody shows no cross-reaction with uninfected erythrocytes as shown by lack of reactivity in contrast to strong rim fluorescence observed in all P falciparum-infected red blood cells.
The shortest truncated protein, K119, was not detected using anti-K1A antibodies, which may be a consequence of basic residues at the C-terminus. To determine if the protein was expressed we performed immunofluorescence assays on 3D7-K119 and 3D7-K(re) transgenic lines. We used 2 antibodies, which were raised against different repeat regions of KAHRP. Anti-K1A antibodies react with the His-rich region, whereas anti-KAHRP(repeats) antibodies react with the 5′ C-terminal repeats. Because the K119 truncated protein consists of the N-terminal 119 amino acids, comprising the His-rich repeat region, and not the further down-stream repeats, this assay distinguished between specific reactivity with KAHRP and cross-reactivity of the antibodies. The 3D7-K(re) parasitized erythrocytes showed rim fluorescence and a dispersed red-cell cytosolic pattern for KAHRP with both antibodies.10,11,32 However, the 3D7-K119 cell line only showed this pattern with antibody recognizing the His-rich region, indicating the protein was expressed and transported into the erythrocyte. From these experiments we conclude all truncated versions of KAHRP are expressed. Even the shortest truncation associates with the red blood cell membrane visible as a faint rim fluorescence. However, light microscopy does not provide the resolution to distinguish between individual knobs and a homogenous distribution of KAHRP underneath the cell surface.
The repeat regions of KAHRP are required for functional knobs in P falciparum-infected erythrocytes
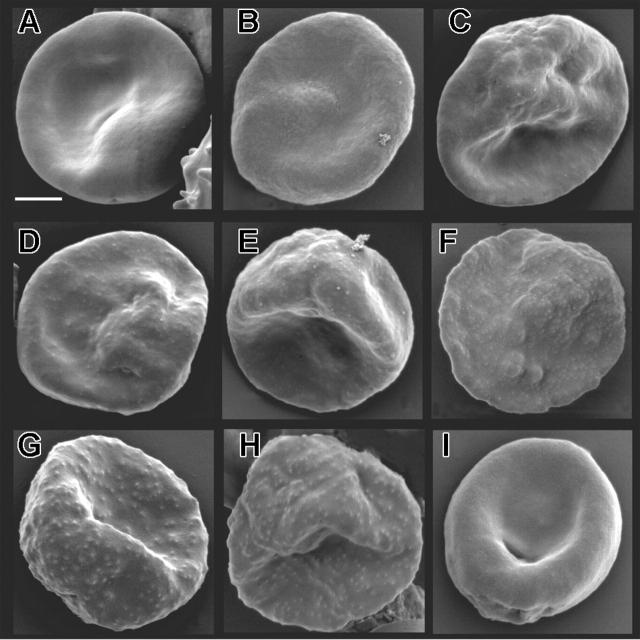
To determine the effect of truncating KAHRP on knob formation and structure we used a combination of high-resolution microscopy. To observe the surface composition of parasitized mutant cells and 3D7 we subjected parasites to SEM. 3D7-K119, -K245, and -K362 (Figure 3A-C) showed a smooth surface similar to the KAHRP KO line lacking expression of full-length KAHRP and knobs (Figure 3I).8 For the 3D7-K405 transgenic line (Figure 3D), which expresses a KAHRP truncation ending within the 5′ repeat region, slight protrusions were observed on the surface, which were sparse and flatter compared to parental 3D7 (Figure 4H). In 3D7-K530 and -K589 (Figure 3E-F) the protrusions were more prominent and in 3D7-K(re) (Figure 3G), normal knobs were formed, confirming that KAHRP function had been reconstituted and the cells displayed the same phenotype as the parent (Figure 3H).
Figure 3.
Surface characteristics of mutant cell lines expressing truncated versions of KAHRP. Scanning electron micrographs of (A) 3D7-K119, (B) 3D7-K245, (C) 3D7-K362, (D) 3D7-K405, (E) 3D7-K530, (F) 3D7-K589, (G) 3D7-K(re), (H) parental 3D7, and (I) KAHRP KO8 parasite lines. The bar represents 2 μm.
Figure 4.
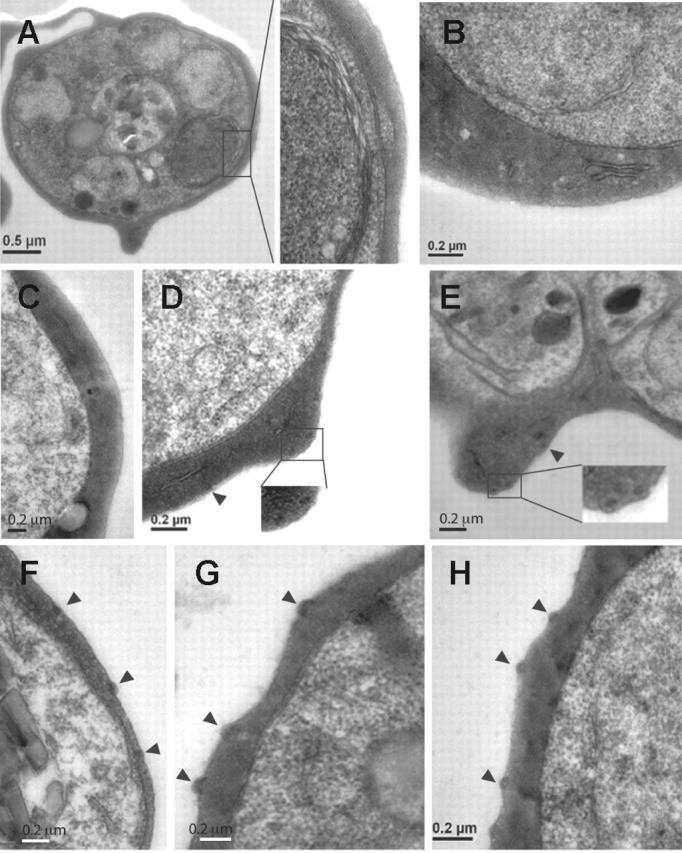
Ultrastructural features of knobs in KAHRP mutant cell lines. Transmission electron micrographs of erythrocytes infected with (A) 3D7-K119, (B) 3D7-K245, (C) 3D7-K362, (D) 3D7-K405, (E) 3D7-K530, (F) 3D7-K589, (G) 3D7-K(re), and (H) 3D7 (parental strain). Arrowheads indicate knob formation. Insets show a higher magnification of the areas of each cell shown in panels A, D, and E.
Knobs have been described as electron-dense, cup-shaped protrusions of the surface of infected erythrocytes.33 To further determine the shape and composition, which might assemble underneath the surface of the mutant cell lines, we performed TEM. 3D7-K119 (Figure 4A), 3D7-K245 (Figure 4B), and 3D7-K362 (Figure 4C) showed no obvious aggregations of dense material within the cytoplasm close to the erythrocyte surface similar to the KAHRP KO parasites (data not shown). In 3D7-K405 cells we saw electron-dense material that accumulated in areas near the erythrocyte membrane (Figure 4D) in accordance with the flat protrusions observed by SEM. In the 3D7-K530 and 3D7-K589 (Figure 4E-F) transgenic lines electron-dense material became more concentrated and was located underneath protuberances of the membrane. 3D7-K(re) (Figure 4G) expressing full-length KAHRP displayed the same shape and distribution of knobs as parental cells (Figure 4H). This suggested self-assembly into electron-dense aggregates in foci underneath the erythrocyte membrane occurred once part of the 5′ repeats were present.
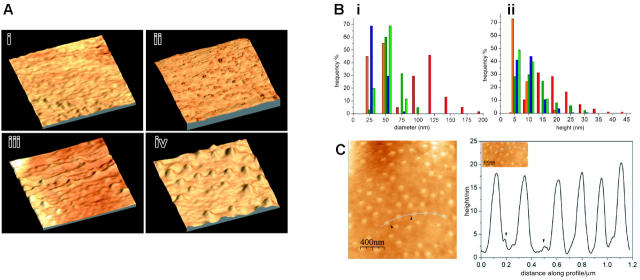
To image the surface with molecular resolution we performed atomic force microscopy on 3 mutant cell lines in comparison with uninfected erythrocytes (Figure 5Ai): 3D7-K119 (Figure 5Aii), which did not display knobs in the SEM studies, 3D7-K405 (Figure 5Aiii) where dense material was present underneath the surface in TEM studies, and 3D7-K(re) (Figure 5Aiv) to examine fully developed knob structures. The purified P falciparum-infected erythrocytes were scanned by a probe in intermittent contact mode following parallel lines using a piezoelectric positioning device (Figure 5A).
Figure 5.
Knob and surface topography of mutant cells lines expressing truncated versions of KAHRP. AFM of (A) uninfected (i), 3D7-K119-infected (ii), 3D7-K405-infected (iii), and 3D7-K(re)-infected (iv) red blood cells. The images show a 3-dimensional reconstruction of an area representative of 1 μm on each studied cell line. (B) Analysis of the frequency of a certain height (ii) and diameter (i) of knobs in uninfected (yellow), K119-infected (green), K405-infected (blue), and K(re)-infected (red) erythrocytes. The pre-knobs of K(re)-infected erythrocytes are shown in orange. (C) Surface profile of K(re)-infected red blood cells. Height of knobs was measured along a distance of 1.2 μm on the surface of the cell shown on the left panel (curved line). The pre-knobs are visible as minor peaks (arrowheads).
The 3-dimensional image of 3D7-K119 (Figure 5Aii) shows a similar ruffled surface to the uninfected erythrocytes (Figure 5 Ai). To determine similarities or differences between elevations on the cells we measured diameter and height of 100 to 300 protrusions (6-10 individual cells from 2 independent sample preparations; Figure 5B). The average diameter (30 nm) and height (7.5 nm) of elevations on the surface of uninfected and 3D7-K119-infected erythrocytes was almost identical (data not shown). It had been shown previously34 that smaller protrusions are sometimes associated with knobs. Interestingly, we found that knobs in the 3D7-K(re) (Figure 5Aiv) mutant line often had small elevations (“pre-knobs”) coupled with the large knobs but these pre-knobs were of similar height to protrusions on uninfected erythrocytes (Figure 5B). Most protrusions on the 3D7-K405-infected erythrocytes (Figure 5Aiii) had a diameter of 25 to 50 nm and a height of 5 to 10 nm, but a few displayed higher elevations of 20 to 30 nm (Figure 5Aiii arrows and Figure 5B) and the average value for diameter (50 nm) and height (10 nm) was higher in this transgenic line. The diameter and height of the knobs on the 3D7-K(re)-infected erythrocytes (Figure 5Aiv) were distributed over a range of 75 to 200 nm and 10 to 45 nm, respectively (Figure 5B) and the average diameter of 120 nm and height of 17.5 nm was significantly higher than all examined samples (data not shown). To determine the distance of knobs from each other on the 3D7-K(re)-infected erythrocytes we plotted height of protrusions versus distance of knobs along the profile over 1.2 μm (Figure 5C). The knobs were distributed evenly on the surface with no obvious pattern of accumulation of these structures.
Mechanical properties of the P falciparum-infected erythrocyte membrane were altered in cells with truncated KAHRP
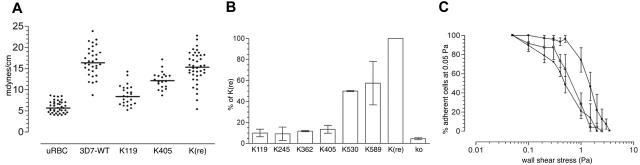
To determine contribution of KAHRP domains on rigidity of the host-cell membrane, we examined the shear elastic modulus of erythrocytes infected with 3D7-K119, -K405, and -K(re) compared to uninfected and 3D7-infected erythrocytes (Figure 6A). Membrane rigidity was studied by aspiration of cell membranes into micropipettes.19 As expected, the transgenic lines with reconstituted KAHRP displayed similar properties to the parent where in both cases the modulus was 3 times higher than in uninfected erythrocytes.31 Interestingly, the transfectants expressing 119 amino acids of KAHRP showed only a 1.5-fold increase in shear elastic modulus and 3D7-K405-infected erythrocytes approximately a 2-fold increase compared to uninfected erythrocytes. The variation of moduli among the cells of each line was similar between the lines tested and therefore comparable.
Figure 6.
Adhesive and mechanical properties of KAHRP mutant cell lines. (A) Membrane shear elastic modulus of erythrocytes infected with 3D7-K119, 3D7-K530, and 3D7-K(re) compared with uninfected red blood cells (uRBCs). Each point represents an individual erythrocyte. (B) Cells were flowed over monolayers of platelets expressing CD36. A physiologically relevant wall shear stress of 0.05 Pa was applied and error bars represent mean values ± SEM. The percentage represents a comparison of the adherence of each mutant cell line with 3D7-K(re)-infected and 3D7-infected erythrocytes. (C) Cells were flowed over monolayers of platelets expressing CD36 at 0.05 Pa. Adherent cells were then exposed to stepwise increases in wall shear stress. The number of infected cells remaining adherent after exposure to each stress was determined and is shown as the proportion of adherent infected cells relative to the number adhering at 0.05 Pa. Each value represents the mean ± SEM for 3 experiments for each parasite line. • indicates 3D7-K119-infected; □, 3D7-K530-infected; and ▪, 3D7-K(re)-infected erythrocytes.
Fully developed knobs are necessary for effective cytoadherence under physiologic flow conditions
Cytoadherence to endothelial plasma membranes is dependent on the interaction of PfEMP1, displayed on the knob platform of infected host cells, with receptors such as CD36.35 Flow-based adhesion assays can detect subtle differences in adhesion properties.36 To determine the effect of KAHRP truncation we quantified the ability of flowing parasitized erythrocytes to adhere to confluent monolayers of human platelets (expressing CD36) at a constant, physiologically relevant wall shear stress of 0.05 Pa. The erythrocytes infected with 3D7-K119, -K245, -K362, and -K405 showed low cytoadherence that was comparable with the KAHRP KO cells (5%-10% of 3D7-K(re); Figure 6B). With the presence of the 5′ repeat region of KAHRP (K530) cytoadherence increased to 50% compared to cells expressing full-length KAHRP. Surprisingly, cells expressing 589 amino acids of KAHRP including both C-terminal repeat regions only bound with approximately 60% efficiency to CD36 under these conditions (Figure 6B).
Trafficking of PfEMP1 is not impaired in KAHRP mutants
PfEMP1 is clustered and elevated in knob structures of parasitized erythrocytes allowing cytoadherence to endothelial cells. Because our mutant parasite lines showed altered adherence compared to cells expressing full-length KAHRP, we determined whether trafficking of PfEMP1 was impaired leading to accumulation inside the parasite or erythrocyte. 3D7-K119- and 3D7-K(re)-infected cells were incubated with αPfEMP1 (αATS) and membrane-associated histidine-rich protein 1 (MAHRP1), a known marker of Maurer clefts.37 Parasite-associated PfEMP1 labeling was detected in both cell lines and in the erythrocyte the protein was localized to Maurer clefts as demonstrated by colocalization with MAHRP (Figure 7A). Also, both transfectants displayed rim fluorescence indicating that trafficking of PfEMP1 to Maurer clefts and the erythrocyte membrane was not impaired in transgenic cells (Figure 7B).
Figure 7.
PfEMP1 trafficking and surface exposure on KAHRP mutant cell lines. (A) Immunofluorescence assay on 3D7-K119- and 3D7-K(re)-infected erythrocytes with anti-ATS antibody detecting PfEMP1 and anti-MAHRP, a Maurer clefts marker. The first panel in each row shows a phase-contrast image, the second panel is nuclear DNA stained with DAPI, the third panel is the reaction with the anti-ATS antibody for PfEMP1 detection, the fourth panel is the reaction with anti-MAHRP antibody, and the fifth panel illustrates an overlay of the previous 4 panels with colocalization shown in yellow. (B) Western blot analysis of trypsin treated (+) or untreated (-) intact erythrocytes infected with all mutant cell lines. The anti-ATS antibody detects cleaved PfEMP1 in case of presence of PfEMP1 on the surface in trypsin-treated samples (arrowhead). The full-length PfEMP1 runs at the same molecular weight as cross-reactive uRBC proteins.
PfEMP1 is exposed on the surface of each mutant cell line
Because surface-exposed PfEMP1 confers adherence to endothelial cells, the most likely explanation for reduced cytoadherence under physiologically relevant flow conditions in transgenic P falciparum-infected erythrocytes would be impairment of PfEMP1 trafficking to the surface. Therefore, we treated the cells with trypsin for cleavage of surface proteins (ie, PfEMP1).38,39 The cytosolic pool in the infected erythrocyte is not digested by trypsin and corresponds to a protein band more than 200 kDa8 using αATS antibodies. In contrast, cleaved PfEMP1 exposed on the erythrocyte surface is detectable with αATS antibody as a band of approximately 80 kDa.38 Uninfected erythrocytes were used in the same assay as a control to detect red-cell cytoskeletal contaminants, which cross-react with the antibody. Furthermore, the same extraction procedure was applied to non-trypsin-treated cells to test for trypsin activity in the assay. All transgenic lines as well as the parent showed similar amounts of cleaved product of the expected size after trypsin treatment, which was absent in untreated samples. These data demonstrated that trafficking and translocation of PfEMP1 onto the erythrocyte surface is neither impaired by truncation of KAHRP nor the absence of knobs. To ensure erythrocytes were intact during the trypsin treatment we stripped the Western blots and reprobed with αKAHRP antibody. The KAHRP antibody detected the same protein pattern as shown in Figure 2B with 1 band for each mutant cell line consistent with the integrity of the cells during trypsin treatment (data not shown).
Discussion
P falciparum induces dramatic modifications of its host cell during development by exporting a large number of proteins and these changes are pivotal for the parasite's survival. One of the most important modifications of the host cell is the development of knob structures, protrusions of the membrane displayed over the surface of P falciparum-infected erythrocytes that act as submembranous attachment points and organizing regions for proteins involved in cytoadherence of infected erythrocytes.40 The parasite ligand, PfEMP1, which is concentrated on knobs, is responsible for adherence to host receptors. Removal or deletion of important regions of KAHRP involved in these interactions and the structure of the knobs would weaken tethering to the membrane and the membrane skeleton. Our results show that the C-terminal repeat region of KAHRP is required for formation of fully functional knobs. Furthermore, elasticity of the membrane changes dramatically suggesting this region is important in cross-linking to other components such as the host-cell cytoskeleton.
To study the role of individual regions of KAHRP in knob formation and cytoadherence in vivo we generated cell lines with mutated versions of the protein. The endogenous gene was disrupted in 3D7-infected erythrocytes and replaced by shorter versions of the KAHRP gene. Our microscopy studies demonstrated the importance of the C-terminal repeats of KAHRP in knob formation. In the presence of the 5′ repeats elevations in certain areas on the surface are initiated but both 5′ and 3′ repeats are necessary for formation of full knobs. These results suggest specific domains are required for full knob formation because these surface protrusions only become prominent when the full 5′ and 3′ domains are present rather than a gradual increase in size of the protrusions as the length of KAHRP increased in the transgenic parasites. We have shown previously that the first 119 amino acids of KAHRP, including the His-rich region, in P falciparum transfectants was sufficient for trafficking to the erythrocyte membrane and partial association with knobs although this was in the presence of endogenous KAHRP.12 Using fluorescence recovery after photobleaching (FRAP) with this GFP chimera, we showed the protein was associated with the membrane or cytoskeleton. In our study, the K119 protein by itself does not form electron dense structures underneath the membrane and these cells do not display knobs, suggesting the protein is distributed evenly underneath the erythrocyte surface. It has been shown previously that the His-rich region of KAHRP associates with the ATS of PfEMP1 and the truncated K119 protein may display a rim fluorescence through interaction with this cytoplasmic domain.20
Earlier immunoelectron microscopy studies have shown KAHRP concentrates as electron-dense material within knobs.10,11 In this work, TEM revealed that in the presence of the His-rich region and part of the 5′ repeat region of KAHRP (K405) electron-dense material accumulated underneath the erythrocyte membrane. This is consistent with the 5′ repeats being the domain required for self-association of KAHRP in certain areas; however, the required elements for elevation of the knob structure allowing protrusion of the host-cell membrane are not present. Once the full 5′ repeat region and part of the intervening sequence were included (K530), elevations were visible. These data are consistent with in vitro studies where the strongest binding region to the major cytoskeletal element spectrin was mapped to the C-terminal end of the 5′ repeats and the intervening region.18 This work showed that 5′ repeats alone were not able to bind spectrin. Also, a recombinant 5′ repeat region of KAHRP forms stable complexes with actin and spectrin, the 2 major components of the red-cell cytoskeletal meshwork.16 This fragment also developed knoblike structures in vitro, which associated with erythrocyte membranes. Our results suggest the 5′ repeat region of KAHRP is an important domain for self-association and linkage to the cytoskeleton of the host cell. The observed foci of electron-dense material underneath the erythrocyte membrane may be nodular regions where cytoskeletal elements such as spectrin, actin, and band 4.1 are joined. The distance between knobs of P falciparum-infected erythrocytes in our AFM studies was 30 to 50 nm and this corresponds to the distance between spectrin-actin band 4.1 junctions.41,42 In isolated cytoskeleton preparations KAHRP localizes to microfilaments and accumulates in these junctional regions.41,42 Another in vitro study demonstrated binding of recombinant KAHRP to isolated spectrinactin band 4.1 complexes17 and the binding region of KAHRP to actin has been mapped.16 The electron-dense material may therefore be where KAHRP interacts with these cytoskeletal proteins and changes its overall density properties. Previous atomic force microscopy on P falciparum-infected erythrocytes has demonstrated the knob structure has a positive charge, whereas the rest of the erythrocyte surface is negatively charged.43 The protrusion of the knob with the cytoadherence molecule PfEMP1 may be necessary to elevate this positive charge for adherence to negatively charged endothelial plasma membranes.44 Other mechanisms of interaction, which are not related to differences in charge, might also be involved.
Our AFM studies show that in absence of C-terminal repeat regions there is no knob structure detectable on the surface of the infected cells. The 3D7-K405 mutants, which contain part of the 5′ repeats, showed a few elevations that were raised slightly above the ruffled surface of uninfected cells and the mutants with a shorter KAHRP (K119). The knobs formed from full-length KAHRP showed an average diameter of 120 nm and height of 17.5 nm, in accordance with earlier studies on P falciparum-infected erythrocytes.45 It has been reported that knobs are not single entities but composed of a small and a larger subunit,43 although a second study suggested these pre-knobs were artifacts from a faulty or double probe tip.46 We have looked at the knob structure formed from full-length KAHRP in detail and found small elevations (preknobs) are often associated with the knob proper suggesting they are not artifacts. In the case of a technical artifact, all pre-knobs should be visible on the same side of the knob and not randomly distributed around the main protuberances. These pre-knobs were hard to distinguish from the rest of the ruffled erythrocyte surface and therefore might not have been noticed in previous studies.
After invasion of merozoites into erythrocytes, the membrane properties of the host cell dramatically change.31,47 The maturation of the parasite inside its host leads to a reduced deformability of the erythrocyte membrane. This is due to the interaction of exported parasite proteins trafficked across the erythrocyte cytosol with various cytoskeletal proteins, which form a dense meshwork underneath the host-cell membrane. The interaction of KAHRP with components of the cytoskeleton influence the rigidity of the membrane.19,31 We show here that 3D7-K119 KAHRP truncation provides half the rigidity of the full-length protein suggesting that the His-rich region interacts with the erythrocyte cytoskeleton either directly or indirectly through the ATS of PfEMP1. Importantly, the 3D7-K405 cells showed a further increase in rigidity consistent with multiple interactions of KAHRP with host-cell and parasite components. These results further support the importance of KAHRP in membrane rigidity at the trophozoite stage. Furthermore, it shows that even before knobs are formed, or in the absence of knobs, the expression of KAHRP has an impact on membrane elasticity. It has been suggested that interaction of KAHRP with spectrin plays a major role in the decreased deformability of P falciparum-infected erythrocytes possibly by cross-linking spectrin tetramers.31 Because the 3D7-K405 mutants express part of the 5′ repeat, which appears not to be involved in spectrin binding of KAHRP,18 other protein-protein interactions have to take part in this process. The interaction of KAHRP with the cytoadherence ligand PfEMP1, which is anchored in the knob structure via this protein, could be important in increased rigidity of the erythrocyte membrane.
PfEMP1 trafficking is not impaired in the P falciparum transfectants expressing different truncated version of the KAHRP protein. Both transfectants with the shortest and full-length KAHRP showed rim fluorescence consistent with erythrocyte membrane association of PfEMP1. Furthermore, we were able to distinguish between cytoplasmic and surface-exposed PfEMP1 and show that lines expressing different KAHRP truncations had similar amounts transferred to the surface of the cell. However, the mutant cells displayed significant differences in abilities to adhere to CD36 and the amount of PfEMP1 on the surface did not account for these differences. Previous studies have shown knobless cells can adhere in static assays; however, under physiologic flow conditions their ability to bind is reduced.8 Furthermore, evidence has been provided in in vitro studies that all 3 repeat regions of KAHRP can bind separately to the cytoplasmic region of PfEMP1.48 We have established that full adherence properties under flow conditions require a KAHRP protein with all 3 repeat regions. Deletion of the 3′ repeat region leads to a 50% decrease in cytoadherence and further disruption of the 5′ repeat region to 80% loss in adherence. Interestingly, even in the absence of the last 65 C-terminal residues the cytoadherence capacity is decreased by about 40% compared to cells expressing full-length KAHRP. An explanation might be the high concentration of basic residues that occurs predominantly in the 5′ repeat region and the C-terminal end. These basic amino acids would form strong electrostatic interactions with the highly acidic ATS region of PfEMP1 and anchor the molecule tightly within the knob. Another explanation may be that various KAHRP regions cross-link more than one PfEMP1 and, therefore, concentrate adherence molecules.
In the absence of KAHRP and knobs PfEMP1 can be transported and displayed on the surface of the P falciparum-infected erythrocyte. The knobs act as a raised platform for display of PfEMP1 molecules attached to the host-cell cytoskeleton perhaps via interaction with KAHRP, which may be linked to spectrin in the erythrocyte cytoskeleton. The physical consequence of these interactions is that extensional forces applied to PfEMP1 are distributed over the surface area of the knob and increase the effective force that can be applied to PfEMP1 without its physical removal from the membrane. This explains the decreased adherence capacity of the mutant P falciparum-infected erythrocytes under flow conditions. Whilst the same amount of PfEMP1 is on the surface of a knob-impaired erythrocyte the protein is not displayed on the raised knob structure and interaction with the cytoskeleton is reduced.
It has been reported that in absence of knobs PfEMP1 is still clustered on the surface of erythrocytes.49 Although this may be due to cross-linking of the antibody used in immunoelectron microscopy, it is possible that PfEMP1 is clustered in some discrete regions of the membrane. It was also observed that the amount of PfEMP1 on the surface of knobless cells was approximately half that of parasites expressing KAHRP and knobs.49 These experiments were performed on P falciparum parasites lacking the subtelomeric region of chromosome 2 including KAHRP and PfEMP3 as well as other telomeric genes. Our results suggest that in the absence of functional KAHRP equivalent amounts of PfEMP1 are trafficked to and displayed on the erythrocyte membrane. Previous results have suggested that PfEMP3 is not required for trafficking and adherence properties of PfEMP1.38 This implies that the proteins responsible for decreased trafficking of PfEMP1 to the erythrocyte surface is neither KAHRP nor PfEMP3 and must be encoded by another gene lost by deletion of the end of chromosome 2. Interestingly, there are 2 genes within this region encoding DNAJ proteins50 that may act as co-chaperones of PfEMP1. We are currently in the process of analyzing these genes further in view of their potential contribution to PfEMP1 transport.
In conclusion, this study demonstrates the essential role for specific domains of KAHRP in formation of knobs, anchoring of PfEMP1 in the structure, and maintaining the physical properties of the P falciparum-infected erythrocyte and is consistent with independent studies using other methodologies.16-18,20,48 Knowledge of parasite-host interactions, especially with regard to the ability of these infectious agents to adhere to specific tissue and the resulting pathologic consequences, may help in development of drugs that interfere with these processes.
Supplementary Material
Acknowledgments
We thank Mike Duffy, Tim Byrne, and Hans-Peter Beck for antibodies and Simon Crawford for assistance with electron microscopy. We would also like to thank the Red Cross Blood Service (Melbourne, Australia) for supplying erythrocytes and serum.
Prepublished online as Blood First Edition Paper, February 28, 2006; DOI 10.1182/blood-2005-11-4624.
Supported by the National Institutes of Health grant R01-A144008-04A1, the Wellcome Trust, and the National Health and Medical Research Council of Australia. A.F.C. is an International Scholar of the Howard Hughes Medical Institute. The Particulates Fluids Processing Centre is established and supported under the Australian Research Council's Research Centres Programme.
M.R. performed experiments, analyzed data, designed experiments, and wrote the manuscript; S.W.P. performed experiments, analyzed data, and designed experiments; K.M.F. performed experiments and analyzed data; B.M.C. designed and performed experiments and analyzed data; and A.F.C. designed experiments and contributed to writing the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434: 214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119: 385-401. [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke BM, Mohandas N, Coppel RL. Malaria and the red blood cell membrane. Semin Hematol. 2004;41: 173-188. [DOI] [PubMed] [Google Scholar]
- 4.Baruch DI, Rogerson SJ, Cooke BM. Asexual blood stages of malaria antigens: cytoadherence. Chem Immunol. 2002;80: 144-162. [DOI] [PubMed] [Google Scholar]
- 5.Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82: 89-100. [DOI] [PubMed] [Google Scholar]
- 6.Baruch DI, Pasloske BL, Singh HB, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82: 77-87. [DOI] [PubMed] [Google Scholar]
- 7.Smith JD, Chitnis CE, Craig AG, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82: 101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crabb BS, Cooke BM, Reeder JC, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89: 287-296. [DOI] [PubMed] [Google Scholar]
- 9.Raventos SC, Kaul DK, Macaluso F, Nagel RL. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985;82: 3829-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culvenor JG, Langford CJ, Crewther PE, et al. Plasmodium falciparum: identification and localization of a knob protein antigen expressed by a cDNA clone. Exp Parasitol. 1987;63: 58-67. [DOI] [PubMed] [Google Scholar]
- 11.Pologe LG, Pavlovec A, Shio H, Ravetch JV. Primary structure and subcellular localization of the knob-associated histidine-rich protein of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1987;84: 7139-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickham ME, Rug M, Ralph SA, et al. Trafficking and assembly of the cytoadherence complex in Plasmodium falciparum-infected human erythrocytes. EMBO J. 2001;20: 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306: 1930-1933. [DOI] [PubMed] [Google Scholar]
- 14.Hiller NL, Bhattacharjee S, van Ooij C, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306: 1934-1937. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Estrano C, Bhattacharjee S, Harrison T, Haldar K. Cooperative domains define a unique host cell-targeting signal in Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 2003;100: 12402-12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilejian A, Rashid MA, Aikawa M, Aji T, Yang YF. Selective association of a fragment of the knob protein with spectrin, actin and the red cell membrane. Mol Biochem Parasitol. 1991;44: 175-182. [DOI] [PubMed] [Google Scholar]
- 17.Oh SS, Voigt S, Fisher D, et al. Plasmodium falciparum erythrocyte membrane protein 1 is anchored to the actin-spectrin junction and knob-associated histidine-rich protein in the erythrocyte skeleton. Mol Biochem Parasitol. 2000;108: 237-247. [DOI] [PubMed] [Google Scholar]
- 18.Pei X, An X, Guo X, Tarnawski M, Coppel R, Mohandas N. Structural and functional studies of interaction between Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and erythrocyte spectrin. J Biol Chem. 2005;280: 31166-31171. [DOI] [PubMed] [Google Scholar]
- 19.Cooke BM, Mohandas N, Coppel RL. The malaria-infected red blood cell: structural and functional changes. Adv Parasitol. 2001;50: 1-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waller KL, Nunomura W, Cooke BM, Mohandas N, Coppel RL. Mapping the domains of the cytoadherence ligand Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) that bind to the knob-associated histidine-rich protein (KAHRP). Mol Biochem Parasitol. 2002;119: 125-129. [DOI] [PubMed] [Google Scholar]
- 21.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature. 2000;403: 906-909. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Kirkman LA, Wellems TE. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc Natl Acad Sci U S A. 1996;93: 1130-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabb BS, Cowman AF. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1996;93: 7289-7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci U S A. 1997;94: 10931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coppel RL, Bianco AE, Culvenor JG, et al. A cDNA clone expressing a rhoptry protein of Plasmodium falciparum. Mol Biochem Parasitol. 1987;25: 73-81. [DOI] [PubMed] [Google Scholar]
- 26.Baruch DI, Gormley JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1996;93: 3497-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rug M, Wickham ME, Foley M, Cowman AF, Tilley L. Correct promoter control is needed for trafficking of the ring-infected erythrocyte surface antigen to the host cytosol in transfected malaria parasites. Infect Immun. 2004;72: 6095-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowakowski R, Luckham P, Winlove P. Imaging erythrocytes under physiological conditions by atomic force microscopy. Biochim Biophys Acta. 2001;1514: 170-176. [DOI] [PubMed] [Google Scholar]
- 29.Cooke BM, Coppel RL, Nash GB. Analysis of the adhesive properties of Plasmodium falciparum-infected red blood cells under conditions of flow. Methods Mol Med. 2002;72: 561-569. [DOI] [PubMed] [Google Scholar]
- 30.Cooke BM, Coppel RL, Nash GB. Preparation of adhesive targets for flow-based cytoadhesion assays. Methods Mol Med. 2002;72: 571-579. [DOI] [PubMed] [Google Scholar]
- 31.Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood. 2002;99: 1060-1063. [DOI] [PubMed] [Google Scholar]
- 32.Triglia T, Stahl HD, Crewther PE, et al. The complete sequence of the gene for the knob-associated histidine-rich protein from Plasmodium falciparum. EMBO J. 1987;6: 1413-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979;76: 4650-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aikawa M. Studies on falciparum malaria with atomic-force and surface-potential microscopes. Ann Trop Med Parasitol. 1997;91: 689-692. [DOI] [PubMed] [Google Scholar]
- 35.Baruch DI, Ma XC, Singh HB, Bi X, Pasloske BL, Howard RJ. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90: 3766-3775. [PubMed] [Google Scholar]
- 36.Cooke BM, Glenister FK, Mohandas N, Coppel RL. Assignment of functional roles to parasite proteins in malaria-infected red blood cells by competitive flow-based adhesion assay. Br J Haematol. 2002;117: 203-211. [DOI] [PubMed] [Google Scholar]
- 37.Spycher C, Klonis N, Spielmann T, et al. MAHRP-1, a novel Plasmodium falciparum histidine-rich protein, binds ferriprotoporphyrin IX and localizes to the Maurer's clefts. J Biol Chem. 2003;278: 35373-35383. [DOI] [PubMed] [Google Scholar]
- 38.Waterkeyn JF, Wickham ME, Davern K, et al. Targeted mutagenesis of Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) disrupts cytoadherence of malaria-infected red blood cells. EMBO J. 2000;19: 2813-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papakrivos J, Newbold CI, Lingelbach K. A potential novel mechanism for the insertion of a membrane protein revealed by a biochemical analysis of the Plasmodium falciparum cytoadherence molecule PfEMP-1. Mol Microbiol. 2005;55: 1272-1284. [DOI] [PubMed] [Google Scholar]
- 40.Luse SA, Miller LH. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971;20: 655-660. [PubMed] [Google Scholar]
- 41.Taylor DW, Parra M, Chapman GB, et al. Localization of Plasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol. 1987;25: 165-174. [DOI] [PubMed] [Google Scholar]
- 42.Taylor DW, Parra M, Stearns ME. Plasmodium falciparum: fine structural changes in the cytoskeletons of infected erythrocytes. Exp Parasitol. 1987;64: 178-187. [DOI] [PubMed] [Google Scholar]
- 43.Aikawa M, Kamanura K, Shiraishi S, et al. Membrane knobs of unfixed Plasmodium falciparum infected erythrocytes: new findings as revealed by atomic force microscopy and surface potential spectroscopy. Exp Parasitol. 1996;84: 339-343. [DOI] [PubMed] [Google Scholar]
- 44.Marchenko SM, Sage SO. Electrical properties ofresting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993;462: 735-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruenberg J, Allred D, Sherman I. Scanning electron microscope-analysis of the protrusions (knobs) present on the surface of Plasmodium falciparum-infected erythrocytes. J Cell Biol. 1983;97: 795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagao E, Kaneko O, Dvorak JA. Plasmodium falciparum-infected erythrocytes: qualitative and quantitative analyses of parasite-induced knobs by atomic force microscopy. J Struct Biol. 2000;130: 34-44. [DOI] [PubMed] [Google Scholar]
- 47.Parker PD, Tilley L, Klonis N. Plasmodium falciparum induces reorganization of host membrane proteins during intraerythrocytic growth. Blood. 2004;103: 2404-2406. [DOI] [PubMed] [Google Scholar]
- 48.Waller KL, Cooke BM, Nunomura W, Mohandas N, Coppel RL. Mapping the binding domains involved in the interaction between the Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and the cytoadherence ligand P. falciparum erythrocyte membrane protein 1 (PfEMP1). J Biol Chem. 1999;274: 23808-23813. [DOI] [PubMed] [Google Scholar]
- 49.Horrocks P, Pinches RA, Chakravorty SJ, et al. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J Cell Sci. 2005;118: 2507-2518. [DOI] [PubMed] [Google Scholar]
- 50.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419: 498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.