Abstract
Despite the central role of xyloglucan (XyG) in plant cell wall structure and function, important details of its biosynthesis are not understood. To identify the gene(s) responsible for synthesizing the β-1,4 glucan backbone of XyG, we exploited a property of nasturtium (Tropaeolum majus) seed development. During the last stages of nasturtium seed maturation, a large amount of XyG is deposited as a reserve polysaccharide. A cDNA library was produced from mRNA isolated during the deposition of XyG, and partial sequences of 10,000 cDNA clones were determined. A single member of the C subfamily from the large family of cellulose synthase-like (CSL) genes was found to be overrepresented in the cDNA library. Heterologous expression of this gene in the yeast Pichia pastoris resulted in the production of a β-1,4 glucan, confirming that the CSLC protein has glucan synthase activity. The Arabidopsis CSLC4 gene, which is the gene with the highest sequence similarity to the nasturtium CSL gene, is coordinately expressed with other genes involved in XyG biosynthesis. These and other observations provide a compelling case that the CSLC gene family encode proteins that synthesize the XyG backbone.
Keywords: nasturtium, cell wall, xyloglucan, polysaccharide
Xyloglucan (XyG) is a major hemicellulose in the primary walls of many land plants (1). It has a β-1,4-glucan backbone that is substituted in a regular pattern with xylosyl residues plus other sugars that vary depending on the plant species (2). Although considerable progress has been made in defining the structure and the biological functions of XyG (1, 2), its biosynthesis is poorly understood. It is known to be synthesized in the Golgi of plants cells before delivery to the cell surface and incorporation into the wall matrix (3). Some of the enzymes that add side chains onto the XyG backbone in the Golgi have been identified and partially characterized (4–7). However, the gene, or genes, encoding the glucan synthase (GS) that makes the backbone of XyG has not yet been identified.
Efforts to identify the GS responsible for XyG biosynthesis by traditional methods of protein purification have not been successful (2, 8). Because of the structural similarity between the β-linked glycan chains of matrix polysaccharides and the β-linked glucan chains of cellulose, the cellulose synthase-like (CSL) genes have been suggested as likely candidates for encoding the Golgi-localized glycan synthases involved in matrix polysaccharide biosynthesis (9), including the GS required for XyG biosynthesis. Recent progress has confirmed that some glycan synthase activities have been found among the CSL proteins (10–12).
The function of a member of the CSLA class of genes was discovered by Dhugga et al. (11), who used expression profiling of developing guar (Cyamopsis tetragonoloba) seeds that deposit large quantities of galactomannan during seed maturation. They made cDNA libraries from several seed developmental stages and sequenced a large number of clones from each library. By looking for genes likely to be involved in polysaccharide biosynthesis, they found that a member of the CSLA family had the expected transcriptional profile. The function of this candidate was confirmed by expressing it in soybean somatic embryos and demonstrating the synthesis of mannan. Other members of the CSLA family were shown to be mannan synthases by using heterologous expression in Drosophila Schneider 2 cells (12). However, Liepman et al. (12) were unable to detect activity of other CSL proteins and thus were unable to identify the XyG GS.
Here, we report efforts to identify the XyG GS by using a transcriptional profiling approach with developing nasturtium (Tropaeolum majus) seeds that accumulate large quantities of XyG as a storage polysaccharide (13). Desveaux et al. (14) demonstrated that, during nasturtium seed development, XyG accumulates rapidly ≈20–25 days postanthesis (DPA). Consequently, our strategy was to sequence random cDNAs from developing seeds in an effort to identify ESTs that are expressed near the time of XyG deposition. Candidate genes were expressed in the yeast Pichia pastoris. We demonstrated that nasturtium and Arabidopsis genes from the CSLC family encode enzymes that produce β-1,4-linked glucans, making them the first group of CSL proteins that was shown to synthesize this important polysaccharide. Finally, evidence and arguments are presented to support the conclusion that the CLSC proteins are involved in synthesis of the XyG backbone.
Results
Transcriptional Profiling of Developing Nasturtium Seeds.
To chose the proper developmental stage from which to construct the cDNA library, we needed a gene known to be involved in XyG biosynthesis. For this purpose, we isolated a nasturtium homolog of MUR3, an Arabidopsis gene encoding an enzyme that adds galactose onto XyG. A nasturtium partial cDNA clone with 95% sequence identity and 98% similarity to the Arabidopsis protein over 58 amino acids was isolated as described in Materials and Methods. A full-length cDNA sequence, called TmMUR3, was obtained by RACE.
We used quantitative PCR to determine the relative levels of the TmMUR3 transcript during seed development from 16 to 25 DPA. This analysis revealed that the TmMUR3 transcript levels reached a maximum at 22 DPA (Fig. 1). We constructed a cDNA library from the mRNA of a single seed having the highest expression of TmMUR3. Before selecting clones for single-pass sequencing, we used a colony hybridization strategy to remove clones encoding seed storage proteins. Because the transcripts for these proteins are abundant, these clones were removed to avoid sequencing them thousands of times.
Fig. 1.
Quantitative analysis by real-time PCR of TmMUR3 transcript abundance during nasturtium seed development. The relative amounts of TmMUR3 mRNA in the various stages of nasturtium seed development were determined by normalizing each sample to the Elongation factor 1-α (TmEF1-α). Ratios are expressed relative to the 20-DPA stage. Error bars are based on three replicate measures of the same RNA sample.
We then performed single-pass sequencing on 10,000 clones from the 22-DPA library and analyzed the sequences to find genes potentially involved in XyG synthesis (Table 1). Approximately 1% of the library consisted of genes encoding endotransglycosylase/hydrolase (XTH), an enzyme involved in XyG remodeling in the cell wall (3). The abundance of XTH is a good indicator that the correct stage of development was chosen to construct the cDNA library. The mRNAs for genes involved in XyG biosynthesis were less abundant, as expected based on analogy with other systems (11). Only five cDNA clones were found that encoded enzymes that add the side-chain xylosyl and galactosyl residues onto XyG (Table 1). Five cDNA clones were found that corresponded to a CSLC homolog. A full-length cDNA for the TmCSLC homolog was obtained by RACE. Sequence analysis demonstrated that all five cDNA clones were derived from the same gene, and that this gene was most closely related to Arabidopsis CSLC4 with 77% identity and 85% similarity [see supporting information (SI) Fig. 5]. Based on the abundance of the CSLC gene in the nasturtium library, we postulate that CSLC genes are the best candidates to encode the XyG GS that is responsible for synthesizing the β-1,4-glucan backbone of XyG.
Table 1.
Frequencies of xyloglucan- and cellulose-related genes in the 22-DPA cDNA library
| Genes | Number of clones |
|---|---|
| XT1 | 2 |
| MUR3-like | 1 |
| MUR3 | 2 |
| CSLC | 5 |
| CesA | 1 |
| XTH | 112 |
| Total EST sequenced | 10,000 |
EST frequencies of xyloglucan- and cellulose-related genes in the 22-DPA nasturtium cDNA library. Sequences of clones from the 22-DPA library were clustered using the STACKPACK software, and the consensus sequences were compared with the predicted proteins from Arabidopsis by using the BLASTX program.
Heterologous Expression of CSLC Genes in the Yeast P. pastoris.
To test the hypothesis that CSLC encodes XyG GS, we sought to express both nasturtium and Arabidopsis CSLC genes in a heterologous system and to detect either new GS activity or the β-1,4-glucan product. TmCSLC full-length cDNA was cloned, as described in Materials and Methods, and cDNAs were obtained from Arabidopsis stock centers. Both TmCSLC and the closest related Arabidopsis homolog, AtCSLC4, were tagged at their N terminus by using T7 peptide to enable detection of heterologously expressed protein (see SI Fig. 6A). One strategy was to express AtCSLC4 or TmCSLC genes in Drosophila Schneider 2 cells and measure enzyme activity as for CSLA genes that possess mannan synthase activity (12); these efforts have not been successful. We were able to express both AtCSLC4 and TmCSLC genes in P. pastoris, as was done earlier for AtXT1 to characterize its xylosyltransferase activity (4). We also coexpressed AtXT1 and AtCSLC4 in P. pastoris, because there is evidence that xylosyltransferase and GS act together during XyG biosynthesis (2, 4). Thus, we postulated that xylosyltransferase (AtXT1) might be required to stimulate the putative GS activity. Although the proteins could be detected in P. pastoris cell extracts (see SI Fig. 6B), it was not possible to measure either glucan or XyG synthase activity in vitro in the same extracts (data not shown). Therefore, we turned our efforts toward investigating whether the transformed and cotransformed Pichia cells were able to synthesize the products, either β-1,4-glucan or XyG.
Characterization of Carbohydrates Produced in Transgenic P. pastoris.
Although P. pastoris cells contain large amounts of a β-1,3-glucan in their walls, they do not contain significant levels of β-1,4-glucan (Fig. 2). Consequently, if CSLC genes encode a β-1,4-GS, it should be possible to detect β-1,4-glucan in the transgenic yeast cells. Because we expected long chains of β-1,4-glucan to be insoluble, we first focused on insoluble material produced in the transgenic lines. We performed linkage analysis on the insoluble fractions isolated from P. pastoris lines expressing TmCSLC, AtCSLC4, coexpressing AtCSLC4 and AtXT1, or GS115 (control) to look for β-1,4-glucan accumulation. Surprisingly, only the line coexpressing AtCSLC4 and AtXT1 showed strong accumulation of 4-linked glucose (4-Glc) (Fig. 2). A second, independently obtained line showed this same accumulation of 4-Glc (data not shown). Despite the significant increase in 4-Glc, the levels of terminal glucose did not increase (Fig. 2), indicating that the insoluble β-1,4-glucan produced in the AtCSLC4/AtXT1 line was long. We also observed that the products produced in the AtCSLC4/AtXT1 line did not contain detectable levels of xylose. The most likely explanation for this observation is that P. pastoris does not produce UDP-xylose, as is the case in Saccharomyces cerevisiae (15).
Fig. 2.
Linkage analysis of the insoluble fraction of transgenic and GS115 P. pastoris lines, by gas chromatography. The main peaks from the chromatograms were integrated, identified based on retention times and fragment ion signatures, and expressed as mol percentage. Errors bars are standard deviation of biological triplicates.
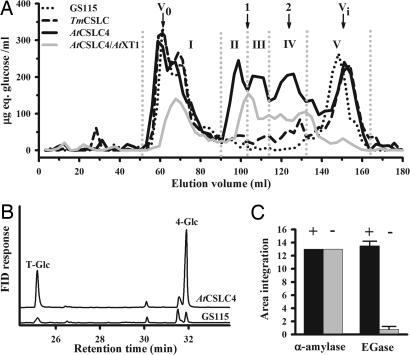
Although insoluble β-1,4-glucan was found only in transgenic lines expressing both AtCSLC4 and AtXT1, we found that lines transformed with only AtCSLC4 or TmCSLC genes produced smaller soluble β-glucans. Carbohydrates from the soluble fraction of transgenic yeast lines were isolated as described in Materials and Methods, and the resulting material was separated by molecular weight by using gel-exclusion chromatography (Fig. 3A). Five main peaks are apparent in the chromatogram, with three (labeled II, III, and IV) being unique to the transgenic lines. The amounts of these peaks are correlated with the amount of CSLC protein present in each line, as determined by immunoblotting (see SI Fig. 6B). The peaks labeled I and V were also present in the control strain. Fractions II and III elute with retention times similar to maltohexaose and maltotriose (Fig. 3A). The material in these fractions was further analyzed by high-performance anion exchange chromatography (HPAEC) (see SI Fig. 7), permethylation analysis to determine the sugar linkages (Fig. 3B), MALDI–linear ion trap mass spectrometry (Fig. 4), and NMR spectroscopy (see SI Fig. 8). As described in more detail below and in SI Text, all of this evidence is consistent with the conclusion that these fractions contain β-1,4-linked glucan oligosaccharides of between four and six residues in length. In many cases, the oligosaccharides have an unidentified molecule attached at the reducing end, and in other cases, the oligosaccharides have a free reducing end.
Fig. 3.
Analysis of the soluble fraction of transgenic and GS115 P. pastoris lines. (A) Gel-permeation chromatography on Biogel-P2 of the boiled soluble fraction from transgenic and GS115 P. pastoris lines. Fractions were analyzed for carbohydrate content by using the anthrone assay. Size markers maltohexaose (1) and maltotriose (2) are indicated, as well as the void (V0) and included (Vi) volumes. Five peak fractions were identified and labeled: I, II, III, IV, and V. (B) Linkage analysis of the oligosaccharide content in the soluble fraction of AtCSLC4-transformed and GS115 Pichia lines, by gas chromatography. Scale of the flame ionization detector (FID) response is the same for both chromatograms. (C) Enzymatic treatment with endo-(1→4)-β-d-glucanase (EGase) or α-amylase of carbohydrates produced in the P. pastoris line expressing AtCSLC4. After enzymatic treatment, oligosaccharides were quantified by using HPAEC-PAD, and two similar experiments were average.
Fig. 4.
Structural analysis by MALDI–linear ion trap of cellohexaose (G6) (A) and the modified oligosaccharide G5-R (B) synthesized in the soluble fraction from the P. pastoris line expressing AtCSLC4. Insets labeled MS in A and B show the mass of the intact G6 and G5-R ions (sodium adducts). The main view represents the collisional-induced fragmentation (MS2) of the G6 ([M+Na]+ = 1,013 Da) and G5-R ([M+Na]+ = 985 Da) ions. The molecule structure deduced from the fragmentation pattern, annotated with the predicted mass, is shown at the top of A and B. The dashed arrows indicate fragment ions that differ by a mass of 162, indicating the loss of a hexose.
HPAEC demonstrated that the material in fractions II (see SI Fig. 7) and III (data not shown) from transgenic lines, but not GS115 Pichia cells, contained oligosaccharides with elution times similar to cellotetraose (G4), cellopentaose (G5), and cellohexaose (G6). Enzymatic digestion of these oligosaccharides from transgenic lines with an endo-(1→4)-β-d-glucanase, but not α-amylase, resulted in their degradation (Fig. 3C). Thus, we conclude that β-linkages were present in these putative oligosaccharides. This conclusion is supported by NMR data collected on these oligosaccharides that show peaks characteristic of β-linked glucose (see SI Fig. 8).
Permethylation analysis was used to determine the sugar linkages present in the soluble oligosaccharides from all three transgenic lines, as well as GS115. This analysis revealed the presence of 4-Glc in all of the samples but with substantially more 4-Glc in the AtCSLC4-expressing lines compared with the GS115 (Fig. 3B). The amount of 4-Glc was correlated with the level of expression of the CSLC protein (see SI Fig. 6B). In addition, the ratio of terminal-glucose to 4-Glc confirms the short size of the β-1,4-glucan found in the soluble fraction from the CSLC-expressing lines (Fig. 3B).
Mass spectrometry of individual peaks separated by HPAEC (see SI Fig. 7) revealed that the peaks corresponding to both cellohexaose and cellopentaose produce fragmentation data consistent with such structures (data not shown). The additional peaks (labeled G6-R, G5-R, and G4-R in Fig. 4B) also produced fragments consistent with hexoses, but they contain a component with a mass of 152. Because there is no shift in mass after reduction with sodium borohydride, this additional component, when present, is located at the reducing end of the oligosaccharide (see SI Fig. 9). Taken together, these results demonstrate that transgenic P. pastoris expressing the CSLC genes specifically synthesize and accumulate oligosaccharides containing β-1,4-glucosyl residues. Therefore, we conclude that both AtCSLC4 and TmCSLC genes encode a GS activity. Because this GS activity does not produce XyG in Pichia, we sought other ways to find support for the hypothesis that it is involved in XyG biosynthesis.
Coordinate Expression of AtCSLC4 and AtXT1 Genes.
Because there is a putative physical interaction between AtCSLC4 and AtXT1 in P. pastoris, we used the BAR expression angler web (16) to determine whether the corresponding genes showed coordinated expression patterns. A large number of publicly available microarray experiments were queried, and selected examples are shown in SI Tables 2 and 3 and SI Fig. 10. In two separate series of microarray experiments, i.e., the abiotic stress and pathogen response series, AtXT1 is among the top 10 genes having the highest correlation coefficients with AtCSLC4. A XyG XTH gene and an expansin gene are also found with correlations ≈0.8 (SI Table 3). Given that there are >22,000 genes represented on the hundreds of microarrays examined, this is extremely unlikely to be a chance event. Thus, this expression pattern provides further support for the hypothesis that CSLC proteins are involved in XyG biosynthesis.
Localization of AtCSLC4 in Tobacco BY-2 Cells.
We presented strong evidence that CSLC proteins have the ability to produce a β-1,4 glucan. This limits the role of these proteins to either the synthesis of cellulose or XyG. The production of cellulose occurs at the plasma membrane (PM), whereas the synthesis of XyG occurs in the Golgi. To investigate the intracellular location of a CSLC protein, we expressed the AtCSLC4 gene containing a T7 epitope tag in BY-2 cells under the control of the 35S promoter. Confocal microscopy demonstrated that the T7 epitope was localized to punctate structures in the cytoplasm with little staining of the PM (SI Fig. 11A). Control experiments by using the JIM-84 antibody, which stains the PM and Golgi apparatus (17), showed that the punctate cytoplasmic spots labeled by T7-tagged AtCSLC4 protein and the JIM-84 epitope overlap (SI Fig. 11 B and C). The apparent Golgi localization of AtCSLC4 provides support for the argument that CSLC proteins are involved in XyG synthesis.
Discussion
Four major hemicellulosic polysaccharides are found in the cell walls of various plant species: mannans, mixed-linkage glucans, arabinoxylans, and XyG, discussed here. Identifying the genes and enzymes required for the synthesis of these four polymers is a fundamentally important problem with significant practical applications. Earlier work by Dhugga et al. (11) and Liepman et al. (12) demonstrated that the CSLA genes are mannan synthases responsible for mannan backbone biosynthesis. More recently, Burton et al. (10) demonstrated that the CSLF genes are involved in the biosynthesis of mixed-linkage glucans in rice and barley. Our evidence presented here indicates that CSLC genes are the most likely candidates to encode XyG GS.
Expression of both nasturtium and Arabidopsis CSLC genes in P. pastoris cells established that the CSLC protein has β-1,4-GS activity. The oligosaccharides produced in transgenic yeast lines producing CSLC proteins contained β-1,4-linked glucosyl residues, as demonstrated by methylation analysis, mass spectrometry, NMR analysis, and susceptibility to enzyme digestion. Several lines of evidence support the conclusion that the GS encoded by CSLC genes is involved in XyG biosynthesis. First is the observation that the TmCSLC gene is specifically expressed at the time when nasturtium seeds are depositing large quantities of XyG (see SI Fig. 12). Second is the observation that coexpression of AtXT1 and AtCSLC4 contributed to the synthesis of a larger β-1,4-glucan by AtCSLC4, indicating that AtXT1 and AtCSLC4 proteins interact directly or indirectly to alter the length of the product. Strong evidence supports the involvement of AtXT1 in XyG biosynthesis (7). Thus, an interaction between these two proteins implicates AtCSLC4 in XyG biosynthesis. Third, a strong correlation exits between the expression of AtCLSC4 and AtXT1. This remarkably strong correlation would be difficult to explain whether AtCSLC4 is involved in cellulose biosynthesis but would be expected if AtCSLC4 is involved in XyG biosynthesis. Fourth, confocal microscopy of BY-2 tobacco cells producing a T7 tagged version of AtCSLC4 showed the protein had a punctuate distribution in the cytoplasm and was coincident with a Golgi epitope (see SI Fig. 11). The punctuate structures are consistent with a Golgi localization, suggesting a role for CSLC proteins in XyG biosynthesis.
One important unresolved issue in polysaccharide biosynthesis is whether a primer is needed to initiate a new chain. Recently Peña et al. (18) demonstrated that Arabidopsis arabinoxylan molecules contain an unusual oligosaccharide at the reducing end and suggested it may serve as a primer during xylan biosynthesis. Many of the oligosaccharides produced by CSLC proteins in Pichia cells contained a 152-Da residue attached at the reducing end. Although the studies reported here were not able to determine the identity of this residue, doing so is an important goal for future studies, because the nature of the primer for XyG biosynthesis is unknown. Because of its location at the reducing end, it may serve as a primer for the action of the plant GS expressed in the yeast cells. Although this same compound may not serve as a primer in plant cells, learning the identity of residues that function as primers in yeast cells may provide valuable clues regarding the types of compounds that function as primers in plants.
Identification of the genes and enzymes involved in the synthesis of plant cell wall polysaccharides is a critical first step to understanding how plants control the composition of their cell walls. Identification of these genes will allow questions about how their expression is regulated, and how the resulting proteins operate to produce the numerous types of cell walls found in plants. Eventually, this information should allow production of plants with improved properties for biofuel production (19).
Materials and Methods
Total RNA Extraction from Nasturtium Seeds.
Seeds were germinated and grown as described by Desveaux et al. (14), with minor modifications. Flowers were self-pollinated, and individual fruits were harvested from 16 to 25 DPA. Individual fruits for the various developmental stages were frozen in liquid nitrogen and stored at −80°C for later use. Total RNA was isolated from the developing nasturtium seeds by means of a modified version of the Pine Tree Method (20) (see SI Text).
Cloning of Partial TmMUR3 and TmEF1-α cDNAs.
A two-step PCR procedure with primers based on Arabidopsis gene sequences was used to obtain partial cDNA sequences of the corresponding nasturtium genes (see SI Text). Based on the sequence from the cDNA library, the 5′- and 3′-ends of TmCSLC sequence were obtained as TmMUR3. Finally, the full-length TmCSLC was generated by using the following gene-specific primers: 5′-CACCATGGCTCCCAACTCAGTTGT-3′ (forward primer, start codon is underlined) and the 5′-CTAACTCATTTGCTCACCAATCAAA-3′ (reverse primer, stop codon is underlined). The CACC nucleotide sequence was put upstream of the initiation codon to allow the directional cloning of TmCSLC in the pENTR-D-TOPO vector.
Expression Analysis of TmMUR3 and TmCSLC During the Development of Nasturtium Seeds by Real-Time PCR.
The relative TmMUR3 and TmCSLC expressions were monitored by using quantitative real-time PCR (21). Primers and Taqman probes were designed by using Primer Express version 1.0 (Applied Biosystems, Foster City, CA) (see SI Text).
Twenty-Two-DPA cDNA Library.
The 22-DPA cDNA library was constructed by using the Creator SMART cDNA Library Construction Kit. DH5α-E ElectroMAX Competent Cells were used for the transformation. A colony hybridization strategy was used to remove clones containing highly abundant sequences (see SI Text).
Construction and Expression of Transgenes in P. pastoris.
PCR was used to construct clones containing the sequence encoding an NH3-terminal T7 epitope tag TmCSL and AtCSLC4 coding region directly upstream and in frame of the start codon (10) with addition of a 5′-CATAATG-3′ Kozak sequence (22). Primer sequences are listed in SI Table 4. The PCR products of TmCSLC and AtCSLC4 were ligated with the pENTR-D-TOPO vector, as described (12). Clones were fully sequenced, and the T7-tagged CSL ORF was recombined, by using Gateway technology, into a modified pPICZ vector. After transformation of methanol-inducible, histidine-auxotrophic GS115 P. pastoris cells with TmCSL or AtCSLC4 construct, positive colonies were selected by PCR, induced, and broken to see protein expression (see SI Text). After breaking, intact cells and debris, representing the insoluble fraction (see SI Fig. 13), were collected by centrifugation (5 min at 800 × g). The insoluble fraction was washed three times with 70% ethanol at 65°C. Triton X-100 (0.1% final vol) was added to the soluble fraction, which was then incubated in boiling water for 5 min. The boiled supernatant was centrifuged for 15 min at 9,000 × g and the resulting supernatant was used to perform characterization of the β-1,4-glucan oligosaccharides. Microsomal membranes were obtained by ultracentrifugation of 1 ml of the soluble fraction (30 min at 100,000 × g), in an RP100 AT4 rotor (Sorvall). Membranes were resuspended in 300 μl of extraction buffer by means of a glass homogenizer. The Bradford assay (23) was used to measure protein content. The microsomal membranes of CSLC-transformed P. pastoris lines were analyzed with the aid of a previously described (12) immunoblot procedure.
The P. pastoris line coexpressing AtCSLC4 and AtXT1 was obtained as follows. An AtXT1 cDNA clone was generated by PCR (for primers see SI Table 4), digested with EcoRI and KpnI, and cloned into the pIB4 vector (24). A histidine auxotrophic P. pastoris line expressing AtCSLC4 protein was transformed with this AtXT1-pIB4 construct, and transformants were selected on minimum dextrose medium containing 100 μg/ml zeocin. Transformants were screened for high expression of AtCSLC4 and AtXT1 proteins by Western blotting by using the anti-T7 epitope antibody (Novagen, San Diego, CA) and a specific anti-XT1 antibody generated in rabbit.
Biogel-P2 Chromatography of the SF of P. pastoris Lines.
Boiled soluble fractions (2 ml) from P. pastoris lines were fractionated on a Biogel-P2 column (2 × 120 cm) eluted with H2O containing 0.02% sodium azide at 0.3 ml/min. The column was calibrated with dextran, maltohexaose, maltotriose, and glucose. Eighty 2.5-ml fractions were collected, and their carbohydrate content was determined by using the anthrone assay (25).
HPAEC-PAD Chromatography.
HPAEC-pulsed amperometric detection chromatography was performed on a DX 600 system (Dionex, Sunnyvale, CA) equipped with a GP 50-gradient pump and a CarboPac PA10 column. Oligosaccharides were separated by using a linear gradient of 50 to 100 mM NaOAc in 100 mM NaOH at a flow rate of 1 ml·min−1. For product characterization, some samples were treated with endo-(1→4)-β-d-glucanase (5 units of endoglucanase in 100 μl of 10 mM sodium acetate buffer, pH 5, for 18 h at 37°C) or α-amylase (100 units in 10 mM KPO4, pH 7, for 18 h at 37°C) before HPAEC-PAD analysis. Chromatogram areas corresponding with the oligosaccharides were integrated, summed, and compared among the various enzymatic treatments. Samples to be further analyzed by mass spectrometry or NMR were desalted on-line, after HPAEC-PAD separation, by using a Carbohydrate Membrane Desalter (CMD; Dionex), according to the manufacturer's recommendations.
Linkage Analysis.
Soluble and insoluble fractions from P. pastoris lines were methylated according to Burton et al. (26) with minor modifications (see SI Text). The derivatives were analyzed by using gas chromatography (model 6890; Hewlett-Packard, Palo Alto, CA) coupled to a mass spectrometer (model 5973). A 30 m × 0.25-mm (i.d.) SP2330 column (Supelco, Bellefonte, PA) was used for all separations. Identification of the derivatives and deduction of the glycosidic linkages were based on both the elution order in relation to standards and fragment ion signatures. The mol percentage of composition of the samples was calculated by normalizing the FID chromatogram peak areas to the molecular masses of the corresponding derivatives. Methylation analyses were performed in triplicate on three different cultures; results were averaged and expressed as ± SD.
MALDI–Linear Ion Trap (LIT) MS and MS2.
MALDI-LIT MS and tandem MS(MS2) data for standard cellohexaose (G6) and purified G5-R molecules were obtained by using a Thermo Electron vMALDI (Thermo Electron, Santa Clara, CA). Preparation of G6 and G5-R molecules is detailed in SI Text.
Supplementary Material
Acknowledgments
We thank David Cavalier for help with the HPAEC-PAD experiments, Linda Danhof for growing tobacco cell cultures, Maricar Posa-Macalincag for assistance with molecular work, Ahmed Faik for characterization of the anti-XT1 antibody, Jim Klug of the Plant Research Laboratory (Michigan State University) greenhouse for providing assistance during the nasturtium culture, the Michigan State University Research Technology Support Facility with Beverley Chamberlin and Jeff Landgraf for the quality of the GC/MS and genomic services respectively provided, Markus Pauly for helpful discussion and comments, Benjamin S. Glick (University of Chicago, Chicago, IL) for the pIB4 vector gift, Will York for assistance with interpreting NMR and MS data, and Karen Bird for editorial assistance. This work was supported in part by grants from the Plant Genome Research Program at the National Science Foundation and the Energy Biosciences Program at the U.S. Department of Energy.
Abbreviations
- XyG
xyloglucan
- CSL
cellulose synthase-like
- DPA
days postanthesis
- 4-Glc
4-linked glucose
- HPAEC
high-performance anion exchange chromatography
- GS
glucan synthase.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703133104/DC1.
References
- 1.Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, et al. Science. 2004;306:2206–2211. doi: 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi T. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:139–168. [Google Scholar]
- 3.Cosgrove DJ. Nat Rev Mol Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 4.Faik A, Price NJ, Raikhel NV, Keegstra K. Proc Natl Acad Sci USA. 2002;99:7797–7802. doi: 10.1073/pnas.102644799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madson M, Dunand C, Li X, Verma R, Vanzin GF, Caplan J, Shoue DA, Carpita NC, Reiter WD. Plant Cell. 2003;15:1662–1670. doi: 10.1105/tpc.009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin RM, DeRocher AE, Bar-Peled M, Zeng W, Norambuena L, Orellana A, Raikhel NV, Keegstra K. Science. 1999;284:1976–1979. doi: 10.1126/science.284.5422.1976. [DOI] [PubMed] [Google Scholar]
- 7.Cavalier DM, Keegstra K. J Biol Chem. 2006;281:34197–34207. doi: 10.1074/jbc.M606379200. [DOI] [PubMed] [Google Scholar]
- 8.White AR, Xin Y, Pezeshk V. Biochem J. 1993;294:231–238. doi: 10.1042/bj2940231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richmond TA, Somerville CR. Plant Mol Biol. 2001;47:131–143. [PubMed] [Google Scholar]
- 10.Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, Stone BA, Newbigin EJ, Bacic A, Fincher GB. Science. 2006;311:1940–1942. doi: 10.1126/science.1122975. [DOI] [PubMed] [Google Scholar]
- 11.Dhugga KS, Barreiro R, Whitten B, Stecca K, Hazebroek J, Randhawa GS, Dolan M, Kinney AJ, Tomes D, Nichols S, Anderson P. Science. 2004;303:363–366. doi: 10.1126/science.1090908. [DOI] [PubMed] [Google Scholar]
- 12.Liepman AH, Wilkerson CG, Keegstra K. Proc Natl Acad Sci USA. 2005;102:2221–2226. doi: 10.1073/pnas.0409179102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards M, Dea I, Bulpin P, Reid JSG. Planta. 1985;163:133–140. doi: 10.1007/BF00395907. [DOI] [PubMed] [Google Scholar]
- 14.Desveaux D, Faik A, Maclachlan G. Plant Physiol. 1998;118:885–894. doi: 10.1104/pp.118.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-Peled M, Griffith CL, Doering TL. Proc Natl Acad Sci USA. 2001;98:12003–12008. doi: 10.1073/pnas.211229198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toufighi K, Brady SM, Austin R, Ly E, Provart NJ. Plant J. 2005;43:153–163. doi: 10.1111/j.1365-313X.2005.02437.x. [DOI] [PubMed] [Google Scholar]
- 17.Horsley D, Coleman J, Evans D, Crooks K, Peart J, Satiat- Jeunemaitre B, Hawes C. J Exp Bot. 1993;44:223–229. [Google Scholar]
- 18.Peña MJ, Zhong R, Zhou G-K, Richardson EA, O'Neill MA, Darvill AG, York WS, Ye Z-H. Plant Cell. 2007;19:549–563. doi: 10.1105/tpc.106.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Jr, Hallett JP, Leak DJ, Liotta CL, et al. Science. 2006;311:484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 20.Chang S, Puryear J, Cairney J. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Kozak M. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- 23.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Sears IB, O'Connor J, Rossanese OW, Glick BS. Yeast. 1998;14:783–790. doi: 10.1002/(SICI)1097-0061(19980615)14:8<783::AID-YEA272>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.Dische Z. In: Methods in Carbohydrate Chemistry. Whistler RL, Green JW, BeMiller JN, Wolfrom ML, editors. Vol I. New York: Academic; 1962. pp. 475–514. [Google Scholar]
- 26.Burton RA, Gibeaut DM, Bacic A, Findlay K, Roberts K, Hamilton A, Baulcombe DC, Fincher GB. Plant Cell. 2000;12:691–706. doi: 10.1105/tpc.12.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.