Abstract
Recombinant hepatitis C virus (HCV)-like particles (HCV-LPs) containing HCV structural proteins (core, E1, and E2) produced in insect cells resemble the putative HCV virions and are capable of inducing strong and broad humoral and cellular immune responses in mice and baboons. Here, we present evidence on the immunogenicity and induction of protective immunity by HCV-LPs in chimpanzees. Chimpanzees (two in each group), were immunized with HCV-LPs or HCV-LPs plus AS01B adjuvant. After immunizations, all animals developed an HCV-specific immune response including IFN-γ+, IL-2+, CD4+, and CD8+ T cell and proliferative lymphocyte responses against core, E1, and E2. Upon challenge with an infectious HCV inoculum, one chimpanzee developed transient viremia with low HCV RNA titers (103 to 104 copies per ml) in the third and fourth weeks after the challenge. The three other chimpanzees became infected with higher levels of viremia (104 to 105 copies per ml), but their viral levels became unquantifiable (<103 copies per ml) 10 weeks after the challenge. After the HCV challenge, all four chimpanzees demonstrated a significant increase in peripheral and intrahepatic T cell and proliferative responses against the HCV structural proteins. These T cell responses coincided with the fall in HCV RNA levels. Four naïve chimpanzees were infected with the same HCV inoculum, and three developed persistent infection with higher viremia in the range of 105 to 106 copies per ml. Our study suggests that HCV-LP immunization induces HCV-specific cellular immune responses that can control HCV challenge in the chimpanzee model.
Keywords: envelope proteins, prevention, protective immunity, vaccine, viral clearance
Hepatitis C virus (HCV) is a major public health problem. Approximately 170 million people are infected by the virus worldwide (1, 2). HCV causes a high rate of chronic infection, which can lead to complications of chronic liver disease such as liver cirrhosis and hepatocellular carcinoma. The efficacy of therapy for chronically infected patients is less than satisfactory. Development of an effective vaccine may hold the key to controlling HCV infection. HCV displays high genetic and antigenic diversities, with at least six different genotypes and diverse quasispecies within infected individuals (1, 2). In addition to this inherent problem, the lack of convenient and robust tissue culture systems and small animal models further hampers the effort to develop an effective HCV vaccine (3). The use of recombinant HCV envelope proteins as a vaccine candidate has been met with variable success. High titers of anti-E1/E2 antibodies could be induced and possibly resulted in lower propensity to chronicity upon HCV challenge (3–5). Recently, a combined modality of plasmid DNA and adenoviral vector to deliver HCV structural and nonstructural antigens induced a strong HCV-specific cell-mediated immunity in chimpanzees and resulted in attenuated HCV infection after challenge (6). However, in this study, no obvious difference was observed in the rate of chronicity between the vaccinated and control chimpanzees. As for HIV, a more realistic goal for an HCV vaccine would be preventing chronicity and disease as opposed to inducing sterilizing immunity.
Virus-like particles are attractive as a recombinant protein vaccine for virus, because they closely mimic the properties of native virions. We have previously reported the synthesis of HCV-like particles (HCV-LPs) by using a recombinant baculovirus containing the cDNA of HCV structural proteins, core, E1, and E2 (7). The HCV-LPs induced virus-specific humoral and cellular immune responses in BALB/c mice (8) and HLA-A 2.1 transgenic (AAD) mice (9, 10). These HCV-LP-induced immune responses protected mice from challenge with recombinant HCV–vaccinia expressing HCV structural proteins (vvHCV.S) in a surrogate HCV–vaccinia challenge model (9). Recently, we also demonstrated the safety and immunogenicity of HCV-LPs in a nonhuman primate model, the baboon (11). The chimpanzee is the only established animal model susceptible to HCV infection (3). In this study, we evaluate the immunogenicity of HCV-LPs in the chimpanzee.
Results
Safety of HCV-LP and Adjuvant in Chimpanzees.
HCV-LP immunizations were well tolerated. All chimpanzees were healthy throughout the immunization period, and there were no adverse signs at the injection sites and no systemic reactions to the HCV-LPs or the adjuvant. Differential blood counts and blood chemistry performed before and after each immunization were normal.
Induction of T Cell Responses by HCV-LP Immunization.
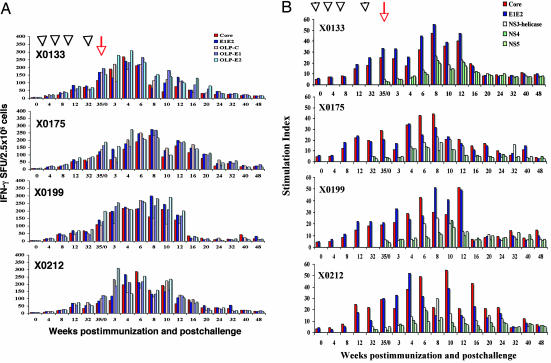
To quantify T cell responses against HCV structural proteins in chimpanzees immunized with HCV-LPs alone or HCV-LPs and adjuvant, we performed enzyme-linked immunosorbent spot (ELISPOT) assays for IFN-γ- and IL-2-secreting cells and proliferation assays for T cells. At weeks −4 and 0, neither IFN-γ- or IL-2-secreting T cells nor proliferative responses were detected against any of the HCV structural proteins or peptides. HCV-specific IFN-γ response was minimally detectable after the first two immunization doses. After the third immunization, the T cell activities to HCV proteins and/or overlapping peptide pools (OLPs) were significantly above baseline values (Fig. 1A). The T cell responses induced after the third dose showed IFN-|gg+ responses targeting multiple HCV antigens. The induced HCV-specific T cell responses persisted for 6 months before the final immunization (Fig. 1A). After the fourth dose of HCV-LPs was administered, a rapid recall response was observed in all animals. Combining the HCV proteins and OLP responses, the total frequency of IFN-producing T cells 3 weeks after the fourth immunization reached ≈0.35% of the peripheral blood mononuclear cell (PBMC) population. No major differences in the numbers of IFN-γ-secreting cells were observed between the HCV-LP and the HCV-LP plus adjuvant groups (Fig. 1A).
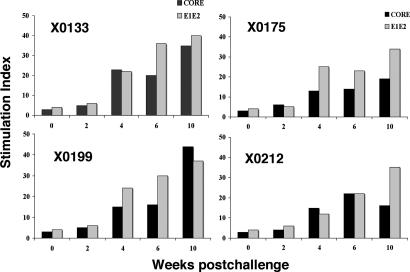
Fig. 1.
Time course and magnitude of HCV-specific T cell immune responses during HCV-LP immunization and after challenge. Four chimpanzees (X0133, X0175, X0199, and X0212) were immunized with HCV-LPs four times over 32 weeks. Arrowheads indicate immunization times, and the arrow indicates the time of HCV-CG1b challenge at week 35 (week 0). (A) IFN-γ response against HCV core, E1/E2, and OLPs. (B) Lymphocyte proliferative responses against HCV core and E1/E2 proteins were assessed over 48 weeks. In each assay, 2 × 105 cells were used. The T cell proliferative response is expressed as stimulation index. (C) IFN-γ responses against HCV nonstructural proteins NS3 helicase, NS4, and NS5A were determined over 48 weeks. NA, not analyzed. SFU, spot-forming unit.
Recent studies have suggested the importance of IL-2-secreting T cells in vaccine-induced protective immunity (12). To investigate the capacity of HCV-LP to induce IL-2 T cell response, PBMCs from the four chimpanzees were stimulated with HCV structural proteins and/or HCV structural protein peptide pools, and were analyzed by IL-2 ELISPOT. The HCV-specific IL-2 T cell response became readily detectable after the third dose of HCV-LP [supporting information (SI)]. The IL-2 response persisted for 6 months and then increased dramatically after the fourth immunization. No major differences in the frequencies of IL-2-secreting cells were observed between the HCV-LP and the HCV-LP plus adjuvant groups. The kinetics and frequencies of both IFN-γ- and IL-2-secreting cells were similar. HCV-specific IL-4-secreting cells were not detected by IL-4 ELISPOT (data not shown).
In addition to the IFN-γ and IL-2 HCV-specific T cell responses generated by the HCV-LP vaccine, we analyzed the proliferative T cell responses to HCV structural proteins. Like the IFN-γ response, a weak T cell proliferative response was detected after the first and second immunizations of the chimpanzees with HCV-LPs (Fig. 1B). The third immunization led to a boost of T helper responses against the HCV core and E1/E2 antigens in all four chimpanzees, which also persisted for 6 months. The HCV-specific T helper responses were further enhanced and an anamnestic immune response was observed after the fourth dose of HCV-LPs (Fig. 1B).
HCV-Specific CD4 and CD8 T Cell Responses.
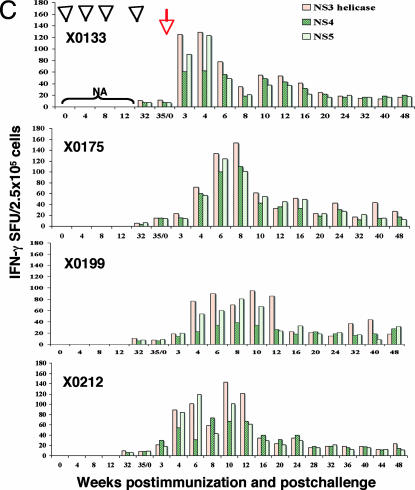
To examine the CD4- and CD8-specific T cell responses induced by HCV-LP, CD4+ and CD8+ T cells were isolated from PBMCs 2 weeks after the fourth immunization with HCV-LP. The isolated CD4+ cells were stimulated with recombinant core or E1/E2 proteins or with four OLPs (core, E1, and two pools of E2). IFN-γ+CD4+ T cell responses, as assessed by ELISPOT, were detected in all of the immunized chimpanzees (Fig. 2A). The frequency of all HCV-specific IFN-γ-producing CD4+ T cells 2 weeks after the fourth immunization was ≈0.27% of the total number of PBMCs.
Fig. 2.
CD4+ and CD8+ T cell responses in chimpanzees immunized with HCV-LPs. Chimpanzees were immunized with HCV-LPs four times. Two weeks after the fourth HCV-LP dose, CD4+ (A) and CD8+ (B) T cells were isolated and stimulated with recombinant core, E1/E2 proteins, and HCV-overlapping peptides as indicated. The HCV-specific IFN-γ SFUs are expressed as numbers of spots per 1 × 105 cells.
The CD8+ T cells were stimulated with HCV core and E1/E2 OLPs and assessed by ELISPOT assay. As shown in Fig. 2B, all of the immunized chimpanzees demonstrated HCV-specific IFN-γ in response to HCV antigens. Combining all of the OLP responses, the frequency of IFN-γ-producing CD8+ T cells 2 weeks after the fourth immunization reached 0.23% of the total number of PBMCs. The use of adjuvant AS01B did not affect either CD4+ or CD8+ T cell responses.
Vaccine-Induced Control of HCV Challenge.
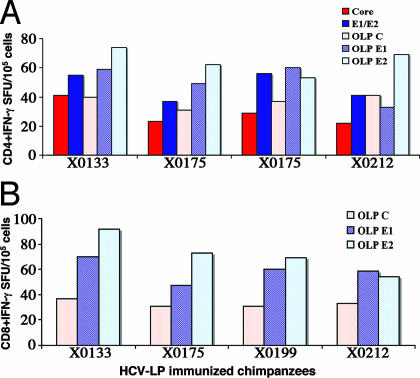
Three weeks after the fourth immunization with HCV-LPs, the animals were challenged with homologous 100 50% chimpanzee-infectious doses (CID50) of HCV-CG1b. All of the HCV-LP-immunized chimpanzees developed HCV viremia 1–2 weeks after the challenge (Fig. 3A). Four naïve animals were inoculated with 3–10 CID50 of the same HCV-CG1b inoculum. Three developed chronic infection and one had self-limited acute infection. The infection courses of the naïve chimpanzees have been described (see refs. 13 and 14). These four chimpanzees represent appropriate comparison as controls for the efficacy of the immunization protocol. In comparison, three vaccinated chimpanzees showed a blunted peak viremia of 104 to 105 genomes per ml from weeks 2 to 12 after the challenge (Fig. 3A and Table 1). The fourth animal, X0133, developed only a transient viremia of 103 to 104 genomes per ml for 2 weeks. These acute-phase viremia levels were lower than those seen in the four control animals (Fig. 3B and Table 1). HCV-LP-vaccinated chimpanzees maintained a low viral level and the average peak viremia was ≈7–20× lower than that of the control group (P = 0.029). The duration of postchallenge viremia in vaccinated chimpanzees was less than that of the control animals. The area under the curve (AUC) representing viremia × duration of viremia for 1 year was significantly lower in the immunized animals compared with the controls (P = 0.0016; Table 1). Some of the follow-up serum samples were positive by ultrasensitive transcription-mediated amplification (TMA) assay (detection limit of 50 copies per ml), despite testing negative by PCR (Fig. 3A). All of the vaccinated chimpanzees were TMA-negative at the end of 1 year, and two of the animals, X0133 and X0199, were repeatedly negative by TMA assay for >2 years.
Fig. 3.
The time course of HCV infection in HCV-LP-immunized chimpanzees. (A) Four chimpanzees were immunized four times over 32 weeks with HCV-LPs. Three weeks after the fourth dose (35/0), the chimpanzees were challenged with homologous HCV-CG1b strain. Arrowheads indicate immunization times and the arrow indicates the time of the HCV-CG1b challenge. HCV RNA titers and alanine transaminase (ALT) levels are shown. Black bars represent HCV RNA in log10(copies per milliliter) (right ordinates) and the lined gray zone represents ALT levels in units/ml (left ordinates). Anti-HCV seroconversion is indicated as − or + at the top of each graph. Some of the samples (negative by PCR) were tested for HCV RNA by transcription-mediated amplification (TMA) and the results are shown. Follow-up samples beyond 1 year after the challenge were available for TMA testing in chimpanzees X0133 and X0199. (B) Comparison of HCV viremia between naïve and HCV-LP-immunized chimpanzees after the challenge. Previously, four control chimpanzees were infected with HCV-CG1b strain at a dose of 3–10 CID50 (15, 32); the courses of HCV viremia are shown (Right). The viral titers were all converted to units per milliliter for ease of comparison because HCV quantification was done with different assays. For comparison, the viremia courses of the four HCV-LP-immunized chimpanzees are shown together (Left).
Table 1.
Viremia comparison of naïve and HCV-LP-immunized chimpanzees
| Chimpanzee | Duration of peak viremia, weeks | Peak viremia, units/ml* | AUC,† duration × peak viremia for 52 weeks |
|---|---|---|---|
| HCV-LP | |||
| X0133 | 2 | 3,970 | 7,840 |
| X0175 | 11 | 14,000 | 41,490 |
| X0199 | 10 | 35,400 | 206,140 |
| X0212 | 12 | 54,600 | 295,090 |
| Naïve | |||
| X0142 | >52 | 78,000 | 667,220 |
| X0190 | 13 | 99,000 | 600,000 |
| X0234 | >52 | 200,000 | 497,000 |
| X0140 | >52 | 257,340 | 746,575 |
*Peak viremia; P< 0.029, naïve vs. HCV-LP-immunized animals.
†Area under the curve; P< 0.0016, naïve vs. HCV-LP-immunized animals.
During the challenge, liver injury was assessed by measuring alanine aminotransferase (ALT) levels. In X0133 and X0175, ALT levels showed a slight elevation (75 units/liter) only in weeks 21 and 26, respectively (Fig. 3A). Otherwise, ALT remained within normal limits (25–40 units/liter) in the four HCV-LP immunized chimpanzees. The level of aspartic aminotransferase (AST) was also monitored during the course of the study and no elevation was observed (data not shown). Liver biopsy showed no histopathological changes.
HCV-Specific T Cell Responses After the Challenge.
PBMCs were analyzed for HCV-specific IFN-γ and IL-2 T cell responses by ELISPOT assay. All four chimpanzees showed early multispecific IFN-γ and IL-2 T cell responses to HCV structural antigens after the HCV challenge. Although each of the animals showed slightly different kinetics, they all displayed a heightened T cell response that corresponded to the viremic phase. The vigorous T cell response was maintained for several weeks beyond the disappearance of viremia, suggesting the importance of this induced immunity in viral clearance.
To investigate the immune response against the HCV nonstructural proteins, IFN-γ production was assessed by ELISPOT assay. Cryopreserved PBMCs, collected at different intervals before and after the challenge, were stimulated with HCV-NS3 helicase, NS4, and NS5A recombinant proteins. As shown in Fig. 1C, a strong IFN-γ response was detectable in X0133 3 weeks after the challenge, whereas the other three animals did not exhibit any positive response until week 4. The frequency of HCV-specific T cells continued to increase to variable duration among the animals but gradually declined to low but detectable levels over 1 year of follow-up (Fig. 1). X0133 had an early and brief peak of IFN-γ response against HCV nonstructural proteins, probably indicative of the transient viremia in this animal.
The lymphoproliferative responses after the challenge were also assessed against various HCV antigens. In contrast to the IFN-γ response, the T cell proliferative response against the HCV structural proteins did not increase significantly beyond the prechallenge level until 4–10 weeks later in most of the immunized chimpanzees (Fig. 1B). The chimpanzees also displayed T cell proliferative responses against HCV nonstructural proteins at later time points (6–8 weeks after the challenge). In all of the animals, the HCV-specific T cell responses declined gradually after viral clearance and the immune responses remained low, but detectable, up to 1 year later.
Antibody Response.
Despite repeated immunizations with HCV-LP, no, or barely detectable, antibody responses to core and E1/E2 were induced. However, chimpanzee X0133 quickly developed anti-HCV with predominantly anti-core antibodies 3 weeks after the challenge, suggesting the priming of B cell response by HCV-LP. Two other chimpanzees, X0175 and X0212, seroconverted to anti-HCV 6 months later, but X0199 never developed anti-HCV (follow-ups were performed for >2 years; Fig. 3A).
Intrahepatic T Cell Proliferative Responses.
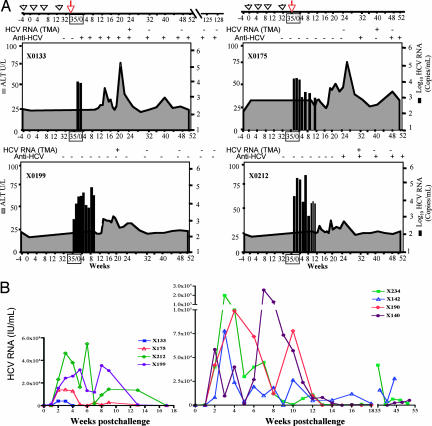
The immune response in the liver was assessed by studying the proliferative response of intrahepatic T cells against HCV structural proteins. Proliferative responses were studied 4, 6, 8, and 10 weeks after the challenge. After expansion in vitro, intrahepatic lymphocytes were stimulated with HCV core and/or E1/E2 proteins. These lymphocytes showed a gradual increase in proliferative response starting 4 weeks and peaking 10 weeks after the challenge (Fig. 4). The high proliferative responses at week 10 after the challenge corresponded to the major decrease in viral titers in the peripheral blood.
Fig. 4.
The magnitude of intrahepatic HCV-specific T cell proliferative responses in vaccinated chimpanzees. Intrahepatic lymphocytes were expanded from liver biopsies collected at multiple time points before immunization and after the challenge. The cells were stimulated with HCV core or E1/E2 recombinant proteins and proliferative responses were determined.
Discussion
Globally, persistent infection with HCV is an important cause of chronic liver disease. The development of vaccines to prevent HCV infection, or at least to prevent progression to chronicity, is a major goal. In this study, we performed a proof-of-principle preclinical experiment to test the immunogenicity and efficacy of the HCV-LPs as a vaccine candidate in chimpanzees. We have previously shown the induction of broadly directed HCV-specific humoral and cellular immune response by HCV-LP immunization in mice and baboons (9–11), and this induced immunity provided protection in a surrogate vaccinia-HCV challenge model (9). We reason that HCV-LPs, as a particulate multivalent antigen, are a potent immunogen that can interact with the immune system for effective antigen presentation in class I and II pathways (15–18).
In this study, we demonstrated that HCV immunization could also induce strong cellular immune response in chimpanzees. The response is broadly directed to all HCV structural proteins and consists predominantly of a T helper 1 response. Shortly after the challenge, the HCV-specific cytokine-producing T cells showed a further increase, reflecting an anamnestic response. The T cell proliferative response also showed a notable increase later. This delay of T cell proliferation in response to HCV challenge could represent the sequence of events in memory T cell response: effector memory cells responding first, followed by expansion of central memory cells. It is interesting to note that T cell response to the nonstructural proteins also emerged rapidly in the vaccinated chimpanzees (Fig. 1C), in contrast with the infected naïve chimpanzees. In our previous study, we showed that cellular immune response against HCV nonstructural proteins was weaker and detected much later during the acute phase of infection of naïve chimpanzees (13). This observation suggests that, upon HCV challenge, the vaccinated chimpanzees were primed to respond strongly to other HCV antigens that were not part of the vaccine. All these heightened T cell responses coincided with the major reduction in viral levels in the vaccinated chimpanzees' blood. Intrahepatic HCV-specific T cell responses also notably increased during the acute phase (4–10 weeks after infection), indicative of the influx of effector T cells to control HCV replication in the liver.
All of the vaccinated chimpanzees became infected upon HCV challenge, but the infection course was apparently attenuated. Although the number of animals in this study is not large, comparison of the HCV infection course in the HCV-LP-immunized animals with that of the naïve control animals suggests two important differences. First, all of the vaccinated animals quickly controlled HCV infection (HCV RNA-negative by PCR), whereas 75% of the naïve animals challenged with a 10- to 30-fold lower dose of HCV (3–10 CID50) became chronically infected with high-level viremia (P = 0.029). Second, the peak viral titers in the vaccinated animals were 5- to 10-fold lower than those of the control chimpanzees. The overall assessment of the HCV infection course, as represented by the area under the curve (AUC) of viremia × duration, showed a highly statistically significant difference between the vaccinated and naïve animals (Table 1). One could argue that the chimpanzees were challenged with different doses of HCV and such a difference could explain the outcome of infection. However, this is highly unlikely, because the rate of chronicity has not been shown to depend on the inoculation dose and a higher inoculation dose in the vaccinated animals would be more likely to be associated with a higher level of initial viremia. The high chronicity rate of HCV-1b-infected chimpanzees has been reported and is consistent with what we have observed in the naïve animals (4, 19–21).
Two of the vaccinated chimpanzees, X0133 and X0199, were repeatedly negative by TMA for >2 years after the challenge. However, some serum samples of the vaccinated chimpanzees were positive for very low-level HCV RNA by this assay during follow-up despite testing negative by PCR. It is not clear what this low level of HCV signifies. Such a low-level HCV has been reported in patients with either spontaneous or treatment-induced recovery from HCV infection (22–24). It is possible that HCV may persist in various tissue reservoirs despite serologic evidence of viral clearance. Periodic exposure to low-level HCV antigens might explain the persistence of HCV-specific T cell response more than 20 years later in individuals who have cleared infection (25).
The absence of significant antibody production against either core or E1/E2 by HCV immunization is surprising because our previous studies in mice and baboons showed induction of anti-HCV antibodies, although the antibody titers induced in baboons were not high (11). It is possible that the immunization dose we used is disproportionally lower than the doses used in the mouse and baboon studies, 20 and 50 μg of HCV-LP per immunization, respectively. In this study, we used 100 μg of HCV-LP. Adult chimpanzees weigh ≈60–70 kg, baboons weigh ≈20–25 kg, and mice weigh only 50–100 g. Therefore, the chimpanzees received a much lower dose relative to weight. Perhaps with a higher dose of HCV-LP, antibodies could be induced. Nevertheless, the “suboptimal” dose of HCV-LP was able to induce cell-mediated immunity sufficient to render partial protection upon HCV challenge. Future studies would have to focus on determining the optimal dose and regimen of HCV-LP immunization that can maximally induce both humoral and cellular immune responses.
The longevity of vaccine-induced T cell responses is an important issue because long-term protection is a requirement for an effective prophylactic vaccine. The first two doses did not induce a detectable HCV-specific immune response. However, the third dose markedly enhanced the response, which remained high for at least 6 months. This sustained HCV-specific T cell response was further boosted by the fourth immunization 6 months later. This regimen of vaccination and boosting is crucial in the activation and expansion of memory T cell responses to elicit a robust and long-term immunity in these animals and might explain the quick and potent anamnestic response in the chimpanzees after the challenge. Overall, the HCV-specific T cell responses rapidly expanded after the challenge and resulted in effective viral control in vaccinated chimpanzees.
The use of the AS01B adjuvant did not enhance the HCV-LP-induced immune response in chimpanzees. This observation is in contrast to the significant effect of this adjuvant in the mouse study (8–10). Although a minor enhancing effect of this adjuvant was observed in our previous baboon study (11), we believe this adjuvant in formulation with HCV-LPs is probably not useful in higher primates, including humans. It is possible that HCV-LPs, as a particulate antigen, interact efficiently with the immune system in antigen presentation and do not require the boosting effect of an adjuvant to induce an immune response.
HCV-LPs are safe and noninfectious, and it is easy to scale up their production. They are an effective immunogen for the induction of cell-mediated immunity. Various approaches to HCV vaccine have been tried (3, 5, 6, 26, 27). Several of them have been tested in chimpanzees and have shown promise (4, 6). Recombinant envelope proteins induced high titers of neutralizing antibodies, but apparently without cellular immune response. A prime–boost approach using DNA immunization followed by recombinant adenovirus expressing HCV nonstructural proteins induced strong T cell responses (6). Both approaches resulted in partial protection that included attenuated acute infection and/or reduced chronicity. Although one cannot compare these studies directly because of the difference in inoculum and vaccine regimens, HCV-LP immunization induces a similarly effective protective immunity in chimpanzees. This potential success of the HCV-LPs, which are a protein-based immunogen without other components, is actually quite encouraging because this approach could be improved by some simple strategies. First, the dose and schedule could be optimized to induce both humoral and cellular immune responses, which are probably equally important in vaccine-induced protective immunity for HCV infection. Second, the HCV-LPs could be engineered to carry sequences of nonstructural proteins that are important for viral clearance, such as the T cell epitopes in the NS3 and NS5 regions. Finally, the HCV-LPs could be combined with other promising candidates in a prime–boost regimen to induce a highly protective and lasting immunity. Future efforts are needed to address these issues and to build on the potential of HCV-LP technology for HCV vaccine development. The virus-like particle strategy has already proven to be a highly promising approach for vaccine development, as evidenced by the recent success of human papillomavirus vaccine based on recombinant virus-like particles (28, 29).
Materials and Methods
Preparation of HCV Proteins, Synthetic Peptides, and HCV-LP.
E1 and E2 proteins were expressed in vvHCV.S-infected BSC-1 cells and purified by lectin column chromatography (30). HCV core protein (amino acids 1–115) was purchased from Mikrogen (Neuried, Germany). Synthetic peptides spanning the whole structural genome of the HCV-CG1b strain were synthesized by Mimotopes (Clayton, Victoria, Australia). Peptides were 15 aa in length with a 10-aa overlap. OLP1 consists of 38 peptides spanning the HCV core sequence, OLP2 consists of 38 peptides spanning the HCV E1 sequence, OLP3 consists of 35 peptides spanning the N-terminal half of the HCV E2 sequence, and OLP4 consists of 36 peptides spanning the C-terminal half of the HCV E2 sequence. Peptide stock solutions were prepared in 5% dimethyl sulfoxide in PBS at a concentration of 1 mg/ml and stored at −20°C. Preparation and purification of HCV-LPs were carried out as described previously (7, 11), with some modifications (details provided in the SI). The AS01B adjuvant is a combination of monophosphoryl lipid A and QS21 saponin (provided by GlaxoSmithKline, Rixensart, Belgium) (11).
Chimpanzee Study.
Four naïve chimpanzees (Pan troglodytes), X0133, X0175, X0199, and X0212, 25–28 years old, were chosen for this study. The animals were housed under special conditions at Southwest Foundation for Biomedical Research (San Antonio, TX) under protocols approved by the institution's Animal Care and Use Committee and the Public Health Service Interagency Model Committee. Hematological and other laboratory values, including liver enzymes such as serum ALT and serum AST, were monitored at regular intervals. All animal experiments were conducted according to the criteria published by the National Institutes of Health (NIH publication 86-23, revised in 1985).
Animals were divided into two groups, two chimpanzees per group. X0133 and X0212 received 100 μg of HCV-LP in 1 ml of saline by injection into the gluteal muscle, whereas X0175 and X0199 received HCV-LP plus AS01B at the same dose, volume, and injection route. The chimpanzees were boosted at weeks 4, 8, and 32. All HCV-LP-immunized chimpanzees were challenged 3 weeks after the fourth immunization dose by an i.v. administration of 100 CID50 of homologous monoclonal HCV-CG1b virus. The virus stock was diluted with PBS containing 10% autologous preimmune plasma. Serum samples were collected weekly after HCV challenge for the first 9 weeks, biweekly for 12 weeks, and every 4 weeks for the remainder of the follow-up. Quantitative RT-PCRs for the determination of HCV viral load and liver enzyme tests were performed on the serum samples. The detection limit of the quantitative PCR was ≈1,000 copies per ml. An ultrasensitive TMA assay (VERSANT HCV RNA Qualitative Assay; Bayer Diagnostics, Berkeley, CA) with a detection limit of <50 copies per ml was also used for detection of HCV in selected samples.
The chimpanzees were followed for at least 1 year after the challenge: physical examinations were performed and vital signs were tested regularly. Body temperature and local and systemic signs were monitored and registered. Differential blood counts and blood chemistry were performed before and after each immunization. Liver enzymes remained within the normal range.
Lymphocyte Samples and Liver Biopsies.
Blood samples were collected before immunization (weeks −4 and 0), during immunization (every 4 weeks after each immunization dose), and after the HCV challenge (every 2 weeks for 3 months, then every month for 9 months). PBMCs were cryogenically preserved at −180°C for various immunological assays. Liver biopsies were obtained before immunization and biweekly for 10 weeks starting 2 weeks after the challenge. Liver biopsies were used for postchallenge assessment of cellular immune response and fixed liver tissue was examined for histological changes.
IFN-γ, IL-2, IL-4 ELISPOT, T Cell Proliferation, and Anti-HCV Assays.
The cryopreserved PBMCs were analyzed by ELISPOT assay for numbers of antigen-specific IFN-γ-, IL-2-, or IL-4-producing cells. Antigen-specific spot-forming units (SFUs) for each sample were calculated by subtracting the average background values (four wells without antigen, typically <10 spots). The sample was considered positive when the background-corrected SFU was >10 and at least twice the mean SFU of the preimmunization samples in the same animal (details provided in the SI). Standard T cell proliferation assays were performed on PBMCs collected at various time points, as described in the SI. Enzyme-linked immunoassay (EIA) for anti-core or E1/E2 has been described previously (8). Anti-HCV seroconversion after the challenge was monitored by the commercially available assay EIA 2.0 (Abbott Laboratories, Abbott Park, IL).
Statistical Analysis.
Total area under the curve was calculated by using GraphPad Prism 4 (GraphPad Software, San Diego, CA). The difference between the two groups was analyzed by using nonparametric Mann–Whitney tests for peak viremia and unpaired two-tailed t tests for area under the curve. The difference in chronicity rates was calculated by χ2 test. P values of <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Barbara Rehermann and Harvey Alter for helpful discussion and assistance and the National Institutes of Health Clinical Center Department of Transfusion Medicine for the virological testing. This work was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and under contract N01-HB-27091 of National Heart, Lung, and Blood Institute.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartic aminotransferase
- CID50
50% chimpanzee-infectious doses
- ELISPOT
enzyme-linked immunosorbent spot
- HCV
hepatitis C virus
- HCV-LP
hepatitis C virus-like particle
- OLP
overlapping peptide pool
- PBMC
peripheral blood mononuclear cell
- SFU
spot-forming unit
- TMA
transcription-mediated amplification.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702162104/DC1.
References
- 1.Lauer GM, Walker BD. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Houghton M, Abrignani S. Nature. 2005;436:961–966. doi: 10.1038/nature04081. [DOI] [PubMed] [Google Scholar]
- 4.Puig M, Major ME, Mihalik K, Feinstone SM. Vaccine. 2004;22:991–1000. doi: 10.1016/j.vaccine.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Youn JW, Park SH, Lavillette D, Cosset FL, Yang SH, Lee CG, Jin HT, Kim CM, Shata MT, Lee DH, et al. Hepatology. 2005;42:1429–1436. doi: 10.1002/hep.20934. [DOI] [PubMed] [Google Scholar]
- 6.Folgori A, Capone S, Ruggeri L, Meola A, Sporeno E, Ercole BB, Pezzanera M, Tafi R, Arcuri M, Fattori E, et al. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 7.Baumert TF, Ito S, Wong DT, Liang TJ. J Virol. 1998;72:3827–3836. doi: 10.1128/jvi.72.5.3827-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechmann M, Murata K, Satoi J, Vergalla J, Baumert TF, Liang TJ. Hepatology. 2001;34:417–423. doi: 10.1053/jhep.2001.26523. [DOI] [PubMed] [Google Scholar]
- 9.Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ, Liang TJ. Proc Natl Acad Sci USA. 2003;100:6753–6758. doi: 10.1073/pnas.1131929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao M, Murata K, Davis AR, Jeong SH, Liang TJ. Hepatology. 2003;37:52–59. doi: 10.1053/jhep.2003.50000. [DOI] [PubMed] [Google Scholar]
- 11.Jeong SH, Qiao M, Nascimbeni M, Hu Z, Rehermann B, Murthy K, Liang TJ. J Virol. 2004;78:6995–7003. doi: 10.1128/JVI.78.13.6995-7003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantaleo G, Koup RA. Nat Med. 2004;10:806–810. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 13.Thomson M, Nascimbeni M, Havert MB, Major M, Gonzales S, Alter H, Feinstone SM, Murthy KK, Rehermann B, Liang TJ. J Virol. 2003;77:862–870. doi: 10.1128/JVI.77.2.862-870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato T, Matsumura T, Heller T, Saito S, Sapp RK, Murthy K, Wakita T, Liang TJ. J Virol. 2007;81:4405–4411. doi: 10.1128/JVI.02334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohm W, Schirmbeck R, Elbe A, Melber K, Diminky D, Kraal G, van Rooijen N, Barenholz Y, Reimann J. J Immunol. 1995;155:3313–3321. [PubMed] [Google Scholar]
- 16.Luo L, Li Y, Chang JS, Cho SY, Kim TY, Choi MJ, Cheong HS, Kim HJ, Ahn HJ, Min MK, et al. Virology. 1998;240:316–325. doi: 10.1006/viro.1997.8922. [DOI] [PubMed] [Google Scholar]
- 17.Greenstone HL, Nieland JD, de Visser KE, De Bruijn ML, Kirnbauer R, Roden RB, Lowy DR, Kast WM, Schiller JT. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barth H, Ulsenheimer A, Pape GR, Diepolder HM, Hoffmann M, Neumann-Haefelin C, Thimme R, Henneke P, Klein R, Paranhos-Baccala G, et al. Blood. 2005;105:3605–3614. doi: 10.1182/blood-2004-05-1952. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto H, Mishiro S, Tokita H, Tsuda F, Miyakawa Y, Mayumi M. Hepatology. 1994;20:1131–1136. [PubMed] [Google Scholar]
- 20.Rollier C, Depla E, Drexhage JA, Verschoor EJ, Verstrepen BE, Fatmi A, Brinster C, Fournillier A, Whelan JA, Whelan M, et al. J Virol. 2004;78:187–196. doi: 10.1128/JVI.78.1.187-196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Hepatology. 2001;33:1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 22.Pham TN, MacParland SA, Mulrooney PM, Cooksley H, Naoumov NV, Michalak TI. J Virol. 2004;78:5867–5874. doi: 10.1128/JVI.78.11.5867-5874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radkowski M, Gallegos-Orozco JF, Jablonska J, Colby TV, Walewska-Zielecka B, Kubicka J, Wilkinson J, Adair D, Rakela J, Laskus T. Hepatology. 2005;41:106–114. doi: 10.1002/hep.20518. [DOI] [PubMed] [Google Scholar]
- 24.Feld JJ, Liang TJ. Hepatology. 2005;41:23–25. doi: 10.1002/hep.20561. [DOI] [PubMed] [Google Scholar]
- 25.Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 26.Forns X, Payette PJ, Ma X, Satterfield W, Eder G, Mushahwar IK, Govindarajan S, Davis HL, Emerson SU, Purcell RH, Bukh J. Hepatology. 2000;32:618–625. doi: 10.1053/jhep.2000.9877. [DOI] [PubMed] [Google Scholar]
- 27.Leroux-Roels G, Depla E, Hulstaert F, Tobback L, Dincq S, Desmet J, Desombere I, Maertens G. Vaccine. 2004;22:3080–3086. doi: 10.1016/j.vaccine.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, Zahaf T, Innis B, Naud P, De Carvalho NS, et al. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 29.Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, Chiacchierini LM, Jansen KU. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 30.Baumert TF, Vergalla J, Satoi J, Thomson M, Lechmann M, Herion D, Greenberg HB, Ito S, Liang TJ. Gastroenterology. 1999;117:1397–1407. doi: 10.1016/s0016-5085(99)70290-8. [DOI] [PubMed] [Google Scholar]
- 31.Sarmiento M, Batterson WW. J Virol Methods. 1992;36:151–157. doi: 10.1016/0166-0934(92)90146-5. [DOI] [PubMed] [Google Scholar]
- 32.Nascimbeni M, Mizukoshi E, Bosmann M, Major ME, Mihalik K, Rice CM, Feinstone SM, Rehermann B. J Virol. 2003;77:4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.