Abstract
Tie2 is a receptor-type tyrosine kinase expressed on hematopoietic stem cells and endothelial cells. We used cultured embryonic stem (ES) cells to determine the function of Tie2 during early vascular development and hematopoiesis. Upon differentiation, the ES cell–derived Tie2+Flk1+ fraction was enriched for hematopoietic and endothelial progenitor cells. To investigate lymphatic differentiation, we used a monoclonal antibody against LYVE-1 and found that LYVE-1+ cells derived from Tie2+Flk1+ cells possessed various characteristics of lymphatic endothelial cells. To determine whether Tie2 played a role in this process, we analyzed differentiation of Tie2-/- ES cells. Although the initial numbers of LYVE-1+ and PECAM-1+ cells derived from Tie2-/- cells did not vary significantly, the number of both decreased dramatically upon extended culturing. Such decreases were rescued by treatment with a caspase inhibitor, suggesting that reductions were due to apoptosis as a consequence of a lack of Tie2 signaling. Interestingly, Tie2-/- ES cells did not show measurable defects in development of the hematopoietic system, suggesting that Tie2 is not essential for hematopoietic cell development.
Introduction
A close cell lineage relationship between hematopoietic and endothelial cells has long been recognized.1,2 During embryogenesis, both cell types emerge in the yolk sac, and primitive erythrocytes differentiate juxtaposed to endothelial precursors by embryonic day 7.5 (E7.5).3 In the mouse embryo, Tie2+ cells in the aorta-gonad-mesonephros (AGM) region generate both blood and endothelial cells.4 From studies of embryonic stem (ES) cell differentiation, vascular endothelial growth factor (VEGF)–responsive bipotent precursors of hematopoietic and endothelial cells, known as hemangioblasts, have been identified.5 Hemangioblast-derived endothelial cells form vascular vessels through vasculogenesis and angiogenesis. Lymphatic development starts when a subset of vascular endothelial cells of the cardinal vein commit to a lymphatic lineage and sprout to form the primary lymph sacs at around E9 or 10.6,7 Mouse molecular genetic experiments indicate that Prox-1 (a homeobox transcription factor) and the VEGF receptor 3 (VEGFR-3) are crucial for the commitment of endothelial cells to a lymphatic lineage.8-10 Since lymphatic vessel–specific molecules are being identified, the molecular mechanisms underlying development of lymphatic cells as well as vascular and hematopoietic cells can now be analyzed.
Many studies report that expression of Flk1 is crucial for early establishment of endothelial and hematopoietic lineages and perhaps for their common progenitor.5,11,12 Flk1 encodes a receptor tyrosine kinase for the vascular endothelial growth factor family of ligands.13 Single Flk1+ cells from embryoid bodies can give rise to blast colonies (blast lymphocyte colony-forming cells [BL-CFCs]), which produce both hematopoietic and endothelial cells in vitro.5 Loss of Flk1 in mice results in selective defects in generating both blood and blood-vessel endothelial cells (BECs).11 In addition to Flk1, Flt1 and Tie2 tyrosine kinases are also expressed in immature hematopoietic cells and BECs.14-17
The expression pattern of Tie2 suggests a function in both vascular endothelial and hematopoietic cells. Recently we determined the function of Ang1/Tie2 signaling, which maintains long-term repopulating hematopoietic stem cells in the bone marrow niche, suggesting that Tie2 signaling is crucial for adult bone marrow hematopoiesis.18 In the mouse vitelline artery at E9.5, Tie2+ hematopoietic cells aggregate and adhere to endothelial cells.19 In vitro culture of Tie2+ cells isolated from the AGM region generates both blood and endothelial cells.4 In fetal liver, the Tie2+ fraction contains an enriched fraction of long-term repopulating cells.20 Based on these findings, Tie2 signaling was thought to regulate embryonic development and differentiation of hematopoietic cells. However, more recently, Puri and Berstein have demonstrated that Tie2 is dispensable for embryonic hematopoiesis using ES cell mouse chimeras.21 This finding suggests that Tie2 function in developmental hematopoiesis differs from its role in bone marrow hematopoiesis.
By contrast, Tie2 function in BECs has been intensively analyzed at both developmental and adult stages. In vivo and in vitro experiments show that Tie2 signaling potently induces sprouting, chemotaxis, and network formation.22,23 Furthermore, the Tie2 ligand Ang1 is a potent survival factor for BECs under serum deprivation.24 A role for Tie2 signaling in blood vessel endothelial survival in vivo has also been illustrated using conditional rescue of Tie2-/- embryos,25 further supporting a role of this signaling system in endothelial cell survival.
Recently it has been demonstrated in mice that Tie2 is expressed and functions in lymphatic vessels embryonically26 and in adults.27 Tie2-deficient mice exhibit severe defects in vascular and heart development and die by E9.5,28,29 making analysis of the lymphatic system difficult. Therefore, here we analyzed the function of Tie2 in lymphatic endothelial cells (LECs) and hematopoietic cells as well as in developing BECs using differentiation of cultured ES cells. We identify ES cell–derived LECs as well as BECs and hematopoietic cells, and demonstrate that Tie2 signaling is essential for development of BECs and LECs, but not for hematopoietic cells.
Materials and methods
Cell preparation and culture conditions
TT2,30 E14,31 and R132 ES cells were maintained on mouse embryonic fibroblast (MEF) feeder cell layers in knockout Dulbecco-modified Eagle medium (Gibco BRL, Carlsbad, CA) containing 15% fetal bovine serum (Intergen, Purchase, NY), 100 U/mL leukemia inhibitory factor (LIF; Chemicon International, Temecula, CA), 0.1 mM nonessential amino acids (Gibco BRL), 1 mM sodium pyruvate (Gibco BRL), 2 mM L-glutamine (Gibco BRL), and 100 μM 2-mercaptoethanol (Sigma-Aldrich, St Louis, MO). After removal of LIF, ES cells were cultured on collagen type IV plates (Becton Dickinson, San Jose, CA) at 1 × 104 cells/mL for 2 days. Cells were then disaggregated by trypsin and seeded on OP9 cells at 1 × 105 cells/mL. After 5 days of culture, OP9 cells were removed from ES cells through a Sephadex G10 column (Amersham Bioscience, Uppsala, Sweden), and the ES cells were fractionated by Flk1 and Tie2 expression using FACSvantage (Becton Dickinson). Sorted cells were seeded on OP9 cells at 1000 to 15 000 cells/mL and cultured in the presence of VEGF-C (100 ng/mL; R&D Systems, Minneapolis, MN), VEGF-D (100 ng/mL; R&D Systems), or the caspase inhibitor Z-VAD-fmk (10-100 nM; Calbiochem, La Jolla, CA). When indicated, sorted cells were cultured with recombinant soluble Tie2-Fc fusion protein (30 μg/mL)19 or recombinant soluble CD4-Fc fusion protein (30 μg/mL).19
Immunocytochemistry
Immunocytochemistry was performed essentially as described.33 Differentiated ES cells cultured on OP9 cells were fixed with 4% paraformaldehyde at 4°C and stained with a rat monoclonal anti-mouse platelet–endothelial cell adhesion molecule 1 (PECAM-1) monoclona antibody (mAb) (MEC13.3; Becton Dickinson) or a rat monoclonal anti-mouse LYVE-1 antibody (ALY726) by the indirect immunoperoxidase method using horseradish peroxidase–conjugated anti-rat IgG. Peroxidase activity was visualized using 3,3′-diaminobenzidine (Dojindo, Kumamoto, Japan). To determine whether Prox-1 was expressed in LYVE-1+ cells, we stained cells with biotinylated ALY7 and anti–Prox-1 antibody (Covance, Berkeley, CA). LYVE-1 and Prox-1 expression was detected by reacting with Alexa 488–conjugated Streptavidin (Molecular Probes, Eugene, OR) and Alexa 546–conjugated goat anti-rabbit antibody (Molecular Probes), respectively. For nuclear staining, cells were treated with TOTO3 (Molecular Probes). Stained cells were visualized by fluorescence microscopy. Stained and unstained cells were visualized by fluorescent microscopy (IX71) with either UplanApo 4×/0.13 NA, 10×/0.40 NA, or 20×/0.70 NA objectives (Olympus, Tokyo, Japan). Images were further processed with Adobe Photoshop (Adobe Systems, San Jose, CA). For DiI labeling, 10 μg/mL DiI-Ac-LDL (Molecular Probes) was added to differentiated ES cells on OP9 cells and adherent cells were incubated for 4 hours at 37°C. After removing the media containing DiI-Ac-LDL, cells were washed and stained with anti–PECAM-1 (FITC-conjugated; Becton Dickinson) or anti–LYVE-1 (biotin-conjugated) plus FITC-conjugated Streptavidin (Becton Dickinson). Uptake of DiI-Ac-LDL by blood vessel endothelial cells (PECAM-1+ cells) and lymphatic endothelial cells (LYVE-1+ cells) was visualized using a standard rhodamine excitation emission filter (Olympus, Tokyo, Japan).
RT-PCR analysis
Total RNA was extracted from cells using RNeasy Kit (Qiagen, Hilder, Germany). Isolated RNA was reverse-transcribed using an reverse transcriptase (RT) for polymerase chain reaction (PCR) Kit (Clontech, Palo Alto, CA). cDNAs were amplified using Taq polymerase (TaKaRa, Kyoto, Japan). Sequences of gene-specific primers for RT-PCR were as follows: GATA2, 5′-(acacaccacccgatacccacctat), 3′-(cctacgccatggcagtcaccatgct); SCL, 5′-(cgcggatccacggagcggccgccgagcgcg), 3′-(cggaattccgcgccgcactactttggtgtg); c-myb, 5′-(gacagaagaggaggacagaatca), 3′-(tctcagggtcttcgtcgttatag); AML1, 5′-(ccagcaagctgaggagcggcg), 3′-(cggatttgtaaagacggtga); Tie2,5′-(ttagttctctgtggagtcag), 3′-(aggccctgagttcttcactc); Flk1,5′-(agaacaccaaaagagaggaacg), 3′-(gcacacaggcagaaaccagtag); VEGFR3, 5′-(gctaccactgctactacaag), 3′-(gataatcccagtcgaaggtg); Prox1, 5′-(aagtggttcagcaatttccg), 3′-(tgaccttgtaaatggccttc); LYVE1, 5′-(ttcctcgcctctatttggac), 3′-(tctgttgtctgcgtttcatcc); Pdpn,5′-(gtgccagtgttgttctgggt), 3′-(tctgttgtctgcgtttcatcc); Evi1, 5′-(aatatgagtcatgccaaccc), 3′-(cttggtgtactgacatcatc); and GAPDH, 5′-(aatcccatcaccatcttcca), 3′-(ccaggggtcttactccttg).
PCR products were separated on a 1.2% agarose gel and gels were stained with ethidium bromide.
Quantitative RT-PCR analysis
Total RNA was isolated from 104 cells and cDNA was reverse-transcribed using Superscript III (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The expression levels of Ang1, Ang2, and Ang3 were analyzed by quantitative (Q) RT-PCR using a LightCycler instrument (Roche Diagnostics, Mannheim, Germany) with LightCycler software version 3.5. cDNA was amplified for Q-PCR using SYBR Green I (Sigma-Aldrich) to detect PCR product. cDNA (2 μL) was used in a 20-μL final volume reaction containing 10 μL SYBR Premix Ex Taq (TaKaRa), 0.4 μM Ang1 forward (5′-GCCTTTGCACTAAAGAAGGTGTTTT-3′), and 0.4 μM Ang1 reverse (5′-ATACATCCGCACAGTCTCGAAATG-3′). The LightCycler was programmed to run an initial denaturation step at 95°C for 10 seconds followed by 45 cycles of denaturation (95°C for 5 seconds) and extension (60°C for 20 seconds), monitoring the synthesis of product at the end of the extension step of each cycle. The same conditions were used with primers Ang2 forward (5′-AGGAGATCAAGGCCTACTGTGACA-3′) and Ang2 reverse (5′-GCTCCCGAAGCCCTCTTTG-3′), and Ang3 forward (5′-GTTCCAGGACTGTGCAGAGATCA-3′) and Ang3 reverse (5′-TCTCCATGTCACAGAACACCTTGAG-3′). The Ang1, Ang2, and Ang3 values were normalized against mouse β-actin (forward 5′-CAGCCTTCCTTCTTGGGTATGG-3′; reverse 5′-CTGTGTTGGCATAGAGGTCTTTACG-3′).
Flow cytometric analysis and cell sorting
The following mAbs used for flow cytometry were purchased from Becton Dickinson: anti-CD34 (RAM34), anti–c-Kit (ACK45), anti–Sca-1 (E13-161.7), anti–CD45 (30-F11), anti–Flk1 (Avas12α1), anti–PECAM-1 (MEC13.3), anti–Mac-1 (M1/70), anti–Gr-1 (RB6-8C5), and anti–TER-119. Also used were anti-Tie2 mAb (TEK4)34 and anti–LYVE-1 mAb (ALY7).26 Fluorescence-activated cell sorting (FACS) analysis was performed on a FACSvantage (Becton Dickinson).
Progenitor assay by methylcellulose culture
Tie2+Flk1+ or Flk1+ cells derived from ES cells were embedded in 1 mL alpha medium containing 1.3% methylcellulose (1500 cp; Sigma-Aldrich), 30% fetal calf serum (FCS), 1% deionized bovine serum albumin (BSA; Sigma-Aldrich), 0.1 mM 2-mercapto-ethanol (Sigma-Aldrich), 10 ng/mL stem cell factor (SCF; PeproTech EC, London, United Kingdom), 10 ng/mL recombinant mouse interleukin-3 (IL-3; PeproTech EC), 10 ng/mL recombinant human IL-6 (PeproTech EC), and 2 U/mL recombinant human erythropoietin (Epo; Chugai Pharmaceutical, Tokyo, Japan). Cells were cultured in a 35-mm culture dish and incubated at 37°C in a humidified atmosphere with 5% CO2.
Statistics
Data are expressed as means plus or minus standard deviation (SD). Statistical analysis was conducted using the Student t test. Statistical significance was defined as a P value less than .05.
Results
In vitro differentiation of ES cells
In order to analyze the function of Tie2 in the development of LECs as well as BECs and hematopoietic cells, we developed a cell culture system for ES cell differentiation. After removal of LIF, E14 ES cells were cultured on collagen type IV plates for 2 days to initiate differentiation to a mesoderm lineage; subsequently, cells were transferred to OP9 stromal cells. Markers of both endothelial and hematopoietic cells, Sca-1, c-kit, and CD34, were expressed in undifferentiated ES cells (Figure 1A). Although 1% of ES cells expressed Flk1 on collagen plates, Tie2 was not expressed in Flk1+ cells (data not shown). Following transfer of cells on collagen plates to OP9 cells, 8% of Flk1+ cells expressed Tie2 on day 3 of culture. Numbers of Tie2+ cells increased until day 5 of culture, when Tie2 expression was maximal; thereafter, both expression levels and numbers of Tie2+ cells gradually decreased. At day 9 of culture, cells cocultured with OP9 cells began expressing CD45, a marker of all hematopoietic cells except mature erythrocytes. Since Tie2+ cells appeared just before CD45+ hematopoietic cells, Tie2+ cells in the Flk1+ cell fraction may represent hematopoietic progenitor cells. We examined other ES strains, such as TT2 and R1 cells, using the same culture conditions, and confirmed that these strains showed a similar mesodermal phenotypes as E14 cells (data not shown).
Figure 1.
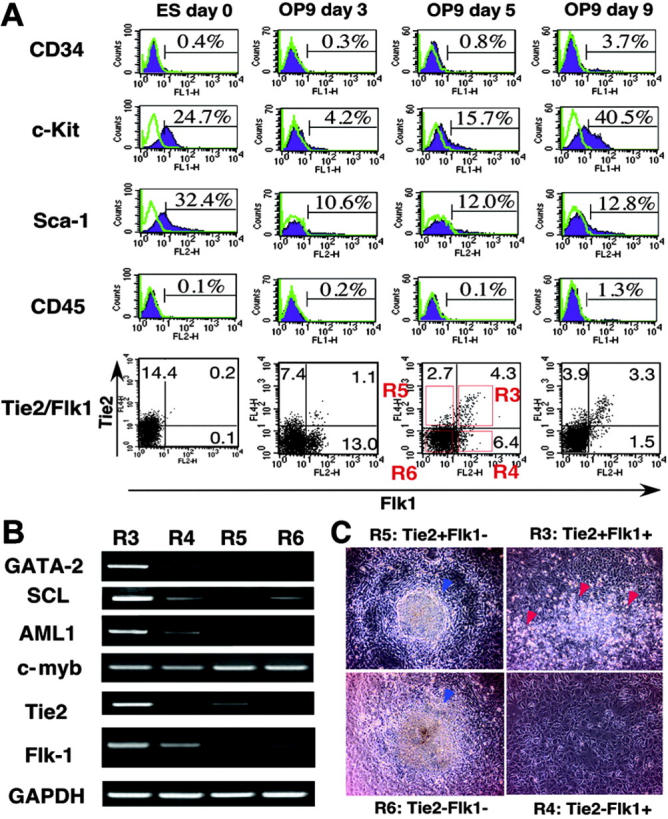
Mesodermal differentiation of ES cells on OP9 stromal cells. (A) E14 ES cells were cultured on collagen type IV plates for 2 days, and then all cells were cultured on OP9 cells for 9 days. The expression of CD34, c-Kit, Sca-1, CD45, Flk1, and Tie2 in ES cell–derived cells was analyzed at the indicated time points by flow cytometry. Stained Tie-/- cells are represented by purple shaded histograms. Unstained controls are represented by green lines. The percentages of cells in each quadrant are indicated. (B) Gene expression of fractionated cells shown in A (R3-R6) was analyzed by RT-PCR. (C) Fractionated cells (20 000; R3-R6) were cultured on OP9 cells. On day 7 of culture, hematopoietic clusters (red arrowheads) were developed only from the R3 fraction. In other fractions (R4-R6) hematopoietic clusters were not developed, but embryoid body–like colonies (blue arrowheads) developed from R5 and R6 fractions. Cells were analyzed at low (×40) magnification.
Development of hematopoietic, lymphatic endothelial, and blood vessel endothelial cells from Flk1+Tie2+ cells
To analyze the differentiating potential of ES-derived cells, we fractionated cells on day 5 of culture using Tie2 and Flk1 mAb as shown in Figure 1A (bottom panel, R1-R4). Expression profiling of transcription factors specific for hematopoietic or endothelial cells was undertaken by RT-PCR (Figure 1B). Expression levels of GATA-2, SCL, and AML-1 in the Tie2+Flk1+ fraction (R3) were 1.7-, 2.5-, and 1.7-fold higher than those in the Tie2-Flk1+ fraction (R4), respectively. In the Flk1- fraction (R5 and R6), expression levels of these genes were much lower compared with the Tie2-Flk1+ fraction (R4), suggesting that the Tie2+Flk1+ fraction may contain committed progenitors of hematopoietic and endothelial lineages. Cells (20 000) fractionated by Flk1 and Tie2 expression were cultured on OP9 cells (Figure 1C), and hematopoietic clusters formed only from the Tie2+Flk1+ fraction (R3) (Figure 1C, red arrowheads). Hematopoietic clusters were not detected in Flk1+Tie2- (R4), Flk1-Tie2+ (R5), or Flk1-Tie2- fractions (R6). The number of hematopoietic progenitors in each fraction was estimated by colony-forming assays in methylcellulose culture (Figure 2A-B). BECs were detected by staining with PECAM-1 mAb (Figure 2C). PECAM-1+ endothelial cells were spindle shaped and took up an acetyl-LDL (Figure 2D). To detect LECs, we generated a mAb against LYVE-1 (ALY7), a receptor for extracellular matrix glycosaminoglycan. Using this mAb, we detected LYVE-1+ cells on OP9 cells (Figure 2E). These cells also took up acetyl-LDL (Figure 2F), and 65% of them expressed Tie2 (Figure 2I). The lymphatic identity of these cells was further demonstrated by RT-PCR, using primers specific for LEC-enriched genes, namely, Pdpn, VEGFR3, and Prox1 (Figure 2J). Furthermore, LYVE-1+ cells coexpressed Prox-1 (Figure 2K). The presence of VEGFR-3 transcripts in these cultures provided the impetus for us to add the lymphatic growth factors, VEGF-C and VEGF-D. The addition of these factors resulted in a marked increase in the number of LYVE-1+ cells (Figure 2L) and an increase in the size of monolayers (VEGF-C, Figure 2G; VEGF-D, Figure 2H). These findings suggest that LYVE-1+ cells derived from ES cell differentiation cultures express many genes that are restricted to or enriched in LECs.
Figure 2.
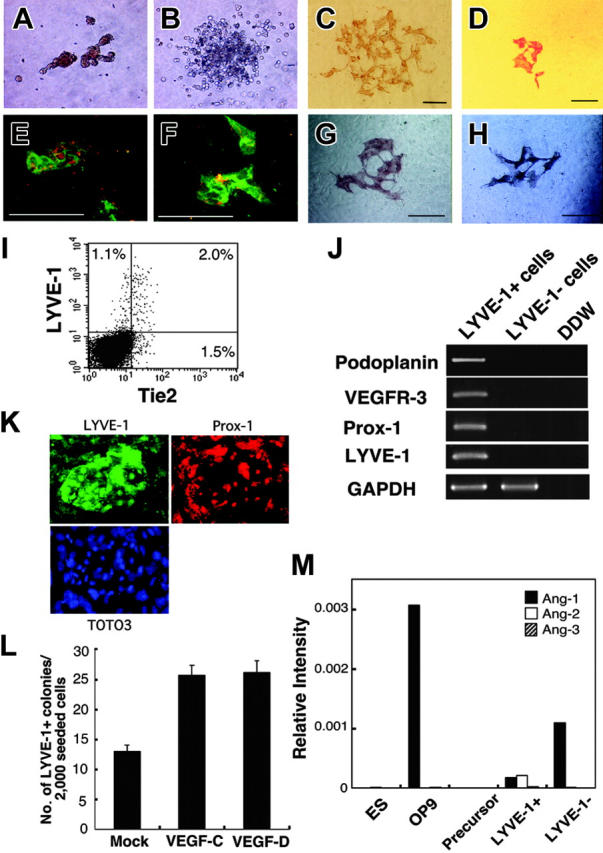
Development of lymphatic endothelial cells from ES cells. The Tie2+Flk1+ fraction of ES cell–derived cells formed erythroid colonies (A) and granulocyte-macrophage colonies (B) in methylcellulose. Vascular and lymphatic endothelial cells were stained with PECAM-1 mAb (C) and LYVE-1 mAb (D), respectively. PECAM-1+ cells (green, panel E) and LYVE-1+ cells (green, panel F) took up acetyl-LDL (red, panels E and F). Scale bars, 10 μm. (I) Expression of LYVE-1 and Tie2 in differentiated cells derived from ES cells on OP9 cells on day 6 of culture was analyzed by flow cytometry. The percentages of cells in each quadrant are indicated. (J) Lymphatic-specific genes, Pdpn, VEGFR-3, and Prox-1 in LYVE-1+ and LYVE-1- cells were analyzed by RT-PCR. (K) Immunostaining at high magnification (× 200) showed that LYVE-1+ cells (green) coexpressed Prox-1 (red). TOTO3 (blue) was used to stain nuclei. (L) In the presence of VEGF-C and VEGF-D (100 ng/mL each), the number of LYVE-1+ colonies increased to 2 times that of mock-treated cells. Colonies were also larger in the presence of VEGF-C and VEGF-D (panels G and H, respectively). Results are expressed as the mean ± SD. (M) OP9 and ES cell–derived LYVE-1- cells expressed Ang1. LYVE-1+ cells expressed low levels of Ang1 and Ang2. Ang1, Ang2, and Ang3 were not detectable in ES cells and lymphatic precursors (Flk1+ cells derived from ES cells).
That Tie2 is required for these cell types is supported by the more than 10-fold increase in hematopoietic, blood vessel endothelial, and lymphatic endothelial colonies from Tie2+Flk1+ cells compared with Tie2-Flk1+ or Tie2-Flk1- cell fractions (Table 1). To determine which cells produce the Tie2 ligands Ang1, Ang2, and Ang3 in order to support Tie2+ cells, we performed quantitative expression analysis for Ang1, Ang2, and Ang3. As shown in Figure 2M, OP9 cells and LYVE-1- ES-derived cells expressed Ang1 but not Ang2 or Ang3. Although low expression levels of Ang1 and Ang2 were detected in LYVE-1+ cells, ES cells and lymphatic precursor cells (ES cell–derived Flk1+ cells) did not express Ang's. These results suggest that Ang1 derived from OP9 cells might affect the growth or survival of Tie2+ cells.
Table 1.
Frequency of hematopoietic and endothelial progenitors of differentiated ES cells
| Erythroid colonies from 30 000 cells | GM colonies from 30 000 cells | Vascular endothelial colonies from 1500 cells | Lymphatic endothelial colonies from 2000 cells | |
|---|---|---|---|---|
| Tie2+Flk1+ | 11.3 ± 6.4 | 11.7 ± 2.1 | 47.5 ± 5.3 | 24.3 ± 2.5 |
| Tie2–Flk1+ | 0 | 0 | 5.2 ± 1.8 | 0 |
| Tie2+Flk1– | 0 | 0 | 0 | 0 |
| Tie2–Flk1– | 0 | 0 | 0 | 0 |
| Bulk | 0 | 0 | 2.7 ± 1.5 | 1.3 ± 0.6 |
Development of hematopoietic and endothelial cells from Tie2-deficient ES cells
To clarify the function of Tie2 during hematopoietic and endothelial differentiation from ES cells, the differentiation capacity of Tie2-/- ES cells was examined using our culture system. Tie2-/- ES cells grew normally on MEF feeder layer cells (data not shown), and differentiated cells grown on OP9 cells were analyzed by flow cytometry. The frequency of cells expressing Flk1 in the Tie2-/- ES cells was similar to that seen in Tie2+/- cells (Figure 3A). RT-PCR of RNA from this fraction for expression of the genes involved in development of hematopoietic and endothelial cells revealed no remarkable differences between Tie2+/- and Tie2-/- cells (Figure 3B). To analyze hematopoietic development of Tie2-/- ES cells, we calculated the number of hematopoietic clusters formed on OP9 cells at day 7 of culture. The number and size of hematopoietic clusters of Tie2-/- ES cells were the same as those of Tie2+/- cells (Figure 3C, and data not shown). When the Tie2-/- hematopoietic clusters were transferred to fresh OP9 cells for an additional week, normal proliferating hematopoietic cells were detected by flow cytometry. Mature Mac-1–, Gr-1–, and Ter119–positive hematopoietic cells were differentiated from Tie2-/- ES cells (Figure 3D), and the frequency of mature hematopoietic cells was similar to that seen in Tie2+/- cells (data not shown), suggesting that Tie2 is not essential for development of hematopoietic cells. By contrast, Tie2-/- ES cells were severely defective in forming blood vessel and lymphatic endothelial colonies on OP9 cells at day 6 of culture. The number of such blood vessel and lymphatic endothelial colonies was approximately one third that observed from Tie2+/- cells (Figure 4A-B), and colony size was smaller than that seen from Tie2+/- cells (Figure 4C-D). These results suggest that Tie2 function is required for development of BECs and LECs, but not of hematopoietic cells. To confirm these findings, we added soluble Tie2-Fc fusion protein to the culture media of wild-type ES cells to block Tie2 signaling. As shown in Figure 4E-G, the number of vascular and lymphatic endothelial cell colonies derived from wild-type ES cells in the presence of soluble Tie2-Fc fusion protein decreased to one half to one third of the mock-treated cells, while the number of hematopoietic clusters was not affected by soluble Tie2-Fc fusion protein. We did not detect such inhibition in the presence of soluble CD4-Fc fusion protein (Figure 4 E-F).
Figure 3.
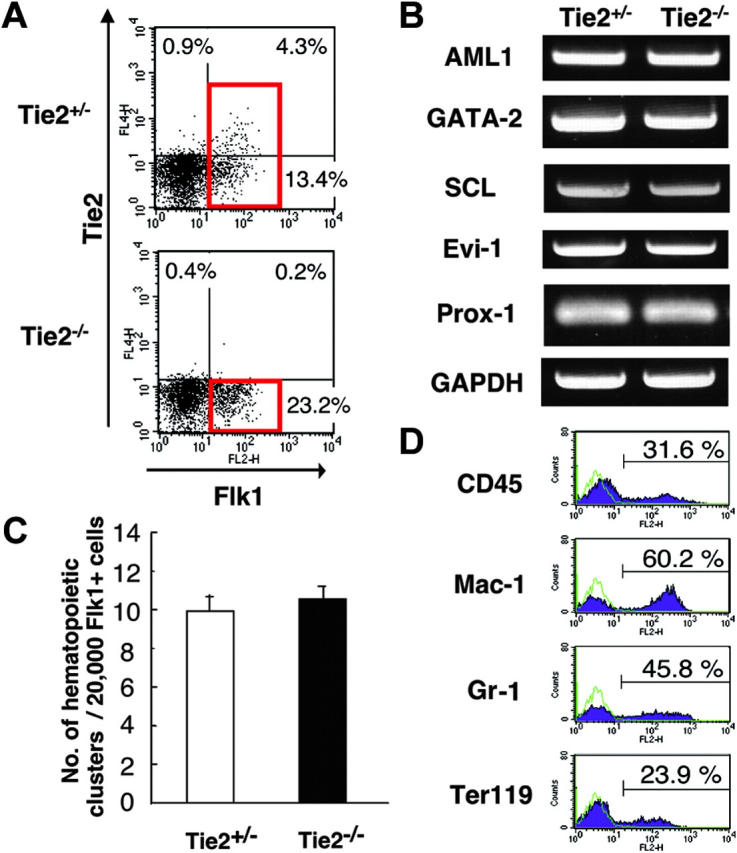
Normal development of hematopoietic cells from Tie2-/- ES cells. (A) FACS analysis of the expression of Flk1 and Tie2 in Tie2-/- and Tie2+/- ES cell–differentiated cells. Red squares show the Flk1+ fraction. The percentages of cells in each quadrant are indicated. (B) Expression of genes associated with mesoderm in Flk1+ cells shown in panel A (red squares) was analyzed by RT-PCR. (C) Flk1+ cells (20 000) were cultured on OP9 cells. The number of hematopoietic clusters from Tie2+/- and Tie2-/- cells at day 7 of culture was calculated. Results are expressed as the mean ± SD. (D) Tie2-/- hematopoietic clusters were cultured for an additional 7 days on fresh OP9 cells. The expression of CD45, Mac-1, Gr-1, and Ter119 was analyzed by flow cytometry. ES cell–derived hematopoietic cells are represented by purple shaded histograms; unstained controls, by green lines.
Figure 4.
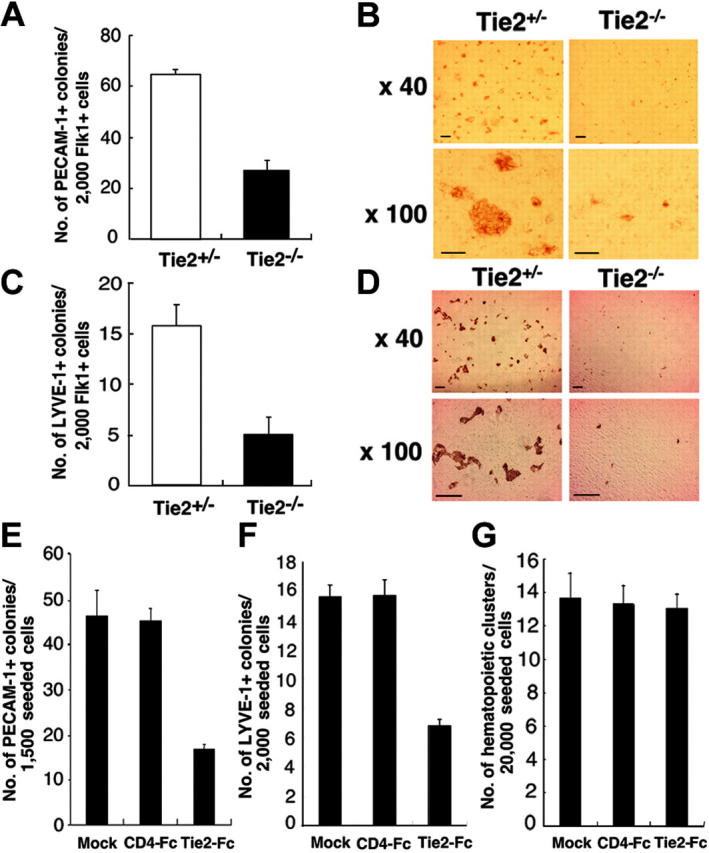
Lymphatic and blood vessel endothelial cell development from Tie2-/- ES cells. (A) Flk1+ cells (2000) were cultured on OP9 cells. At day 7 of culture, the number of vascular endothelial colonies developed from Tie2+/- (□) and Tie2-/- (▪) ES cells was calculated. (B) Vascular endothelial colonies were stained with PECAM-1 mAb and analyzed at low (× 40) and high (× 100) magnification. (C) The number of lymphatic endothelial colonies developed from Tie2+/- (□) and Tie2-/- (▪) ES cells was calculated. (D) Lymphatic endothelial colonies were stained with LYVE-1 mAb and analyzed at low (× 40) and high (× 100) magnification. Scale bars in panels B and D, 20 μm. In the presence of Tie2-Fc (30 μg/mL), the number of vascular and lymphatic endothelial colonies was decreased (panels E and F, respectively). (G) Hematopoietic cluster formation was not affected by exogenous soluble Tie2-Fc (30 μg/mL). Exogenous soluble CD4-Fc (30 μg/mL) served as the control. Results in panels A, C, and E-G are expressed as the mean ± SD.
Tie2 signaling is crucial for antiapoptotic signaling in the development of lymphatic and blood vessel endothelial cells
To further analyze the mechanisms underlying defective proliferation of BECs and LECs from Tie2-/- ES cells, we analyzed expression of blood vessel– and lymphatic-specific markers PECAM-1 and LYVE-1, respectively, by flow cytometry at days 2, 4, and 6 of culture (Figure 5A). Cells expressing PECAM-1 and LYVE-1 in Tie2-/- ES cells decreased over 6 days. PECAM-1+ cells in Tie2-/- cells were approximately one third the number of those in Tie2+/- cells at day 6 of culture, as was the case with LYVE-1+ cells (Figure 5B-C). This finding suggested that Tie2-/--mediated signaling protects endothelial cells from cell death. To test this possibility, we treated Tie2-/- cells with the caspase inhibitor Z-VAD-fmk. As shown in Figure 5B and C, lymphatic and blood vessel endothelial colony formation was rescued in the presence of Z-VAD-fmk in a dose-dependent fashion. In the presence of 50 nM of Z-VAD-fmk, the number of lymphatic endothelial colonies from Tie2-/- cells was rescued to 60% of that from Tie2+/- cells (Figure 5C), although the size of Tie2-/- lymphatic endothelial colonies remained small (Figure 5D). The formation of blood vessel endothelial colonies was similar in the presence of Z-VAD-fmk (50 nM; Figure 5C). These findings suggest that Tie2 signaling is crucial for LEC and BEC development and mediates antiapoptotic signaling during ES cell differentiation.
Figure 5.
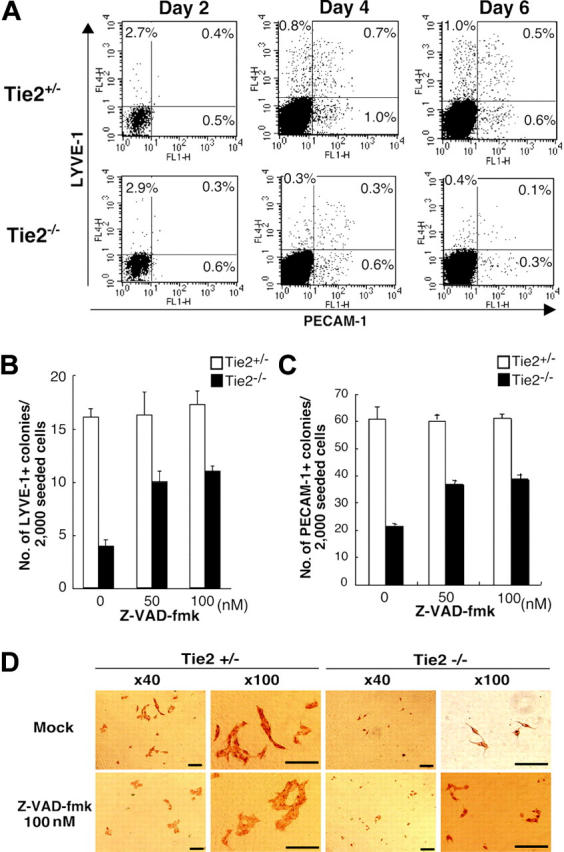
Tie2 signaling in blood vessels and lymphatic endothelial cells protected from apoptosis. (A) Expression of PECAM-1 and LYVE-1 in ES cell–derived cells was analyzed by flow cytometry at days 2, 4, and 6 of culture. The percentages of cells in each quadrant are indicated. (B) Addition of the caspase inhibitor Z-VAD-fmk (50 nM) to the culture media rescued the number of LYVE-1+ colonies from Tie2-/- ES cells (▪). (C) The number of PECAM-1+ colonies from Tie2-/- ES cells (▪) was also rescued in the presence of Z-VAD-fmk (50 nM). Results are expressed as mean ± SD. (D) In the presence of Z-VAD-fmk, the size of Tie2-/- lymphatic endothelial cells was unchanged, although the number of lymphatic colonies was partially rescued. Scale bars, 20 μm.
Discussion
Although we have demonstrated that Tie2 is expressed in the vitelline artery,19 the AGM region,4 and fetal liver,20 the function of Tie2 in development has not been elucidated. The early death of Tie2-/- embryos precludes detailed analysis of the role of Tie2 in development of hematopoietic and endothelial lineages. Using an ES cell differentiation system, we have analyzed the development of hematopoietic cells as well as BECs and LECs from Tie2-/- ES cells. At day 8 of culture on OP9 cells, the normal formation of hematopoietic clusters from Tie2-/- cells suggests that Tie2 signaling does not contribute to development of hematopoietic cells from precursors. Furthermore, differentiation of myeloid cells from Tie2-/- hematopoietic clusters was normal. These results indicate that Tie2 is not essential for development of hematopoietic cells from ES cells. Recently, Puri and Bernstein21 used combined mosaic analysis to demonstrate that Tie receptors are not required for differentiation and proliferation of definitive hematopoietic lineages in the embryo and fetus. Their findings are consistent with what we show in this study. Although our in vitro differentiation experiments suggest that Tie2 is not essential for hematopoiesis during development, Ang1/Tie2 signaling in hematopoietic cells has been reported to function to maintain hematopoietic stem cells in the bone marrow niche where Ang1 is expressed by osteoblasts.18 Thus Tie2 function in hematopoiesis would seem to be specific for adult bone marrow.
Although much is known about normal and pathologic development of the vascular system,35 the lack of specific markers has made it difficult to follow the development of the lymphatic system. In order to identify LECs, we recently generated a LEC-specific mAb against LYVE-1.26 This mAb allowed us to detect LECs in mouse embryos at midgestation and purify these cells. ES cell–derived LYVE-1+ cells express LEC-specific genes, such as Prox1, Pdpn, and VEGFR3, and exhibit DiI-Ac-LDL uptake. Furthermore, VEGF-C and VEGF-D, lymphatic growth factors, increased the number of LYVE-1–positive endothelial colonies from ES cells. These findings indicate that LYVE-1+ cells isolated from these cultures have many characteristics of LECs. Lymphatic vasculature of the thoracic and abdominal viscera have been proposed to arise by endothelial spreading from lymph sacs.6,36 This proposal is supported by the finding that budding of endothelial cells from the veins of Prox-1 mutant embryos is arrested.9,10 To clarify the mechanisms of lymphangiogenesis in vitro, we analyzed the function of Tie2. In this study LECs were differentiated from ES cell–derived Tie2+Flk1+ cells, and approximately 1% of Tie2+Flk1+ cells formed lymphatic endothelial colonies on OP9 cells. Our in vitro differentiation assay allows us to clarify the mechanisms of LEC development from its precursor.
Thus far, Tie2 signaling has been reported to be required for proper development and function of the vascular system. Mice lacking Ang2 exhibit lymphatic vessel defects, strongly suggesting a role of Tie2 signaling in lymphangiogenesis.37 In this study we have shown that LECs and BECs can be differentiated from Tie2+Flk1+ cells, and that 65% of LYVE-1+ LECs express Tie2. To analyze the function of Tie2 in the development of lymphatic endothelial cells, we performed FACS analysis and immunocytochemistry during differentiation of Tie2-/- ES cells. FACS analysis revealed that Tie2-/- cells expressing LYVE-1 decreased over time as did PECAM-1+ BECs. Treatment with a caspase inhibitor, which specifically inhibits apoptosis, partially rescued defective formation of lymphatic and blood vessel endothelial colonies from Tie2-/- cells, suggesting that both endothelial cells undergo apoptosis in the absence of Tie2 signaling. Although Tie2 signaling contributed to the survival of both LECs and BECs on OP9 cells, treatment with the caspase inhibitor did not affect the size of Tie2-/- colonies. Based on these findings, we propose that Tie2 cooperates with other signaling pathways involved in growth. Although we do not identify these pathways, OP9 cells are known to secrete several growth factors, including VEGF-C and VEGF-D.38 FACS analysis also revealed that at day 2 of culture, the frequency of LYVE-1+ and PECAM-1+ cells derived from Tie2-/- cells was comparable with frequencies seen in Tie2+/- cells, suggesting that lymphatic and blood vessel endothelial precursors develop normally from Tie2-/- cells. A gene expression study clearly showed that expression levels of Prox-1 in lymphatic precursors (the Flk1+ cell fraction) of Tie2-/- cells were comparable with those seen in Tie2+/- cells, suggesting that Tie2 deficiency does not affect Prox-1 expression. These findings suggest that Tie2 is not essential for development of lymphatic and blood vessel endothelial precursors, although both types of endothelial cells from Tie2-/- cells undergo apoptosis due to lack of Tie2 signaling.
Regarding vascular endothelial cells, in vivo study has shown that mice lacking Ang1 and Tie2 develop a fairly normal primary vasculature, but that this vasculature fails to undergo further normal remodeling.28,39,40 We have demonstrated that lymphatic endothelial cells express Tie2 in both embryonic and adult settings, and that the activation of Tie2 signaling by Ang1 stimulates both in vivo lymphatic angiogenesis in mouse cornea and in vitro colony formation of lymphatic endothelial cells.26 Data from both in vitro ES cell differentiation and in vivo embryonic development suggest that Ang/Tie2 signaling may contribute to regulation of lymphatic vessel formation in the development of lymphatic vessels.
In summary, our results derived from induction studies of ES cells have revealed that Tie2 is expressed in the precursors of mesodermal lineages, hematopoietic cells, and endothelial cells. We have also shown that Tie2 is not essential for development of hematopoietic cells, but that it plays an important role in antiapoptotic signaling in lymphatic and blood vessel development.
Acknowledgments
We thank Ms Ayami Ono for expert assistance in the FACS analysis.
Prepublished online as Blood First Edition Paper, October 11, 2005; DOI 10.1182/blood-2005-05-1823.
Supported in part by Grants-in-Aid from Ministry of Education, Science, Technology, Sports, and Culture, Japan; and by a research grant from the Human Frontiers Science Program Organization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
- 1.Sabin F. Studies on the origin of blood vessels and red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Contribut Embryol. 1920;9: 213-262. [Google Scholar]
- 2.Kubo H, Alitalo K. The bloody fate of endothelial stem cells. Genes Dev. 2003;17: 322-329. [DOI] [PubMed] [Google Scholar]
- 3.Haar JL, Ackerman GA. A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat Rec. 1971;170: 199-223. [DOI] [PubMed] [Google Scholar]
- 4.Hamaguchi I, Huang XL, Takakura N, et al In vitro hematopoietic and endothelial cell development from cells expressing TEK receptor in murine aorta-gonad-mesonephros region. Blood. 1999;93: 1549-1556. [PubMed] [Google Scholar]
- 5.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125: 725-732. [DOI] [PubMed] [Google Scholar]
- 6.Sabin FR. On the origin of the lymphatic system from the veins, and the development of the lymph hearts and thoracic duct in the pig. Am J Anat. 1902;1: 367-389. [Google Scholar]
- 7.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16: 773-783. [DOI] [PubMed] [Google Scholar]
- 8.Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276: 1423-1425. [DOI] [PubMed] [Google Scholar]
- 9.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98: 769-778. [DOI] [PubMed] [Google Scholar]
- 10.Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21: 1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376: 62-66. [DOI] [PubMed] [Google Scholar]
- 12.Shalaby F, Ho J, Stanford WL, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89: 981-990. [DOI] [PubMed] [Google Scholar]
- 13.Millauer B, Wizigmann-Voos S, Schnurch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72: 835-846. [DOI] [PubMed] [Google Scholar]
- 14.Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203: 80-92. [DOI] [PubMed] [Google Scholar]
- 15.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255: 989-991. [DOI] [PubMed] [Google Scholar]
- 16.Dumont DJ, Gradwohl GJ, Fong GH, Auerbach R, Breitman ML. The endothelial-specific receptor tyrosine kinase, tek, is a member of a new subfamily of receptors. Oncogene. 1993;8: 1293-1301. [PubMed] [Google Scholar]
- 17.Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterization of mouse TIE and TEK receptor tyrosine kinase genes and their expression in hematopoietic stem cells. Biochem Biophys Res Commun. 1993;195: 301-309. [DOI] [PubMed] [Google Scholar]
- 18.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118: 149-161. [DOI] [PubMed] [Google Scholar]
- 19.Takakura N, Huang XL, Naruse T, et al. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity. 1998;9: 677-686. [DOI] [PubMed] [Google Scholar]
- 20.Hsu HC, Ema H, Osawa M, Nakamura Y, Suda T, Nakauchi H. Hematopoietic stem cells express Tie-2 receptor in the murine fetal liver. Blood. 2000;96: 3757-3762. [PubMed] [Google Scholar]
- 21.Puri MC, Bernstein A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc Natl Acad Sci U S A. 2003;100: 12753-12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8: 529-532. [DOI] [PubMed] [Google Scholar]
- 23.Papapetropoulos A, Garcia-Cardena G, Dengler TJ, Maisonpierre PC, Yancopoulos GD, Sessa WC. Direct actions of angiopoietin-1 on human endothelium: evidence for network stabilization, cell survival, and interaction with other angiogenic growth factors. Lab Invest. 1999;79: 213-223. [PubMed] [Google Scholar]
- 24.Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448: 249-253. [DOI] [PubMed] [Google Scholar]
- 25.Jones N, Voskas D, Master Z, Sarao R, Jones J, Dumont DJ. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2: 438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105: 4649-4656. [DOI] [PubMed] [Google Scholar]
- 27.Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105: 4624-4648. [DOI] [PubMed] [Google Scholar]
- 28.Dumont DJ, Gradwohl G, Fong GH, et al Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8: 1897-1909. [DOI] [PubMed] [Google Scholar]
- 29.Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14: 5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yagi T, Tokunaga T, Furuta Y, et al. A novel ES cell line, TT2, with high germline-differentiating potency. Anal Biochem. 1993;214: 70-76. [DOI] [PubMed] [Google Scholar]
- 31.Hooper M, Hardy K, Handyside A, Hunter S, Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326: 292-295. [DOI] [PubMed] [Google Scholar]
- 32.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90: 8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takakura N, Yoshida H, Ogura Y, Kataoka H, Nishikawa S. PDGFR alpha expression during mouse embryogenesis: immunolocalization analyzed by whole-mount immunohistostaining using the monoclonal anti-mouse PDGFR alpha antibody APA5. J Histochem Cytochem. 1997;45: 883-893. [DOI] [PubMed] [Google Scholar]
- 34.Yano M, Iwama A, Nishio H, Suda J, Takada G, Suda T. Expression and function of murine receptor tyrosine kinases, TIE and TEK, in hematopoietic stem cells. Blood. 1997;89: 4317-4326. [PubMed] [Google Scholar]
- 35.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13: 1055-1066. [DOI] [PubMed] [Google Scholar]
- 36.Gray H. In Anatomy of the human body. In: Clemente CD, ed. The Lymphatic System. Philadelphia, PA: Lea and Febiger; 1985: 866-932.
- 37.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3: 411-423. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura K, Hirashima M, Ogawa M, et al. Modulation of VEGFR-2-mediated endothelial-cell activity by VEGF-C/VEGFR-3. Blood. 2003;101: 1367-1374. [DOI] [PubMed] [Google Scholar]
- 39.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87: 1171-1180. [DOI] [PubMed] [Google Scholar]
- 40.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376: 70-74. [DOI] [PubMed] [Google Scholar]


