Abstract
Synaptotagmin (Syt) I , an abundant synaptic vesicle protein, consists of one transmembrane region, two C2 domains, and a short C terminus. This protein is essential for both synaptic vesicle exocytosis and endocytosis via its C2 domains. Although the short C terminus is highly conserved among the Syt family and across species, little is known about the exact role of the conserved C terminus of Syt I. In this paper, we report a function of the Syt I C terminus in synaptic vesicle docking at the active zones. Presynaptic injection of a peptide corresponding to the C-terminal 21 amino acids of Syt I (named Syt-C) into the squid giant synapse blocked synaptic transmission without affecting the presynaptic action potential or the presynaptic Ca2+ currents. The same procedure repeated with a mutant C-terminal peptide (Syt-CM) had no effect on synaptic transmission. Repetitive presynaptic stimulation with Syt-C produced a rapid decrease in the amplitude of the postsynaptic potentials as the synaptic block progressed, indicating that the peptide interferes with the docking step rather than the fusion step of synaptic vesicles. Electron microscopy of the synapses injected with the Syt-C peptide showed a marked decrease in the number of docked synaptic vesicles at the active zones, as compared with controls. These results indicate that Syt I is a multifunctional protein that is involved in at least three steps of synaptic vesicle cycle: docking, fusion, and reuptake of synaptic vesicles.
Neurotransmitter release from nerve terminals in the brain is regulated by several families of Ca2+-binding proteins having tandem C2 domains (1). These domains were originally found in the C2 regulatory region of protein kinase C and are thought to sense a rapid increase in intracellular Ca2+ concentrations (up to 200 μM) (2) via a Ca2+-dependent phospholipid-binding site (1, 3). Syt, a member of such protein family, consists of at least six separate domains: a short intravesicular (or extracellular) N terminus, a single transmembrane domain, a C2A domain, a C2B domain, and a short C terminus (1, 4–6). Among these domains, two C2 domains and a C terminus (WHXL motif; red letters in Fig. 1A) are highly conserved among the Syt family and along phylogeny (from nematoda to human) (7, 8). Synaptotagmin (Syt) I, a well characterized isoform abundant in synaptic vesicles, has been shown to be essential for efficient Ca2+-dependent neurotransmitter release as well as synaptic vesicle endocytosis by analysis of knockout animals (9–13) and by antibody- or peptide-injection experiments (14–21). In particular, we have shown that the C2A domain is essential in the vesicle fusion step (16, 18, 19, 22), whereas the C2B domain is involved in synaptic vesicle reuptake (17), and that neurotransmitter release is regulated by inositol high-polyphosphate binding to the C2B domain of Syt I (17–19, 23–25). The in vivo function of the conserved C terminus of Syt family in vesicular trafficking has been addressed (8), and it is known to interact with latrotoxin receptors (26), presently known as neurexin polymorphic neuronal cell surface proteins, in vitro (26–28).
Figure 1.
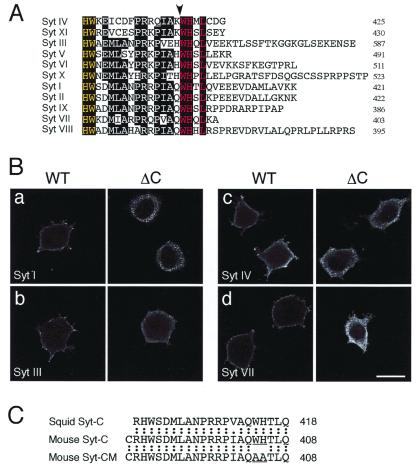
Conserved WHXL motif is essential for plasma membrane association of Syt family. (A) Comparison of the C terminus of mouse Syts I–XI. Although the length of the C-terminal tail varies, the HWX(D/E)X5RX5WHXL sequence is highly conserved among mouse Syts I–XI. Arrowhead indicates the deletion point of SytΔC constructs. The WHXL motif is colored in red, and its mirror image (HW) is shown by yellow. (B) Plasma membrane association of Syt cytoplasmic domain. FLAG-tagged Syt-cytos (Left) or SytΔC-cytos (Right) was expressed in PC12 cells as described in Materials and Methods. PC12 cells were fixed, permeabilized, and stained with anti-FLAG (rabbit) antibody. Note that deletion of the WHXL motif of Syts I, III, IV, and VII completely prevented plasma membrane localization. Bar = 20 μm. (C) Amino acid sequence of mouse Syt-C and Syt-CM peptides and corresponding sequence of squid Syt I. Amino acid substitutions (WH to AA) are indicated by underlining. The Syt-C peptide sequence is highly conserved during evolution, and only one conserved amino acid substitution (Ile to Val) is found between mouse and squid Syt I. The Syt-C and Syt-CM peptides contain an artificial Cys residue at the N terminus.
In this study, we showed that the conserved WHXL motif of the C terminus of mouse Syts I–XI is essential for plasma membrane association in PC12 cells and suggested a presence of a common plasma membrane receptor for the Syt-C terminus. We further examined the conserved C terminus of Syt I in neurotransmitter release by introduction of peptides corresponding to the C terminus of Syt I (Syt-C peptide; Fig. 1C) into squid giant preterminals. Electrophysiological and ultrastructural analyses indicated that the Syt-C peptides interfere with synaptic vesicle docking onto the presynaptic plasma membrane. On the basis of these results, we proposed that the conserved C terminus of Syt I is involved in synaptic vesicle docking step by binding to a presynaptic plasma membrane receptor, possibly neurexin.
Materials and Methods
Materials.
Recombinant Taq DNA polymerase and restriction enzymes were obtained from Toyobo Biochemicals (Osaka). All other chemicals were commercial products of reagent grade. Syt-C (mouse Syt I C-terminal peptide, amino acid residues 388–408; CRHWSDMLANPRRPIAQWHTLQ) and Syt-CM (mouse Syt I C-terminal mutant peptide; CRHWSDMLANPRRPIAQAATLQ) peptides were synthesized by Sawady Technology (Tokyo). Solutions were made up in deionized water prepared with an Elix10 Water Purification System and Milli-Q Biocel A10 System (Millipore, Bedford, MA).
Expression Constructs, Transfection, and Immunocytochemistry.
pEF-FLAG-Syts I-XIΔC-cyto were essentially constructed by PCR as described previously (7, 29, 30) by using the following primers with appropriate restriction enzyme sites (underlined) and termination codons (bold letters): Syt IΔC primer, 5′-GCACTAGTCACTGGGCGATGGGTCGCCG-3′ (antisense; amino acids 398–403); Syt II ΔC primer, 5′-GCACTAGTCACTGGGCAATGGGCCTCCG-3′ (antisense; amino acids 399–404); Syt IIIΔC primer, 5′-GCACTAGTTCAGTGTTCCACAGGCTGCG-3′ (antisense; amino acids 555–560); Syt IVΔC primer, 5′-GCACTAGTTCACTTGGCTATGGGCTTGCG-3′ (antisense; amino acids 418–423); Syt VΔC primer, 5′-GCACTAGTCATGCGATGGGCTTCCGAGGAT-3′ (antisense; amino acids 476–482); Syt VIΔC primer, 5′-GCACTAGTCAGTGCGCAATGGGCTTCG-3′ (antisense; amino acids 488–493); Syt VIIΔC primer, 5′-GCACTAGTCACTGGGCCACAGGCTGCCGG-3′ (antisense; amino acids 391–397); Syt VIIIΔC primer, 5′-GCACTAGTCACTGGGCGATGGGCCGCCGAG-3′ (antisense; amino acids 362–368); Syt IXΔC primer, 5′-GCACTAGTCACTGGGCAATGGGGCGCCTGG-3′ (antisense; amino acids 364–370); Syt XΔC primer, 5′-GCACTAGTCATGTGATTGGCTTTCGGTGAT-3′ (antisense; amino acids 487–493); and Syt XIΔC primer, 5′-GCACTAGTTCACTTGGCTATGGGCTTGCG-3′ (antisense; amino acids 418–423). In the case of FLAG-tagged Syt IXΔC, for example, the Syt IXΔC fragment was amplified by the Syt IXΔC primer and the SP6 primer by using pGEM-T-FLAG-Syt IX as a template (7). Purified PCR products were digested with BamHI/SpeI and inserted into the BamHI/SpeI site of pGEM-T-FLAG-Syt IX. The Syt IXΔC cytoplasmic fragment with FLAG tags was then excised from the pGEM-T Easy vector (Promega) by NotI digestion, subcloned into the NotI site of modified pEF-BOS vector (named pEF-FLAG-Syt IXΔC-cyto) (23, 31), and verified by DNA sequencing with a Hitachi SQ-5500 DNA sequencer. Other expression constructs (pEF-FLAG-Syt I-XI-cyto) were prepared as described previously (7, 32). Plasmid DNA was prepared by using Wizard-mini preps (Promega) or Qiagen (Chatsworth, CA) Maxi prep kits.
Glass-bottom dishes (35 mm-dish, MatTek, Ashland, MA) were coated with collagen type IV (Becton Dickinson). PC12 cells (0.8–1 × 105 cells, the day before transfection) were cultured on these dishes in DMEM containing 10% horse serum and 10% FBS at 37°C, 5% CO2 (29, 33). Transfection of pEF-FLAG-SytΔC-cyto (or Syt-cyto) constructs into PC12 cells was achieved by using the LipofectAmine Plus reagent according to the manufacturer's notes (Life Technologies, Rockville, MD).
Three days after transfection, PC12 cells (35-mm dish) expressing each SytΔC-cyto (or Syt-cyto) protein were stained with anti-FLAG (0.5 μg/ml) rabbit polyclonal antibody and anti-rabbit Alexa 488 antibody (Molecular Probes) as described previously (29, 33). Immunoreactivity was analyzed with a fluorescence microscope (TE300, Nikon) attached to laser confocal scanner unit CSU 10 (Yokogawa, Tokyo) and a HiSCA charge-coupled device camera (C6790, Hamamatsu Photonics, Hamamatsu City, Japan).
Pressure Injection into the Squid Giant Synapse.
The isolation of the stellate ganglion of the squid and the electrophysiological techniques used in this paper are the same as described previously (18, 22). In each case, the presynaptic terminal was impaled at different distances from the synaptic contact by the injecting electrode. The injection fluid, containing 20 mM of the Syt-C peptide or 20 mM of the Syt-CM control peptide, was dissolved in 500 mM potassium acetate/20 mM Hepes, pH 7.2, to a final concentration of 5 mM. Injection was then implemented with the diffusion of peptide into the preterminal digit being monitored by fluorescence imaging (Argus-100, Hamamatsu) of the coinjected rhodamine dextran. Total volume injected with this technique is of the order of 0.5 to 1 pl, which represents close to 1% of total presynaptic volume given that the injection was generally done at the main presynaptic axon immediately proximal to the preterminal digits. Given these parameters, final peptide concentration at the terminal digit is calculated at 50 μM. The correlation between the diffusion of the injected peptide into the presynaptic terminal and the change in postsynaptic response amplitude was then determined against time after injection (34).
Ultrastructure.
After each electrophysiological recording, the ganglia were removed from the recording chamber and fixed by immersion in glutaraldehyde followed by postfixation in osmium tetroxide and in-block staining with uranyl acetate. Dehydration was done in ethanol, substituted with propylene oxide, and embedded in Araldite resin (CY212) or Embed 812 (EM Science). Ultrathin sections were collected on Pyoloform (Ted Pella, Redding CA) and carbon-coated single-sloth grids, contrasted with uranyl acetate and lead citrate, observed, and photographed in a Philips (Eindhoven, The Netherlands) 208 transmission electron microscope. Electron micrographs were taken at an initial magnification of ×12,000 and photographically enlarged to a magnification of ×36,000. Morphometry and quantitative analysis of the synaptic vesicles were performed with a Zidas digitizing system (Zeiss) interfaced with a Macintosh G3 computer. Vesicle density was determined as number of vesicles per cluster. Synaptic vesicles in the docking state were considered a percentage of the total vesicle count in the cluster. Processing of the data was performed by using lotus 1-2-3 software for Macintosh.
Results
Conserved WHXL Motif in the C Terminus of Syt Family Is Essential for Plasma Membrane Association.
Previously, we had shown that an alternatively splicing variant of Syt VI (Syt IVΔTM) lacking a transmembrane domain is associated with plasma membrane via the C-terminal 29 amino acids in PC12 cells (29). Because the C terminus of mouse Syts I-XI is highly conserved and contains a HWX(D/E)X5RX5WHXL sequence (Fig. 1A), we first examined whether the C terminus of Syts I-XI is involved in plasma membrane association. Expression of the cytoplasmic domains of Syts I-XI in PC12 cells resulted in an enriched concentration of this protein on the inner surface of the plasmalemma [Fig. 1B (WT) and data not shown]. By contrast, when the WHXL motif of Syts I-XI was deleted (arrowhead in Fig. 1A), such plasma membrane association was completely prevented [Fig. 1B (ΔC) and data not shown]. All the Syts I-XIΔC-cyto proteins seemed to be present in the cytosol. Thus, receptors that interact with the C-terminal WHXL motif of the Syt family protein must be present at the plasmalemma.
Taking advantage of the structural conservation of the C terminus of Syt I during phylogeny (only one amino acid substitution between squid and mouse Syt I; see Fig. 1C), we next sought to determine whether the conserved C terminus (i.e., WHXL motif) is involved in neurotransmitter release in vivo by introduction of the peptide corresponding to the 21 amino acids of the C terminus of mouse Syt I into the squid giant preterminal. We also prepared a mutant peptide carrying the WH-to-AA substitution (named Syt-CM peptide) as a control.
Effect of Syt-C Peptide on Transmitter Release.
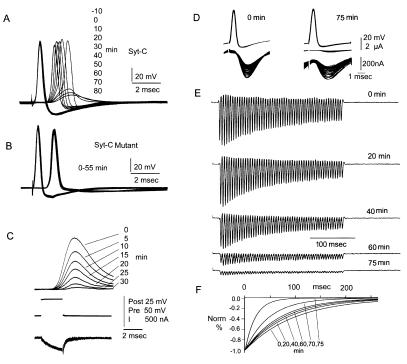
Because Syt has been conserved throughout phylogeny (6), we examined the effect of the Syt-C peptide on neurotransmitter release in squid giant synapse. After presynaptic impalement and injection of the Syt-C peptide (confirmed by fluorescence imaging of the preterminal), the immediate postsynaptic response consisted of a rapidly rising action potential arising from a rapid postsynaptic potential (Fig. 3A, 0 time). After 5–10 min of Syt-C peptide diffusion into the preterminal, the latency of the postsynaptic spike generation increased, with a subsequent decrease of postsynaptic potential amplitude. At 45 min, only a subthreshold synaptic potential was evident, which decreased further at 80 min after injection (Fig. 3A). Similar results were obtained in 10 other synapses. The time course and magnitude of the reduction were always proportional to the amount of injected peptide observed diffusing into the preterminal. Each of these injections was made with a freshly prepared peptide that was in the high-salt buffer for less than 10 min before use. It was noted, however, that some injections using Syt-C peptide prepared at least 1 hr before use showed no effects on postsynaptic potential amplitude. In these cases, the injected electrode often showed distinct aggregated material that resisted injection at pressures up to 100 psi (normal, 40 psi). Biochemical studies, allowing the Syt-C peptide to sit for a comparable period in the high-salt concentration used in the injection fluid, showed a largely reduced interaction between Syt-C and neurexin Iα, explaining the lack of effect by the “old” Syt-C peptide.
Figure 3.
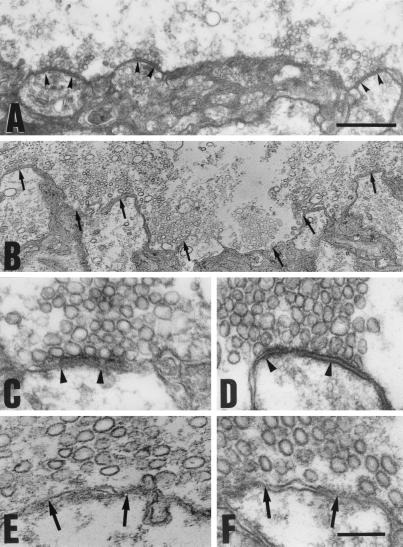
Ultrastructure showing reduced synaptic vesicular docking. After presynaptic injection of the Syt-CM peptide, presynaptic active zones showed the usual set of docked vesicles on, or in tight contact with, the presynaptic membrane (A, C, and D, arrowheads). By contrast, similar presynaptic injection of the Syt-C peptide produced a marked reduction of docked vesicles. In latter case, the clusters of vesicles appear as lingering above the plasmalemma (B, E, and F), resulting in a 10 − 75-nm-wide space empty of vesicles along most of the active zones (arrows). (Bar in A = 1 μm in A and B; bar in F = 0.2 μm in C–F.)
By contrast to the above, preterminal injection with the control Syt-CM peptide showed no reduction in postsynaptic potential amplitude or latency (Fig. 2B). This was the case in all synapses injected (n = 6) even up to 1 hr after injection. The lack of effect by the Syt-CM mutant highlights the essential role of the conserved Syt I C-terminal WHXL motif in neurotransmitter release.
Figure 2.
Presynaptic injection of the Syt-C peptide reduces transmitter release in the squid giant synapse. (A) Pre- and postsynaptic recordings after Syt-C injection. (A) Reduction of postsynaptic potential without affecting the amplitude or duration of the presynaptic spike. Time after injection is indicated on the right. (B) Syt-CM peptide showed no reduction in postsynaptic potential amplitude. (C) Syt-C injection reduces transmitter release without affecting presynaptic calcium current at the preterminal. Top traces postsynaptic amplitude; Middle traces presynaptic voltage step; Lower traces presynaptic calcium current. For all records, time interval after injection is indicated in minutes (Right). (D) Kinetics of transmitter release reduction after Syt-C peptide injection. Presynaptic spike trains at 200 Hz generate a rapid PSC decrease within each train (Right). The amplitude of the PSC is reduced, and the rate of reduction is increased with time after injection (Left, at 75 min after injection). PSC were amplified 10× (Bottom). (E) Set of PSC produced by a train of stimuli at 200 Hz. (F) Plot of the time course for PSC amplitude against time for each set of spike trains. Note the increase rate of decline of PSC with time after injection.
Because the block of transmitter release can also be produced by a reduction in the voltage-gated presynaptic Ca2+ conductance, injections using a presynaptic voltage clamp paradigm (n = 3) were performed to rule out the possibility of indirect blocking effect, via Ca2+ conductance, on transmitter release. As no reduction or delay in the presynaptic Ca2+ current accompanied the reduction in the postsynaptic potential (Fig. 3C), we concluded that the Syt-C peptide had no effect on Ca2+ channel-dependent inward current. These studies support the assertion that the Syt-C peptide directly affects the neurotransmitter release mechanism at the synapse without an accompanying effect on presynaptic Ca2+ current.
To determine the kinetics of transmitter release reduction after Syt-C peptide injection, a set of experiments was implemented by using presynaptic spike recording and postsynaptic current (PSC) amplitude measurements (Fig. 3D) to trains of presynaptic stimuli. This set of experiments (n = 4) shows two features: (i) a rapid decrease in PSC within each train of 52 presynaptic stimuli without change in the amplitude of the presynaptic action potential; and (ii) a gradual decrease of PSC over the time course of the synaptic block. Fig. 3D compares the initial amplitude and range and reduction of postsynaptic current amplitudes (Top) during the stimulus train immediately after injection, 0 min (Left), and at 75 min (Right) after injection, during a single tetanic stimulation train (Bottom). At 75 min, the postsynaptic currents were small (Middle) and were enlarged 10 times (bottom trace) for detailed analysis. This lower trace shows a PSC reduction of more than 10 times for the PSC generated by the first stimulus of the train at 75 min (300 μA), compared with the unexpanded trace shown at 0 min (4 μA) for the same stimulus.
In addition to the gradual change described above, the rates of PSC reduction within a train increase with time after injection (Fig. 3F). At 75 min, the postsynaptic current drops close to 0% change within 100 ms. In contrast, immediately after injection (0 min), the same reduction takes more than 250 ms. The rate of this decline increases directly with the observed diffusion of the Syt-C peptide into the preterminal and with the amount of time elapsed after presynaptic injection. These results suggest that the “immediately available” store of transmitter becomes more rapidly depleted as more of the Syt-C peptide binds its target in the preterminal. In contrast to the Syt-C peptide's effects on vesicular availability, a block of vesicular release or fusion events would not lead to a decrease in postsynaptic current amplitudes within a train of presynaptic stimulation. Instead, the synaptic response amplitude would remain close to constant for the duration of the tetanic stimulation and would show a uniform overall decline over the time course of the block. From the above, we conclude that the Syt-C peptide blocks neurotransmitter release through a direct effect on vesicular availability.
Effect of Syt-C Peptide on Synaptic Morphology.
Ultrastuctural study of the synapses injected with Syt-C peptide demonstrates a marked reduction in the number of docked synaptic vesicles. Morphologically, docked vesicles have been defined as those found immediately in contact with the presynaptic plasma membrane (35). Following that definition, we quantified synaptic vesicle clusters in the Syt-C-injected terminals and compared the results with those electron micrographs obtained after Syt-CM-injected experiments. In the Syt-C-injected set, the results showed areas void of vesicles forming a band of 150–200 nm in front of the postsynaptic active zone profiles. Indeed, the vesicular clusters seemed “suspended” above the presynaptic active zones (Fig. 3 B, E, and F). This void area corresponds to the space normally occupied by one to two vesicle rows at the active zone in control synapses (Fig. 3 A, C, and D).
Morphometry and quantitative analysis of vesicle density was determined as stated in Materials and Methods. The percentage of docked vesicles per cluster in terminals injected with Syt-C peptide was compared with that of those injected with Syt-CM (Table 1). The results indicate a dramatic reduction in the percentage of docked vesicles in the Syt-C-injected terminals, with a similar number of vesicles per cluster. This latter measurement is complicated by the large number of scattered vesicular profiles in the Syt-C-injected terminals, which made total vesicular content difficult to quantify. Nevertheless, the results indicate a reduced number of docked vesicles rather than a reduction of vesicles number at the presynaptic terminal.
Table 1.
Effects of Syt-C and Syt-C M on vesicle number and percentage of docked vesicles per cluster morphometrical analysis
| Mean number of vesicles per millimeter squared | Percent docked vesicles per cluster | |
|---|---|---|
| Syt-C1 | 46.00 ± 7.5 | 6.48 |
| Syt-C2 | 48.00 ± 2.1 | 8.32 |
| Syt-C M1 | 43.2 ± 7.8 | 23.9 |
| Syt-C M2 | 49.5 ± 6.2 | 27 |
| Mean Syt-C | 47.00 ± 4.8 | 7.4 |
| Mean Syt-CM | 46.35 ± 7.0 | 25.45 |
Vesicle number is the mean number of synaptic vesicles per micromillimeter squared clustered near the active zones (mean ± SEM). Docked vesicles are those in direct contact with the presynaptic membrane. Mean Syt-C and average controls are the mean from the two Syt-C injected and two Syt-CM injected synapses, respectively.
Discussion
In a previous set of studies, we addressed the role of two C2 domains of Syt I in synaptic vesicle trafficking. Indeed, the C2A domain was shown to be involved in synaptic vesicle fusion, whereas the C2B domain is involved in synaptic vesicle reuptake (16, 17). In the present study, we describe a function of the WHXL motif of the Syt I C terminus, which is highly conserved among the vertebrate Syt family as well as across species. The WHXL motif of the Syt family proteins is involved in plasmalemmal association in PC12 cells, indicating the presence of Syt receptor at the plasmalemma (Fig. 1B). To test in vivo significance of this association, we prepared the peptide corresponding to the C-terminal 21 amino acids of the mouse Syt I (Syt I-C) and examined its effect on neurotransmitter release by injecting it into the giant presynaptic terminal in the squid stellate ganglion.
Electrophysiological analysis of the squid giant synapse injected with Syt-C peptide indicate that the reduction of synaptic transmission is more in line with a change of transmitter availability than with synaptic release interference (Fig. 2 D, E, and F). Moreover, the effect does not modify presynaptic Ca2+ currents (Fig. 2C). At the ultrastructural level, the Syt I peptide injection markedly reduces synaptic vesicles docking at the active zones, in agreement with electrophysiological findings, suggesting a decreased vesicular availability. This effect of the Syt-C peptide is quite different from that of the anti-Syt-C2A or anti-Syt-C2B antibodies reported previously (16, 17). Indeed, it is evident that the Syt-C peptide prevented the docking of the synaptic vesicles on the presynaptic terminal and also the approach to docking, considering the void areas between the vesicular clusters and the presynaptic active zones (Fig. 3).
Together, these findings indicate that Syt I is a multifunctional protein with each domain having different roles in the synaptic vesicle cycle. The first C2 domain (C2A), which binds phospholipids or syntaxin in a Ca2+-dependent manner, functions as a Ca2+-sensor for neurotransmitter release (16, 22). The second C2 domain (C2B) is involved in vesicular reuptake, probably through its interaction with the clathrin assembly protein, AP-2 (36, 37). The conserved WHXL motif, found in the C terminus, is essential for the docking of synaptic vesicles to active zones. This latter finding is consistent with the recent report (13) showing a reduced number of docked synaptic vesicles in Drosophila Syt mutants.
Because mammalian neurexin Iα acts as a Ca2+-dependent α-latrotoxin receptor (38–40), it will be interesting to see whether α-latrotoxin has some effect on neurotransmission in squid giant synapse. Indeed, it is likely that Syt binds to the plasma membrane via neurexins, because Syt-C peptides, but not Syt-CM, peptides effectively disrupt the Syt I-neurexin Iα interaction in vitro (data not shown). A second issue will be whether Syt I-neurexin Iα interaction will be affected by α-latrotoxin binding to neurexin.
In summary, we showed that the Syt I C terminus is essentialfor synaptic vesicle docking. Because the C termini of the Syt family (the WHXL motif) reported so far are highly conserved, we propose that the conserved C terminus of Syt family is important for stabilizing transport vesicles to the plasma membrane by binding to a plasma membrane receptor, probably neurexin.
Acknowledgments
We thank Dr. Shigekazu Nagata for the expression vector (pEF-BOS), Eiko Kanno for expert technical assistance, and Gabriel Arisi for morphometrical analysis. This work was supported in part by grants from the Science and Technology Agency of Japan (K.M.), from the Ministry of Education, Science, and Culture of Japan (11780571 and 12053274, M.F.), from Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil, Grants 97/3026 and 11097–0, and Conselho Nacional de Desenvolvimento Científico e Tecnológico Fellowship 300420/84–6 to J.E.M., and from National Institutes of Health Grant NS 13742.
Abbreviations
- Syt(s)
synaptotagmin(s)
- PSC
postsynaptic current
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260491197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260491197
References
- 1.Südhof T C, Rizo J. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 2.Llinás R, Sugimori M, Silver R B. Science. 1992;256:379–388. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 3.Nalefski E A, Falke J J. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiavo G, Osborne S L, Sgouros J G. Biochem Biophys Res Commun. 1998;248:1–8. doi: 10.1006/bbrc.1998.8527. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda M, Mikoshiba K. BioEssays. 1997;19:593–603. doi: 10.1002/bies.950190710. [DOI] [PubMed] [Google Scholar]
- 6.Marquèze B, Berton F, Seagar M. Biochimie. 2000;82:409–420. doi: 10.1016/s0300-9084(00)00220-0. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M, Kanno E, Mikoshiba K. J Biol Chem. 1999;274:31421–31427. doi: 10.1074/jbc.274.44.31421. [DOI] [PubMed] [Google Scholar]
- 8.Krasnov P A, Enikolopov G. J Cell Sci. 2000;113:1389–1404. doi: 10.1242/jcs.113.8.1389. [DOI] [PubMed] [Google Scholar]
- 9.Geppert M, Goda Y, Hammer R E, Li C, Rosahl T W, Stevens C F, Südhof T C. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 10.Nonet M L, Grundahl K, Meyer B J, Rand J B. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 11.Littleton J T, Bellen H J. Trends Neurosci. 1995;18:177–183. doi: 10.1016/0166-2236(95)93898-8. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen E M, Hartwieg E, Schuske K, Nonet M L, Jin Y, Horvitz H R. Nature (London) 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- 13.Reist N E, Buchanan J, Li J, DiAntonio A, Buxton E M, Schwarz T L. J Neurosci. 1998;18:7662–7673. doi: 10.1523/JNEUROSCI.18-19-07662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elferink L A, Peterson M R, Scheller R H. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 15.Bommert K, Charlton M P, DeBello W M, Chin G J, Betz H, Augustine G J. Nature (London) 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- 16.Mikoshiba K, Fukuda M, Moreira J E, Lewis F M T, Sugimori M, Niinobe M, Llinás R. Proc Natl Acad Sci USA. 1995;92:10703–10707. doi: 10.1073/pnas.92.23.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda M, Moreira J E, Lewis F M T, Sugimori M, Niinobe M, Mikoshiba K, Llinás R. Proc Natl Acad Sci USA. 1995;92:10708–10712. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llinas R, Sugimori M, Lang E J, Morita M, Fukuda M, Niinobe M, Mikoshiba K. Proc Natl Acad Sci USA. 1994;91:12990–12993. doi: 10.1073/pnas.91.26.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohara-Imaizumi M, Fukuda M, Niinobe M, Misonou H, Ikeda K, Murakami T, Kawasaki M, Mikoshiba K, Kumakura K. Proc Natl Acad Sci USA. 1997;94:287–291. doi: 10.1073/pnas.94.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang J, Fukuda M, Zhang H, Mikoshiba K, Wollheim C B. EMBO J. 1997;16:5837–5846. doi: 10.1093/emboj/16.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas D M, Elferink L A. J Neurosci. 1998;18:3511–3520. doi: 10.1523/JNEUROSCI.18-10-03511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugimori M, Tong C-K, Fukuda M, Moreira J E, Kojima T, Mikoshiba K, Llinás R. Neuroscience. 1998;86:39–51. doi: 10.1016/s0306-4522(97)00689-1. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda M, Aruga J, Niinobe M, Aimoto S, Mikoshiba K. J Biol Chem. 1994;269:29206–29211. [PubMed] [Google Scholar]
- 24.Fukuda M, Kojima T, Aruga J, Niinobe M, Mikoshiba K. J Biol Chem. 1995;270:26523–26527. doi: 10.1074/jbc.270.44.26523. [DOI] [PubMed] [Google Scholar]
- 25.Ibata K, Fukuda M, Mikoshiba K. J Biol Chem. 1998;273:12267–12273. doi: 10.1074/jbc.273.20.12267. [DOI] [PubMed] [Google Scholar]
- 26.Petrenko A G, Perin M S, Davletov B A, Urhkaryov Y A, Geppert M, Südhof T C. Nature (London) 1991;353:65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- 27.Hata Y, Davletov B, Petrenko A G, Jahn R, Südhof T C. Neuron. 1993;10:307–315. doi: 10.1016/0896-6273(93)90320-q. [DOI] [PubMed] [Google Scholar]
- 28.Perin M S. Biochemistry. 1996;35:13808–13816. doi: 10.1021/bi960853x. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda M, Mikoshiba K. J Biol Chem. 1999;274:31428–31434. doi: 10.1074/jbc.274.44.31428. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda M, Mikoshiba K. J Biol Chem. 2000;275:28180–28185. doi: 10.1074/jbc.M001376200. [DOI] [PubMed] [Google Scholar]
- 31.Mizushima S, Nagata S. Nucleic Acids Res. 1990;18:5332. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda M, Mikoshiba K. J Biochem (Tokyo) 2000;128:637–645. doi: 10.1093/oxfordjournals.jbchem.a022796. [DOI] [PubMed] [Google Scholar]
- 33.Ibata K, Fukuda M, Hamada T, Kabayama H, Mikoshiba K. J Neurochem. 2000;74:518–526. doi: 10.1046/j.1471-4159.2000.740518.x. [DOI] [PubMed] [Google Scholar]
- 34.Llinás R, Gruner J, Sugimori M, Mc Guinness T, Greengard P. J Physiol. 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heuser J E, Reese T S. J Cell Biol. 1981;88:564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J Z, Davletov B A, Südhof T C, Anderson R G W. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 37.Haucke V, De Camilli P. Science. 1999;285:1268–1271. doi: 10.1126/science.285.5431.1268. [DOI] [PubMed] [Google Scholar]
- 38.Geppert M, Khvotchev M, Krasnoperov V, Goda Y, Missler M, Hammer R E, Ichtchenko K, Petrenko A G, Südhof T C. J Biol Chem. 1998;273:1705–1710. doi: 10.1074/jbc.273.3.1705. [DOI] [PubMed] [Google Scholar]
- 39.Sugita S, Khvochtev M, Südhof T C. Neuron. 1999;22:489–496. doi: 10.1016/s0896-6273(00)80704-7. [DOI] [PubMed] [Google Scholar]
- 40.Shoji-Kasai Y, Yoshida A, Ogura A, Kuwahara R, Grasso A, Takahashi M. FEBS Lett. 1994;353:315–318. doi: 10.1016/0014-5793(94)01069-2. [DOI] [PubMed] [Google Scholar]