Abstract
Organ allografts have been shown to provide a syngeneic microenvironment for organ-based donor hematopoietic stem cells to maintain long-lasting chimerism after transplantation. We hypothesized that organ allografts would also support engraftment and hematopoiesis of adjunctively infused donor marrow stem cells, syngeneic to organ grafts, in nonmyeloablated recipients. In BN-to-LEW and GFP-to-ACI rat combinations, donor bone marrow (BM) infusion together with small intestine transplantation (SITx) under short-course tacrolimus immunosuppression resulted in persistent macrochimerism (more than 5%) for 150 days. In contrast, after BM infusion or SITx alone, chimerism was temporary and disappeared by day 100. Y-chromosome polymerase chain reaction (PCR) in sex-mismatched male BM plus female intestine or female BM plus male intestine transplantation into female recipients suggested that persistent macrochimerism was derived from infused BM. BM infusion together with lymphoid-depleted intestine grafts also supported macrochimerism development; however, third-party intestine grafts did not. After GFP-positive BM plus wild-type (WT) SITx into ACI, large numbers of GFP-positive leukocytes were found in WT intestine grafts. Isolated cells from WT intestine grafts developed GFP-positive CFU-Cs and propagated multilineage GFP-positive leukocytes when adoptively transferred into lethally irradiated WT recipients. These findings suggest that intestine allograft supports simultaneously infused donor (syngeneic to organ grafts) marrow stem cell engraftment, differentiation, and persistence of chimerism.
Introduction
Long-lasting microchimerism was found in organ allograft recipients treated with conventional immunosuppression in clinical and experimental studies.1,2 The longevity (decades in humans) and multilineage features of persisting donor leukocytes in organ allograft recipients suggest the presence of hematopoietic stem cells in organ allografts and their proliferation/differentiation after transplantation.3,4 This finding is consistent with recent numerous reports demonstrating that stem cells are present in multiple adult tissues; however, the identification, behavior, and characterization of the specific stem cells in adult tissues outside of the bone marrow (BM) have not been established.5,6
Apart from microchimerism associated with organ transplantation, the establishment of hematopoietic (macro)chimerism has been long known to associate with stable donor-specific immunologic tolerance,7,8 and the creation of hematopoietic chimera using donor marrow stem cells has been an attractive approach in the field of organ transplantation to obtain drug-free allograft acceptance.9-11 Because this procedure requires the repopulation of the hematopoietic stem cell compartment with infused donor marrow cells after host myeloablative conditioning, the engraftment of donor stem cells into host marrow microenvironment is the crucial element in success of the strategy. In this regard, the marrow microenvironment, recognized as “niches,” has important roles by providing a proximate relationship of stem cells with stroma cells and extracellular matrix.12,13 Previous experiments demonstrated that transplantation of the microenvironment together with marrow stem cells permitted stable marrow stem cell engraftment without a recipient conditioning regimen; bone fragments under the kidney capsule, vascularized bone grafts as a part of composite hind limb, or vascularized sternum grafts resulted in stable hematopoietic chimerism.14-16 The comparable finding in organ transplantation is that the organ allograft parenchyma provides a syngeneic microenvironment for organ-based hematopoietic stem cells for the maintenance of multilineage chimerism after solid organ transplantation.17
Considering the significance of the relationship between stem cells and the microenvironment, we hypothesized that organ allografts would provide a syngeneic microenvironment not only for the organ-based donor stem cells but also to adjunctively infused donor marrow cells. Accordingly, using a small intestine transplantation (SITx) model, this study examined the roles of allograft parenchyma in supporting the engraftment of simultaneously infused allogeneic marrow hematopoietic stem cells. The results demonstrated that the engraftment was negligible when allogeneic marrow cells were infused alone into noncytoablated recipients under the coverage of tacrolimus immunosuppression; however, donor marrow stem cell engraftment and persistent multilineage macrochimerism were achieved when donor BM was infused together with intestine allografts. Simultaneously infused donor marrow stem cells preferably engrafted into intestine grafts. The study suggests that the presence of intestine allografts supports the engraftment of allogeneic marrow hematopoietic stem cells into major histocompatibility complex (MHC)–matched microenvironment of intestine grafts.
Materials and methods
Animals
Green fluorescent protein (GFP)–transgenic and wild-type (WT) Sprague Dawley (SD) rats originally generated by Dr Masaru Okabe (University of Osaka, Japan)18-20 were obtained from Japan SLC (Hamamatsu, Japan). The expression of GFP was under the control of the cytomegalovirus enhancer and the chicken β-actin promoter derived from an expression vector, pCAGGS.18-20 Inbred BN (RT1n), LEW (RT1l), and ACI (RT1a) rats weighing 180 to 250 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). All animals were maintained in a laminar flow animal facility at the University of Pittsburgh and fed with a standard diet ad libitum. All procedures in this experiment were performed according to the guidelines of the Council on Animal Care at the University of Pittsburgh and the National Research Council's Guide for the Humane Care and Use of Laboratory Animals.
Transplantation procedures
Orthotopic SITx with caval drainage was performed as previously described.21 The donor small intestine from the ligament of Treitz to the ileocecal valve was isolated on a vascular pedicle consisting of the portal vein and of the superior mesenteric artery in continuity with a segment of aorta. The graft was perfused via the aortic segment with 5 mL chilled lactated Ringer solution, and the intestinal lumen was irrigated with 20 mL cold saline solution containing 0.5% neomycin sulfate (Sigma, St Louis, MO) The entire recipient intestine, including gut-associated lymphoid tissue (GALT), was removed and end-to-side anastomoses between the graft aorta and the recipient infrarenal aorta and between the graft portal vein and host vena cava were performed. Enteric continuity was restored by proximal and distal end-to-end intestinal anastomoses. All recipient animals were given 20 mg/d prophylactic cefamandole nafate for 3 postoperative days.
Bone marrow isolation and infusion
Unfractionated BM cells were obtained by flushing the tibias and femurs. Cells were processed with RPMI 1640; supplemented with 25 mM HEPES, 2mM l-glutamine, 50 μg/mL gentamicin, and 10% fetal bovine serum (all from Life Technologies, Grand Island, NY); and then passed through nylon mesh to separate the remaining connective tissue fragments.22 Trypan blue exclusion testing uniformly showed more than 95% cell viability. A total of 2.5 × 108 viable cells were intravenously injected into the recipient via the jugular or penile vein.22,23
Reagents and procedures
Tacrolimus (TAC) (Astellas Pharma, Tokyo, Japan) was administered intramuscularly with a daily dosage of 1.0 mg/kg on day 0 to day 13 with additional single doses on day 20 and day 27.22 Rabbit anti–rat lymphocyte serum (ALS) (Accurate Chemical & Scientific, Westbury, NY) was intraperitoneally injected for 3 days with a daily dose of 1.0 mL. This dose of ALS effectively decreased circulating T cells to less than 5%.24 Ex vivo irradiation of the harvested intestine graft (9.5 Gy) in cold lactate Ringer solution in a 50 mL Falcon tube was performed using a 137Cs source (Gammacell 1000 Elite; Nordion International, Kanata, ON, Canada).23
Flow cytometry
Affinity-purified biotinylated rat monoclonal antibodies (mAbs) 163 (rat IgG2b) and 42 (rat IgG2a) were used to detect RT1Al (MHC class I) on LEW and RT1An antigens on BN, respectively.25 Phycoerythin (PE)–conjugated streptavidin (PharMingen, San Diego, CA) was used as a secondary antibody. Lineages of leukocytes were analyzed with fluorescent-conjugated mAbs R7.3 (αβTCR; PharMingen), anti–rat IgM (Organon Teknika, Durham, NC), OX33 (CD45RA, B cells; PharMingen), NK3.2.3 (NKR-P1, natural killer [NK] cells; PharMingen), ED1 (macrophages CD68; Serotec, Kidlington, Oxford, United Kingdom), ED2 (macrophage CD163; Serotec), and OX42 (CD11b; Serotec). The samples were fixed in paraformaldehyde and analyzed on a Coulter Elite ESP (Coulter, Miami, FL). Isotype-matched nonspecific antibodies were used for the control.
Routine histopathology and immunohistopathology
Formalin-fixed tissues were embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. The slides were blindly reviewed by one of the authors (M.A.N.) without knowledge of experimental groups. The degree of epithelial injury of intestine allografts was determined by the frequency of apoptosis in crypt epithelial cells. The numbers of lymphoid nodules and Peyer patches (PPs) in the intestine were counted and expressed as the number per section. The presence of mesenteric fibrosis in mesenteric lymph nodes (MLNs) and severity of mesenteric arteriopathy were graded as 0 (none), 1 (mild), and 2 (severe).
Cryosections were stained with immunofluorescent technique using OX27 (1:200; Serotec) that recognized BN, but not LEW, MHC class I antigens. Double staining was with OX27 followed by PE-conjugated anti–mouse IgG and FITC-conjugated anti–rat IgM. To detect GFP-positive cells in tissues, samples were obtained by perfusing animals via the abdominal aorta with phosphate-buffered saline (PBS) followed with 2% paraformaldehyde in PBS. Tissue samples were stored in 2% paraformaldehyde for several hours at 4°C, cryoprotected in 2.3 M sucrose in PBS overnight, embedded in OCT compound, and frozen in liquid nitrogen–cooled isopentane. Samples were cut into 6-μm sections, nuclear DNA stained with Hoechst dye (bisbenzimide), and visualized with an Olympus BX51 epifluorescence microscope (Malvern, NY) equipped with an Olympus MagnaFire™ CCD camera (Goleta, CA) and interfaced with MagnaFire image software. GFP-positive cells were counted per 500 nuclei in 8 to 10 high-power fields (HPF) in each section, and the result was expressed as the percentage of GFP-positive cells.
Real-time PCR
Real-time polymerase chain reaction (PCR) for sex-determining region of Y chromosome (SRY) gene was used to determine the concentration of male cells in the sample. Genomic DNA of peripheral blood mononuclear cells (PBMCs) and recipient tissues were prepared using QIAamp kit (Qiagen, Chatsworth, CA) as described by the manufacturer. PCR reaction mixture was prepared using SYBR green PCR master mix (Perkin Elmer, Foster City, CA) using the primers of AAGTCAAGCGCCCCATGA (sense) and TGAGCCAACTTGTGCCTCTCT (antisense).26,27 The reactions were performed using an ABI7000 Prism Sequence Detection System (Perkin Elmer). The thermal cycler was configured as the following: incubation (95°C, 10 minutes), 40 cycles of denaturation (95°C, 15 seconds), and annealing and extension (60°C, 60 seconds). The standard curves for the presence of SRY were prepared by mixing male and female DNA in various proportions (100%, 20%, 4%, 0.8%, 0.16%). Each run consisted of standard and a negative control without template.
CFU-C assay
Leukocytes from MLNs were isolated by injecting RPMI 1640 into MLNs and by filtration through nylon mesh. Lymphocytes in PPs were obtained as previously described.28 Briefly, after the mucosal layer of the intestine was disrupted, PPs were excised; cut into small pieces; digested in a water bath for 30 minutes at 37°C with RPMI 1640 containing 0.05% collagenase (type B; Boehringer Mannheim, Mannheim, Germany), 2% FBS, 10 mM HEPES, and 50 μg/mL gentamicin (Life Technologies); and passed through nylon mesh to separate the remaining connective tissue fragments from leukocyte fraction. Isolated cells were washed twice with RPMI 1640 containing 5% FBS and further purified by centrifugation over Ficoll-Paque (specific gravity, 1.077; Amersham Biosciences, Uppsala, Sweden). The interface was collected and washed twice with RPMI 1640 containing 5% FBS.
Isolated leukocytes from MLNs and PPs were resuspended in Iscove modified Dulbecco medium with 2% FBS (2% IMDM) (StemCell Technologies, Vancouver, BC, Canada). The cells (1.5 × 104) in 0.1 mL of 2% IMDM were mixed with 1.0 mL methylcellulose-based media in the presence of stem cell factor, GM-CSF, and IL-3 (MethoCult, StemCell Technologies). A total suspension volume of 1.1 mL was plated in a 35-mm dish. These dishes were placed in 150-mm dishes with 3 to 4 mL sterile water for humidity and incubated at 37°C with 5% CO2 and at least 95% humidity. At 10 days of culturing, colonies (more than 30 cells per aggregate) were counted as CFU-C counts by inverted microscope and an Olympus SZ×12 fluorescence dissecting microscope for confirmation of GFP-positive colonies.
Statistical analysis
All data in this study were expressed as mean ± SD. Results of flow cytometry and histopathology were analyzed by 1-way analysis of variance (ANOVA) and the Fisher projected least significance difference (PLSD) test. A P value of less than .05 was considered to be significant.
Results
Development of macrochimerism with simultaneous BM and intestine transplantation
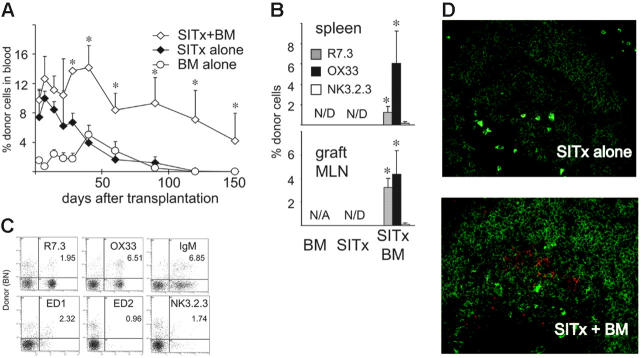
LEW recipients received the intestine, BM, or intestine plus BM from fully allogeneic BN donors under short-course TAC immunosuppression, and the percentages of donor cells in the peripheral blood were sequentially studied by flow cytometry. In recipients of SITx alone, donor cells during the first week after SITx were a mean of 9.9%. However, they gradually decreased and disappeared by day 120. After BM infusion alone, donor cells slowly increased and reached 5.0% ± 1.3% during the second month. Thereafter, chimerism was not maintained, and donor cells disappeared by day 90. In contrast, when BM was infused together with SITx, higher levels of donor cells (10% to 15%) were detected early after SITx, and stable blood chimerism more than 5% was maintained for more than 150 days (Figure 1A).
Figure 1.
Development of multilineage chimerism after simultaneous BM plus SITx, but not after BM infusion or SITx alone, in BN-to-LEW combination under short-course TAC immunosuppression. (A) Flow cytometric analysis of blood chimerism with mAbs for donor MHC class I. Transplantation of intestine alone (n = 10) showed an early high-level chimerism at day 14. Donor cells gradually decreased and disappeared at day 120. After BM cell injection alone (n = 6), donor cells gradually increased with a peak (2% to 6%) at day 40, slowly decreased, and became undetectable by day 90. On the contrary, when BM and intestine were transplanted together (n = 12), augmented macrochimerism (3% to 10%) was maintained for 150 days. *P < .05 versus BM or SITx alone. (B) B-cell–dominant multilineage chimerism was seen in both host spleen and intestinal graft MLNs 150 days after BM infusion plus SITx. Donor cells were not identified in recipients of BM or SITx alone; n = 3 to 7 for each group. *P < .05 versus BM or SITx alone. N/D indicates not detected; N/A, not applicable. (C) Representative flow cytometry of the blood taken at day 150 from 1 of the recipients of BM plus intestine transplantation. Result demonstrates multilineage macrochimerism, including donor T, B, and NK cells and macrophages. (D) Double fluorescent immunohistochemical staining of host spleen with OX27 (BN MHC class I, red) and anti–rat IgM (B cell, green). Abundant OX27-positive donor cells were found in the B-cell area of the spleen at day 150 after BM infusion plus SITx but not after SITx alone (40×/0.75 numeric aperture [NA] oil-immersion objection). Images are representative of 3 animals per group.
Numbers of donor cells in recipient spleen at day 150 correlated well with peripheral blood chimerism levels. No donor cells were found in the recipient spleen after BM or SITx alone, while multilineage donor cells (2% to 8%) were found in the spleens of simultaneous BM plus SITx recipients. The multilineage feature of macrochimerism at day 150 suggests the engraftment of donor stem cells in recipients of BM plus SITx. Interestingly, B cells were a predominant population among donor cells (Figure 1B-C). Immunohistochemistry of the spleen confirmed abundant OX27-positive donor B cells in the splenic B-cell follicles of simultaneous BM plus SITx recipients (Figure 1D).
Intestine allografts are chronic rejection–free and maintain donor leukocytes when BM and intestine are simultaneously transplanted
Histopathologic analysis of allografts at day 150 revealed the development of chronic rejection (CR), including the depletion of lymphoid components and fibrotic changes of GALT, and the presence of arteritis, when the intestine was transplanted alone (Table 1). In accordance with our previous studies,23,24 these changes were completely prevented in intestine allografts with simultaneous BM infusion. The numbers of PPs and lymphoid nodules in intestine grafts of SITx plus BM recipients were similar to those seen in normal intestine, and there was no fibrosis or arteritis in graft MLNs with simultaneous BM infusion.
Table 1.
Histopathologic changes of intestine allografts
|
Allograft histopathology
|
|||||||
|---|---|---|---|---|---|---|---|
|
Intestine
|
MLNs
|
||||||
| Transplant | No. of recipients | Blood chimerism, day 150, % | No. of apoptosis per 10 crypts | No. of PPs per section | No. of lymphoid nodules per section | Fibrosis grade | Arteritis grade |
| Normal intestine | 4 | NA | 0.5 ± 0.6 | 0.30 ± 0.50 | 1.65 ± 0.42 | 0 | 0 |
| Intestine alone | 10 | 0 | 2.0 ± 1.1 | 0.03 ± 0.06 | 0.56 ± 0.50 | 1.00 ± 1.07 | 0.20 ± 0.42 |
| Intestine and BM | 12 | 4.2 ± 3.8* | 1.2 ± 0.5 | 0.28 ± 0.21* | 1.24 ± 0.47 | 0* | 0* |
| Irradiated intestine alone | 5 | 0 | 1.8 ± 0.8 | 0.02 ± 0.02 | 0.48 ± 0.32 | 1.80 ± 0.83 | 0.20 ± 0.45 |
| Intestine from ALS-pretreated donor alone | 7 | 0 | 2.0 ± 1.2 | 0.02 ± 0.05 | 0.84 ± 0.46 | 0.86 ± 0.70 | 0.14 ± 0.24 |
| Irradiated intestine and BM | 8 | 1.9 ± 1.0* | 1.3 ± 0.8 | 0.19 ± 0.16* | 1.00 ± 0.36 | 0* | 0* |
| Intestine from ALS-pretreated donor and BM | 11 | 5.0 ± 3.0* | 2.1 ± 0.5 | 0.20 ± 0.14* | 1.12 ± 0.58 | 0* | 0* |
BN-to-LEW SITx under TAC 1.0 mg/kg on days 0-13, 20, and 27 after SITx. Data represent mean ± SD.
NA indicates not applicable.
P < .05 versus intestine alone (1-way ANOVA and Fisher PLSD).
To investigate levels of chimerism in intestine allografts, single-cell suspension was prepared from graft MLNs. Because of the severe fibrosis of graft MLNs in the SITx-alone group, the numbers of lymphocytes recovered from graft MLNs were not sufficient for flow cytometric analysis. In contrast, graft MLNs in the SITx plus BM group were well populated, and flow cytometry showed a total of 9.3% ± 0.76% donor cells, including donor T, B, and NK cells (Figure 1B).
Origin of macrochimerism after simultaneous intestine and BM transplantation
After we observed that infusion of allogeneic BM with SITx in noncytoablated recipients resulted in persistent macrochimerism, we next determined whether chimeric donor cells in these recipients were derived from the intestine or BM graft using sex-mismatched transplantation. In these experiments, female LEW recipients received either male BN intestine plus female BN BM or female BN intestine plus male BN BM. Using flow cytometry, female LEW recipients of sex-mismatched BN intestine and BM grafts were confirmed to have the similar levels of macrochimerism at day 150 as seen in male recipients. Levels of chimerism derived from male grafts were determined using quantitative real-time PCR for SRY. When male intestine and female BM were transplanted, male DNA was detected early after transplantation and disappeared by 90 days, while flow cytometry detected 3.9% ± 2.0% donor cells at day 150 (Figure 2A). On the contrary, after transplantation of male BM and female intestine, the male DNA concentration gradually increased and became nearly equal to the level of chimerism detected with flow cytometry by day 150. These results indicate that most chimeric donor cells found early after simultaneous intestine and BM transplantation were from intestine allografts. In contrast, BM-derived donor cells gradually increased with time, and donor chimeric cells in the blood at day 150 were largely from the infused BM (Figure 2A-B).
Figure 2.
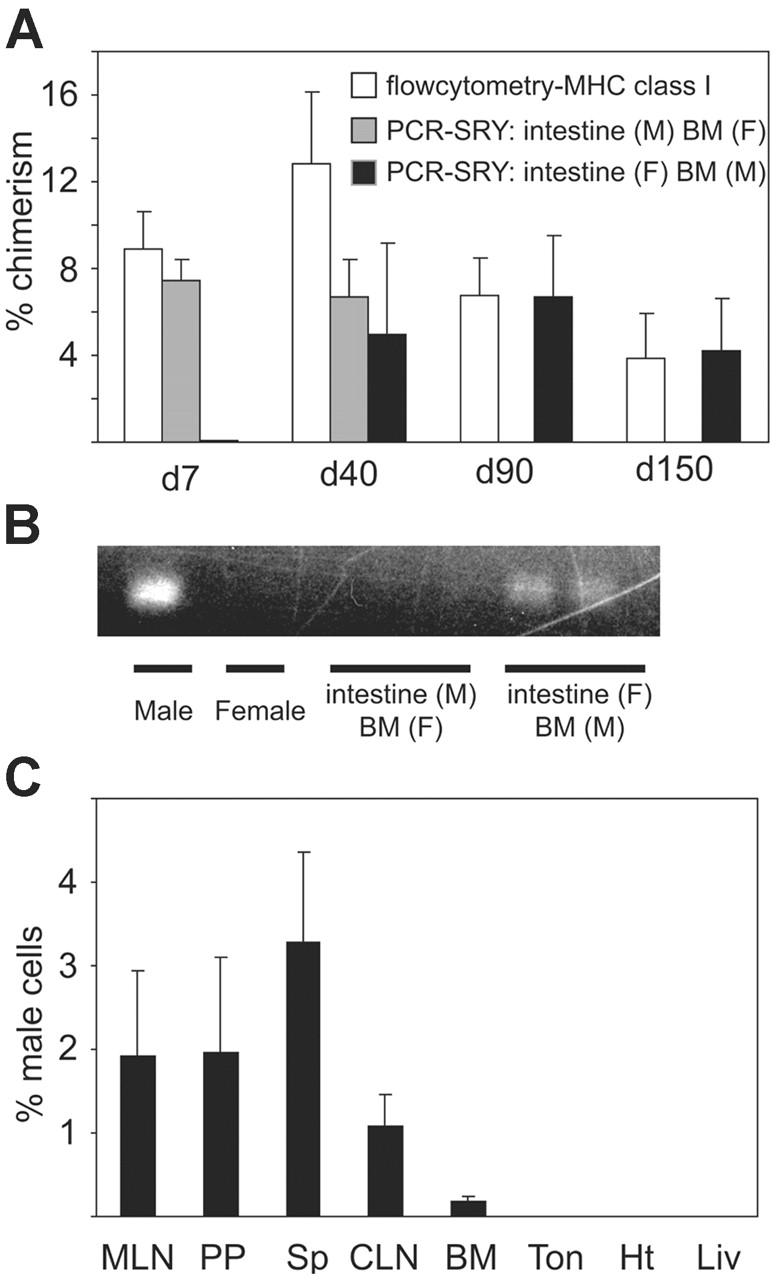
Origin of donor cells in peripheral blood after BN-to-LEW BM plus intestine transplantation. (A) Peripheral blood chimerism in female recipients of male BM plus female intestine or female BM plus male intestine grafts was analyzed by flow cytometry (BN MHC class I) and quantitative real-time Y-chromosome PCR. Results demonstrated that most donor cells in the blood at 7 days after transplantation consisted of passenger leukocytes from intestine allografts. However, intestine graft–derived cells disappear by day 90. In contrast, BM-derived donor cells gradually increased, and almost all donor cells were of infused BM origin at day 90 and day 150; n = 3 to 4 for each group. (B) PCR analysis of PBMCs with primers for SRY at day 150. When female BN intestine and male BN BM were transplanted into female LEW recipients, male DNA band was identified. On the other hand, transplantation of male intestine and female BM resulted in undetectable SRY band in the blood at day 150, while flow cytometry detected 3.9% donor cells, suggesting that macrochimeric donor cells at day 150 were mainly derived from infused donor BM cells. Blot is representative of 2 independent experiments from 3 to 4 rats per group. (C) Real-time PCR analysis for SRY in various female LEW host organs at day 150 after female BN intestine and male BN BM transplantation. Male DNA derived from BN BM was mainly found in graft GALT (PP and MLN), cervical lymph node (CLN), and host spleen (Sp). Very few infused BM-derived cells were found in host heart (Ht), liver (Liv), tongue (Ton), or bone marrow (BM); n = 3 for each group.
BM-derived cells distribute into intestine grafts as well as recipient lymphoid tissues
Distribution of BM-derived BN donor cells in various LEW recipient tissues at day 150 after simultaneous female intestine plus male BM transplantation was analyzed by PCR for SRY. BM-derived male signals were mainly found in graft (female) GALT, including PPs and MLNs. Host spleen also had male signals. On the other hand, very few male signals were detected in host nonlymphoid organs, such as the liver, heart, and tongue. BM contained mostly progenitors with small numbers of mature cells, and recipient BM showed less than 1% donor BM-derived male signals at day 150 (Figure 2C).
Depletion of intestine graft passenger leukocyte does not prevent macrochimerism development
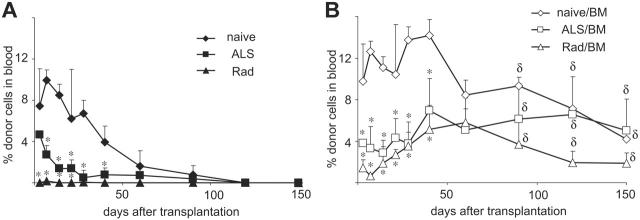
To investigate whether GALT leukocytes in intestine grafts play roles in developing macrochimerism after SITx plus marrow infusion, we next examined sequential changes of blood chimerism after BM infusion and SITx when intestine leukocytes were eliminated by donor ALS pretreatment or ex vivo graft irradiation.23,24 Early chimerism was significantly reduced after transplantation of leukocyte-eliminated intestine grafts alone (0.15% ± 0.01% with ex vivo graft irradiation and 2.75% ± 0.85% with ALS donor pretreatment) compared with those of nondepleted intestine grafts (9.94% ± 0.99%). In all recipients without BM infusion, donor cells quickly disappeared (Figure 3A). When BM was infused together with leukocyte-depleted intestine grafts, early chimerism levels were low; however, donor cells gradually increased, and macrochimerism was maintained with 1.4% to 11.5% at day 150 (Figure 3B). Histopathologically, leukocyte-depleted intestine grafts also were chronic rejection–free when BM was simultaneously infused (Table 1).
Figure 3.
Sequential blood chimerism after BM infusion with leukocyte-depleted intestine grafts. (A) Donor BN cells in LEW recipients of leukocyte-depleted intestine grafts without donor BM infusion became undetectable by day 120. (B) In contrast, donor BM infusion together with transplantation of leukocyte-depleted intestine grafts resulted in persistent macrochimerism. ALS indicates donor ALS pretreatment for 3 days (1.0 mL; day –3 to –1); Rad, ex vivo 9.5 Gy graft irradiation. *P < .05 versus naive SITx (without lymphoid depletion); δP < .05 versus corresponding group without donor BM at the same time point.
GFP BM cells induce macrochimerism when a syngeneic intestine graft is simultaneously transplanted
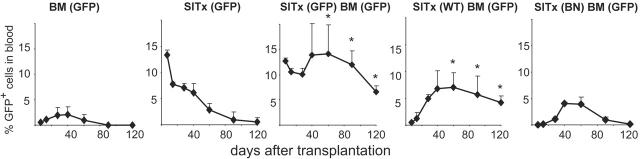
To further confirm the engraftment of BM-derived stem cells and to determine the site of engraftment, we next employed BM plus SITx experiments using GFP transgenic rats. GFP BM was infused into fully allogeneic ACI rats with or without WT intestine grafts under brief TAC immunosuppression. Sequential flow cytometric analysis of GFP-positive cells in host blood samples confirmed the finding in the BN-to-LEW combination model that GFP-positive–infused BM-derived cells gradually increased in recipients and maintained levels above 5% for more than 120 days when BM was infused simultaneously with SITx. After GFP BM infusion or GFP SITx alone, GFP-positive cells were only temporarily seen in the blood and stable macrochimerism was not established (Figure 4).
Figure 4.
Sequential blood chimerism of ACI recipients after transplantation of GFP, WT, or BN (third-party) intestine plus GFP-positive BM under TAC immunosuppression. Similar to results of BN-to-LEW SITx experiments, donor-derived GFP-positive cells gradually decreased with time in recipients that received GFP-positive BM or GFP-positive SITx alone. Transplantation of GFP-positive intestine and GFP-positive BM resulted in stable macrochimerism for 120 days. Stable long-term macrochimerism was also achieved when WT intestine graft and GFP-positive BM were transplanted together. However, cotransplantation of third-party intestine (BN) and GFP-positive BM did not result in a long-lasting macrochimerism. *P < .05 versus BM or SITx alone; n = 4 to 13.
Further, stable long-term macrochimerism was achieved only when BM cells and simultaneously transplanted intestine grafts were syngeneic, because GFP BM infusion together with third-party intestine (BN) did not maintain stable macrochimerism. The result suggested that syngeneic intestine graft supported the engraftment of simultaneously infused marrow stem cells and the development of macrochimerism (Figure 4).
Infused BM-derived GFP-positive cells repopulate intestine grafts and host secondary lymphoid organs
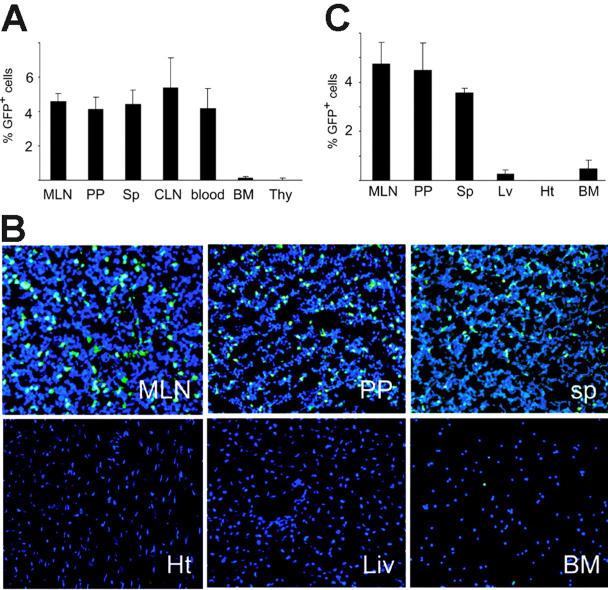
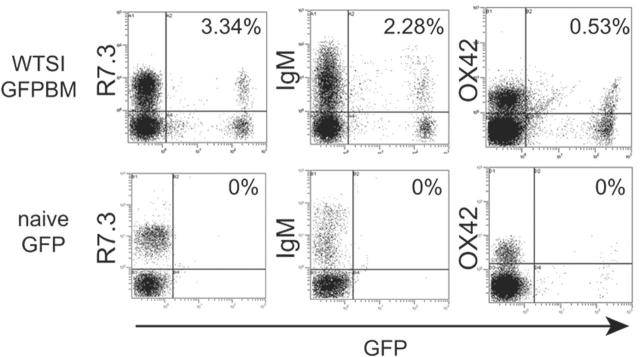
In animals with stable blood macrochimerism at day 120 after GFP BM plus WT SITx, GFP-positive cells were detected by flow cytometry at similar levels (about 5%) in graft GALT (eg, MLNs, PPs) of WT intestine graft as well as host spleen and lymph nodes (Figure 5A). However, GFP-positive cells were remarkably less in host bone marrow and thymus. Location of GFP-positive cells was studied with fluorescent microscopic analysis, and results revealed a homogeneous distribution of GFP-positive cells in the graft lymphoid organs. Few GFP-positive BM-derived cells were seen in the recipient liver, heart, or BM, probably due to less frequency of mature leukocytes in these tissues (Figure 5B-C).
Figure 5.
Distribution of BM-derived GFP-positive cells after GFP-positive BM plus WT intestine transplantation. (A) Flow cytometric analysis of lymphoid organs in recipients of WT intestine plus GFP-positive BM at day 120. From 4% to 6% of GFP-positive cells were seen in the mesenteric lymph nodes (MLN) and Peyer patches (PP) of WT intestine grafts. Host spleen (sp), cervical lymph nodes (CLN), and blood also showed similar levels of macrochimerism. On the other hand, there were few GFP-positive cells in recipient BM and thymus (Thy); n = 4 to 5. (B) Fluorescent microscopic analysis of various organs in the recipients of WT intestine plus GFP-positive BM at day 120. Numerous GFP-positive cells were seen in graft MLNs and PPs as well as host spleen. Only few GFP-positive BM-derived cells were identified in recipient liver (Liv), heart (Ht), or BM. Blue indicates nuclear stain; 40×/0.75 NA oil-immersion objective was used. (C) Percentages of GFP-positive cells. GFP-positive positive cells were counted in the section and expressed as the percentage to the number of nucleus.
Infused marrow-derived stem cells engraft into simultaneously transplanted intestine grafts, which are syngeneic to stem cells
Based on the findings that infused BM-derived multilineage macrochimerism was maintained for more than 150 days when the intestine graft (syngeneic to marrow cells) was simultaneously transplanted, we hypothesized that marrow-derived donor hematopoietic stem cells engrafted into the syngeneic microenvironment provided by intestine grafts for differentiation. To investigate this possibility, we conducted in vitro and in vivo assays to detect infused marrow-derived hematopoietic stem cells in intestine grafts. At day 120 after GFP BM and WT intestine grafts were transplanted into ACI rat recipients under brief TAC immunosuppression, leukocytes were obtained from GALT of WT graft intestine as well as host BM. When BM cells taken from ACI recipients were cultured in a methylcellulose-based culture system for CFU-C assay, about 150 colonies per 1.5 × 105 cells were developed. However, there were very few GFP-positive colonies (1.1 ± 0.32 per 1.5 × 105 cells), and most of the colonies were GFP negative. In contrast, when isolated cells from PPs and MLNs of WT grafts were cultured for CFU-C assay, most of the developed colonies were GFP positive (more than 90%) with frequencies of 6.5 ± 4.1 and 0.9 ± 0.1 per 1.5 × 105 cells, respectively. Although total CFU-C counts of GALT leukocytes were low compared with those of BM cells, GALT of transplanted intestine tended to have more CFU-Cs than naive WT intestine (Figure 6). Nevertheless, the frequency of GFP-positive colonies remained at a similar low level in both host bone marrow and graft GALT. In vitro propagated colonies were mostly composed of ED2-positive macrophages, and negative stain with mAb R7.3 (αβTCR) excluded the possibility that the colonies were due to mixed lymphocyte reactions (data not shown).
Figure 6.
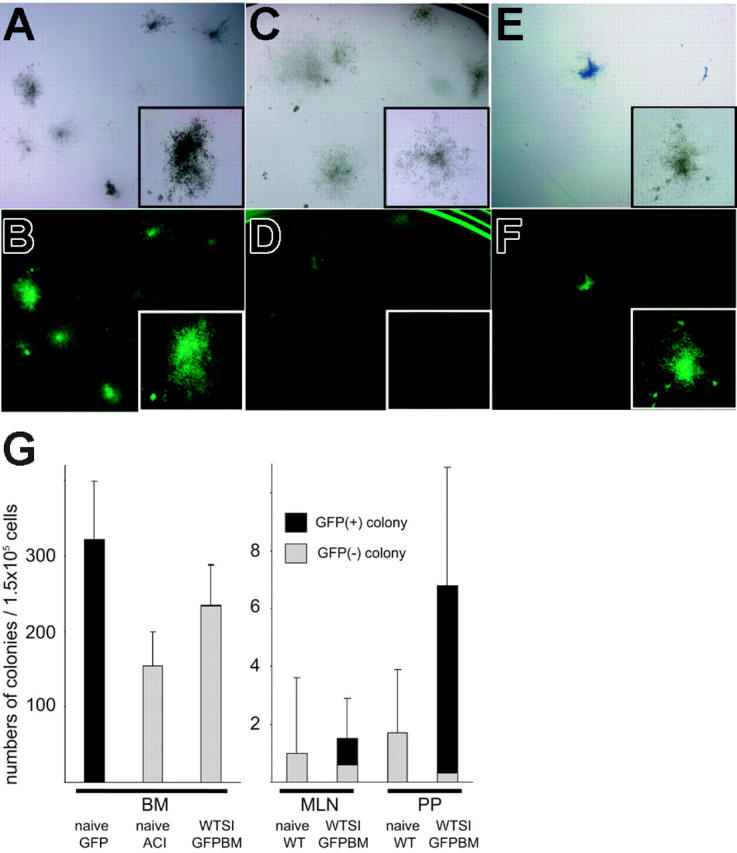
Colony-forming unit in culture (CFU-C) assay of isolated cells from WT intestine grafts cotransplanted with GFP-positive BM. (A-B) Naive GFP-positive BM formed more than 300 colonies per 1.5 × 105 cells, and all of them were GFP positive under fluorescent microscopy. (C-D) BM cells taken from the recipient of WT intestine plus GFP-positive BM formed comparable numbers of colonies as normal ACI animals, but very few GFP-positive colonies were found despite stable macrochimerism. (E-F) Cells isolated from PPs and MLNs of WT intestine grafts transplanted with GFP-positive BM developed GFP-positive colonies with a frequency of 2 to 6 per 1.5 × 105 cells. CFU-Cs of cells isolated from naive WT intestinal PPs and MLNs were 1 to 3 per 1.5 × 105 cells. (G) Mean numbers of colonies per 1.5 × 105 cells (n = 3). Panels A, C, and E are under an inverted microscope; B, D, and F are under an Olympus SZ×12 fluorescence dissecting microscope with magnification wheel set at 7×, insets at 30×. SI indicates small intestine.
To further confirm the engraftment of marrow-derived GFP-positive stem cells into intestine grafts, GALT leukocytes were obtained from WT graft after GFP BM plus WT intestine transplantation. Isolated cells were adoptively transferred into lethally (9.5 Gy whole body irradiation, 137Cs Gammacell 40) irradiated WT animals together with naive WT BM cells (2 × 106 per animal). Flow cytometric analysis of peripheral blood was performed at day 100 to evaluate whether GFP-positive stem cells in WT intestine grafts propagated in these animals. When host BM cells (120 days after GFP-positive BM plus WT SITx) were transferred into irradiated WT recipients, 1.1% ± 0.8% GFP-positive cells were found in the peripheral blood. Transfer of cells from WT intestine graft MLNs and PPs resulted in 5.9% ± 0.4% GFP-positive cells, while cells from naive GFP rat MLNs and PPs resulted in 0.8% ± 0.9% GFP-positive cells (Table 2). Propagated GFP-positive cells included T cells, B cells, and monocytes/macrophages. Although some GFP-positive T cells could be long-lived T cells that were originally adoptively transferred, multilineage hematopoietic reconstitution in secondary recipients suggests the engraftment of GFP-positive hematopoietic stem cells in GALT of WT intestine grafts transplanted simultaneously with GFP-positive BM (Figure 7).
Table 2.
Propagation of GFP-positive cells after adoptive transfer of cells isolated from intestine grafts into lethally irradiated WT recipients
| Source and number of cells transferred | No. of recipients | Survival, d | % GFP-positive cells in the blood, day 100 |
|---|---|---|---|
| Without additional BM cells | |||
| None | 5 | 8, 9, 11, 11, 12 | NA |
| Naive GFP BM, 2 × 106 | 2 | > 100 | > 96 |
| Naive WT BM, 2 × 106 | 2 | > 100 | 0 |
| ACI host BM 100 d after transplantation of WT intestine and GFP BM, 2 × 106 | 3 | > 100 | 1.1 ± 0.8 |
| With 2 × 106 WT BM cells | |||
| Naive GFP MLNs and/or PPs, 10 × 106 | 4 | > 100 | 0.8 ± 0.9 |
| WT graft MLNs and/or PPs 100 d after transplantation of WT intestine and GFP BM, 8 × 106 to 12.4 × 106 | 3 | > 100 | 5.9 ± 0.4 |
Data represent mean ± SD.
NA indicates not applicable.
Figure 7.
Propagation of BM-derived GFP-positive cells engrafted in the graft GALT. Lethally irradiated WT rats were adoptively transferred with cells isolated from GALT of WT intestine grafts simultaneously transplanted with GFP-positive BM. Flow cytometric analysis of peripheral blood was performed 100 days after irradiation and transfer. After adoptive transfer of cells isolated from MLNs and PPs of naive GFP rats, a GFP-positive population was not identified. On the other hand, multilineage GFP-positive cells including T, B, and monocytes/macrophages (about 6%) were detected after infusion of cells from WT intestine grafts transplanted with GFP-positive BM at day 120. Pictures are representative of 3 different animals of each group.
Discussion
The current study demonstrated sustained macrochimerism for more than 150 days in noncytoablated but conventionally immunosuppressed rat recipients of simultaneous BM and intestine transplantation. Macrochimerism was only seen when both marrow and intestine were transplanted together and either graft alone failed to develop persistent macrochimerism. Further analyses of in vivo propagation and in vitro CFU formation of cells isolated from intestine grafts proved the presence of infused marrow hematopoietic stem cells in simultaneously transplanted intestine allografts (syngeneic to marrow stem cells) for at least 150 days. The development of macrochimerism was not hindered with the depletion of leukocytes from the GALT of intestine allografts. Thus, the study suggests that the intestinal graft parenchyma plays roles in infused marrow stem cell engraftment and subsequent donor-type hematopoiesis.
Marrow or blood stem cells are known to home and lodge in the bone marrow for differentiation. Previous dogma has indicated that the space needs to be created in the bone marrow using myelotoxic procedures for infused stem cells to engraft. However, marrow stem cells could engraft and maintain long-term chimerism in immunocompetent syngeneic recipients without any myelotoxic procedure,1,29-32 suggesting that there probably are open bone marrow niches available for infused stem cells. Without immunologic barriers, frequency of engraftment is shown to correlate with the number of infused stem cells competing with the number of host stem cells. Myelotoxic therapy in syngeneic recipients has been thus suggested to play beneficial roles in eliminating the host stem cells and increasing the competitive ratios of infused stem cells over host cells to home and engraft.33 On the other hand, engraftment of allogeneic marrow stem cells into host bone marrow of nonmyeloablated recipients is extremely limited. Although the primary role of conditioning remains controversial, higher rates of initial engraftment failure of MHC-mismatched allogeneic stem cells, compared with MHC-identical or syngeneic stem cells, indicate that considerable immunologic barrier exists for the engraftment of infused allogeneic marrow cells into the marrow microenvironment. In fact, numbers of studies showed increased allogeneic marrow stem cell engraftment by inhibiting alloreactivity using CD4+ and CD8+ T-cell depletion, cytoxan, and costimulatory blockade without profound myeloablation.9-11,34,35
Thus, allogeneic marrow stem cell engraftment into the host marrow microenvironment is a complicated task influenced by the degree of MHC disparity, quality and quantity of stem cells, immunosuppression or pretransplantation conditioning regimens, and alloimmune reactivity. An additional potentially critical factor is MHC disparity between donor stem cells and the host marrow microenvironment. To clarify whether an MHC restriction exists between stem cells and the marrow microenvironment, several in vivo and in vitro experiments examine the engraftment and differentiation of stem cells with MHC-matched or -mismatched stromal cells.36,37 Although in clinical practice of bone marrow transplantation the significance of a syngeneic marrow microenvironment for the infused stem cells is uncertain, these experimental studies demonstrate the increased numbers of infused hematopoietic progenitor cells in an MHC-matched microenvironment and suggest the existence of an MHC restriction between marrow stem cells and supporting microenvironment.
Hematopoietic stem cells have been long known to exist outside of bone marrow in the adult life.3,4,38,39 Recent studies reveal that mesenchymal stem cells also are present in a variety of tissues besides in the bone marrow.40,41 Although exact roles of stem cells outside bone marrow in normal and disease conditions are not elucidated, these studies indicate that adult extra-bone marrow tissues are capable of providing microenvironment for stem cells. Infused marrow stem cells in this study engraft into both the allogeneic marrow environment and syngeneic extra-marrow environment (intestine graft). Considering that marrow is the primary site of stem cell engraftment, although the number is low, a relatively higher frequency of infused marrow-derived GFP-positive CFU-Cs in graft PPs and MLNs than host bone marrow (6.5 ± 4.1 and 0.9 ± 0.1 versus 1.1 ± 0.3 colonies per 1 × 105 cells, respectively) at day 120 suggests that intestinal graft microenvironment is suitable for marrow stem cell engraftment. Although the study did not analyze earlier time points, early enhanced chimerism in the bone marrow plus intestine transplantation group compared with intestine or bone marrow alone transplantation group might suggest the migration and temporary engraftment of donor hematopoietic stem cells in the host bone marrow during the early posttransplantation period.
Transplantation-induced early graft injury might result in the local production of growth factors and cytokines/chemokines, and such milieus could facilitate the homing of infused stem cells. However, third-party intestine grafts in this study failed to support macrochimerism development, indicating that the intestine graft syngeneic to infused bone marrow, but not the soluble factor, is crucial for marrow stem cell engraftment into intestine grafts in noncytoablated recipients. Thus, the preferential migration of infused marrow stem cells toward syngeneic intestine grafts might suggest the advantage of an MHC-identical microenvironment.
In summary, this study demonstrates that infused allogeneic marrow cells induce macrochimerism when an intestine graft is simultaneously transplanted. Although macrochimerism after simultaneous transplantation of BM and intestine tended to decrease over the prolonged period of 12 months, persistence of macrochimerism for a significantly prolonged time after transplantation indicates active roles of intestine allografts in supporting the engraftment of infused marrow stem cells that are syngeneic to intestine grafts. By using molecular and innovative tools, recent extensive efforts significantly advanced the understanding of hematopoietic stem cell behavior, growth factors, adhesion molecules, and other molecules (eg, extracellular matrix proteins).12,42 However, the biology of hematopoietic stem cell engraftment into BM niches remains largely unknown. Although further studies are certainly required, data in this study provide new information in understanding the behavior of marrow stem cells.
Acknowledgments
The authors are grateful to Alison Logar and Mike Tabacek for their expert technical support, Dr Masaru Okabe for providing GFP rats, and Carla Forsythe for expert manuscript organization and preparation.
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2006-02-004341.
Supported by the National Institutes of Health grants DK 29961, RO1 AI/DK 38899, and R01 DK54232.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339: 1579-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17: 1127-1152. [PMC free article] [PubMed] [Google Scholar]
- 3.Murase N, Starzl TE, Ye Q, et al. Multilineage hematopoietic reconstitution of supralethally irradiated rats by syngeneic whole organ transplantation. With oarticular reference to the liver. Transplantation. 1996;61: 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2: 198-203. [DOI] [PubMed] [Google Scholar]
- 5.Krause DS, Theise ND, Collector MI, et al. Multiorgan, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105: 369-377. [DOI] [PubMed] [Google Scholar]
- 6.Chan RJ, Yoder MC. The multiple facets of hematopoietic stem cells. Curr Neurovasc Res. 2004;1: 197-206. [DOI] [PubMed] [Google Scholar]
- 7.Billingham RE, Brent L, Medawar PB. “Actively acquired tolerance” of foreign cells. Nature. 1953; 172: 603-606. [DOI] [PubMed] [Google Scholar]
- 8.Main JM, Prehn RT. Successful skin homografts after the administration of high dosage X radiation and homologous bone marrow. J Natl Cancer Inst. 1955;15: 1023-1028. [PubMed] [Google Scholar]
- 9.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157: 2820-2829. [PubMed] [Google Scholar]
- 10.Wekerle T, Sayegh MH, Hill J, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187: 2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89: 3048-3054. [PubMed] [Google Scholar]
- 12.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106: 1901-1910. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson SK, Simmons PJ. Transplantable stem cells: home to specific niches. Curr Opin Hematol. 2004;11: 102-106. [DOI] [PubMed] [Google Scholar]
- 14.Bingaman AW, Waitze SY, Alexander DZ, et al. Transplantation of the bone marrow microenvironment leads to hematopoietic chimerism without cytoreductive conditioning. Transplantation. 2000;69: 2491-2496. [DOI] [PubMed] [Google Scholar]
- 15.Santiago SF, de Faria W, Khan TF, et al. Heterotopic sternum transplant in rats: a new model of a vascularized bone marrow transplantation. Microsurgery. 1999;19: 330-334. [DOI] [PubMed] [Google Scholar]
- 16.Lukomska B, Janczewska S, Durlik M, Olszewski WL. Kinetics of bone marrow repopulation in lethally irradiated rats after transplantation of vascularized bone marrow in syngeneic hind limb. Ann Transplant. 2000;5: 14-20. [PubMed] [Google Scholar]
- 17.Sakamoto T, Ye Q, Lu L, Demetris AJ, Starzl TE, Murase N. Donor hematopoietic progenitor cells in nonmyeloablated rat recipients of allogeneic bone marrow and liver grafts. Transplantation. 1999;67: 833-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyokawa H, Nakao A, Stolz DB, et al. 3D-confocal structural analysis of bone marrow-derived renal tubular cells during renal ischemia/reperfusion injury. Lab Invest. 2006;86: 72-82. [DOI] [PubMed] [Google Scholar]
- 19.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. `Green mice' as a source of ubiquitous green cells. FEBS Lett. 1997;407: 313-319. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Suzuki A, Imai E, Okabe M, Hori M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12: 2625-2635. [DOI] [PubMed] [Google Scholar]
- 21.Murase N, Demetris AJ, Matsuzaki T, et al. Long survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK 506. Surgery. 1991;110: 87-98. [PMC free article] [PubMed] [Google Scholar]
- 22.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft-versus-host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to brown Norway rats. Transplantation. 1995;60: 158-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murase N, Ye Q, Nalesnik MA, et al. Immunomodulation for intestinal transplantation by allograft irradiation, adjunct donor bone marrow infusion, or both. Transplantation. 2000;70: 1632-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakao A, Nalesnik MA, Ishikawa T, Azhipa O, Demetris AJ, Murase N. Chimerism and tolerance in rat recipients of intestinal allografts from ALS-treated donors with and without adjunct naive-donor-strain bone-marrow cells. Transplantation. 2003;75: 1575-1581. [DOI] [PubMed] [Google Scholar]
- 25.Gill TJ III, Kunz HW, Misra DN, Hassett AL. The major histocompatibility complex of the rat. Transplantation. 1987;43: 773-785. [PubMed] [Google Scholar]
- 26.Yano Y, Hara M, Miyahara T, et al. Microchimeric cells from the peripheral blood associated with cardiac grafts are bone marrow derived, long-lived and maintain acquired tolerance to minor histocompatibility antigen H-Y. Transplantation. 2001;71: 1456-1462. [DOI] [PubMed] [Google Scholar]
- 27.Kiyomoto T, Toyokawa H, Nakao A, et al. The difficulty of eliminating donor leukocyte microchimerism in rat recipients bearing established organ allografts. Transplantation. 2006;81: 438-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murase N, Demetris AJ, Woo J, et al. Graft-versus-host disease after brown Norway-to-Lewis and Lewis-to-Brown Norway rat intestinal transplantation under FK506. Transplantation. 1993; 55: 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micklem HS, Clarke CM, Evans EP, Ford CE. Fate of chromosome-marked mouse bone marrow cells transfused into normal syngeneic recipients. Transplantation. 1968;6: 299-302. [PubMed] [Google Scholar]
- 30.Saxe DF, Boggs SS, Boggs DR. Transplantation of chromosomally marked syngeneic marrow cells into mice not subjected to hematopoietic stem cell depletion. Exp Hematol. 1984;12: 277-283. [PubMed] [Google Scholar]
- 31.Stewart FM, Crittenden RB, Lowry PA, Pearson-White S, Quesenberry PJ. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81: 2566-2571. [PubMed] [Google Scholar]
- 32.Brecher G, Ansell JD, Micklem HS, Tjio JH, Cronkite EP. Special proliferative sites are not needed for seeding and proliferation of transfused bone marrow cells in normal syngeneic mice. Proc Natl Acad Sci U S A. 1982;79: 5085-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quesenberry PJ, Colvin G, Abedi M. Perspective: fundamental and clinical concepts on stem cell homing and engraftment: a journey to niches and beyond. Exp Hematol. 2005;33: 9-19. [DOI] [PubMed] [Google Scholar]
- 34.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000; 6: 464-469. [DOI] [PubMed] [Google Scholar]
- 35.Adams AB, Durham MM, Kean L, et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001;167: 1103-1111. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto F, Sugiura K, Inoue K, Ikehara S. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vivo. Blood. 1997;89: 49-54. [PubMed] [Google Scholar]
- 37.Sugiura K, Hisha H, Ishikawa J, et al. Major histocompatibility complex restriction between hematopoietic stem cells and stromal cells in vitro. Stem Cells. 2001;19: 46-58. [DOI] [PubMed] [Google Scholar]
- 38.Hays EF, Hays DM, Golde DW. Hemopoietic stem cells in mouse liver. Exp Hematol. 1978;6: 18-27. [PubMed] [Google Scholar]
- 39.Decker T, Lohmann-Matthes ML, Baccarini M. Liver-associated macrophage precursor cells proliferate under impairment of regular hemopoiesis. Eur J Immunol. 1988;18: 697-703. [DOI] [PubMed] [Google Scholar]
- 40.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283: 534-537. [DOI] [PubMed] [Google Scholar]
- 41.Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002; 30: 1339-1345. [DOI] [PubMed] [Google Scholar]
- 42.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23: 879-894. [DOI] [PubMed] [Google Scholar]