Abstract
Male mice with a knockout of the estrogen receptor (ER)-α gene, a ligand-activated transcription factor, showed reduced levels of intromissions and no ejaculations whereas simple mounting behavior was not affected. In contrast, all components of sexual behaviors were intact in male mice lacking the novel ER-β gene. Here we measure the extent of phenotype in mice that lack both ER-α and ER-β genes (αβERKO). αβERKO male mice did not show any components of sexual behaviors, including simple mounting behavior. Nor did they show ultrasonic vocalizations during behavioral tests with receptive female mice. On the other hand, reduced aggressive behaviors of αβERKO mice mimicked those of single knockout mice of ER-α gene (αERKO). They showed reduced levels of lunge and bite aggression, but rarely showed offensive attacks. Thus, either one of the ERs is sufficient for the expression of simple mounting in male mice, indicating a redundancy in function. Offensive attacks, on the other hand, depend specifically on the ER-α gene. Different patterns of natural behaviors require different patterns of functions by ER genes.
Keywords: testosterone, mounts, intromissions, ejaculation, aggression
Intracellular estrogen receptors (ERs) play key roles in the neuroendocrine regulation of reproduction. Binding of ovarian steroid, estradiol, to ERs in female rodents triggers a series of molecular and neurochemical processes that lead to the induction of lordosis behavior, an essential behavioral component for successful reproduction (1). Normal expression of male reproductive behaviors also may depend on activation of ER, because the male gonadal steroid, testosterone, acts not only through androgen receptors (ARs) in its original form or 5α-reduced form (dihydrotestosterone), but also through ERs after being aromatized to estradiol.
Two types of ERs, ERα and ERβ, which bind to estradiol with a similar affinity, are identified in the brain (2–4). This field of work is well enough developed that we can attempt to move beyond the classical Beadle and Tatum “one gene, one enzyme” formulation, to discover patterns of gene activation influencing patterns of behavior.
In knockout (KO) mice that lack the gene for either ERα (αERKO) or ERβ (βERKO) individually (5, 6), male sexual behaviors are only partially disrupted or even virtually normal. αERKO mice, although they rarely ejaculated and were infertile, showed almost normal levels of mounts and just reduced levels of intromissions (7). In contrast, all three components of sexual behaviors were present and robust in intact βERKO males (8). Because earlier findings indicated some unique ovarian phenotype in double KO mice compared with either αERKO or βERKO (9), it was necessary to determine what pattern of behavioral deficits would appear in KO mice that lack both ERα and ERβ genes (αβERKO).
In aromatase KO (ArKO) male mice sexual behaviors are strongly modified including the reduction of mount frequency and the prolonged mount latency (10). However, male ArKO mice are known to be fertile (11). It is assumed that their ejaculatory behaviors are intact, although no detailed analysis of ArKO male sexual behaviors has been reported.
Do these results suggest that ER activation may only be partially responsible for the induction of male sexual behaviors, or in αERKO and βERKO mice, is the missing gene compensated by the other ER? To answer this question, we used double KO mice that lack both ERα and ERβ genes, αβERKO (9).
In contrast to sexual behavior, aggressive behavior was greatly reduced in αERKO male mice (7, 12). Particularly, male-typical offensive attacks were almost completely abolished in both gonadally intact and testosterone-treated gonadectomized αERKO, whereas lunge and bite attacks (mild and short-lasting aggressive behaviors) were still present. In βERKO male mice, on the other hand, aggressive behavior was not at all reduced but rather elevated depending on age‖ and social experiences (8). These findings suggest that activation of ERβ might be inhibitory to male aggressive behavior when facilitated by activation of AR and/or ERα. How might the deletion of both ERα and ERβ gene function affect male aggressive behavior? In the present study, we assessed aggressive behavior of αβERKO mice in two different paradigms, resident-intruder and homogeneous set tests, and compared results with those of single KO (αERKO and αERKO) and wild-type (WT) (αβWT) litter mates.
Materials and Methods
Mice.
Gonadally intact male mice (12–45 weeks old) lacking genes for both ERα and ERβ (αβERKO; n = 8), ERα (αERKO; n = 2), or ERβ (βERKO; n = 9), and their WT (αβWT; n = 7) littermates from a mixed background of C57BL/6J and 129 were used. They were obtained from the breeding colony maintained at the National Institute of Environmental Health Sciences. All mice were individually housed in plastic cages (30 × 20 × 13 cm) throughout the extent of the studies and maintained on a 12/12-h light/dark cycle (light off at 10 a.m.) at constant temperature (22°C). Food and water were available ad libitum. Mice were tested for sexual and aggressive behaviors. All tests were done during the dark phase of light/dark cycle starting at 2 h after lights off, under red light and videotaped for further analysis. It should be noted that although only two αERKO mice were used in the present study, we found that their sexual and aggressive behavioral phenotypes were consistent with those found in our previous studies (7, 12).
Sexual Behavior Tests.
Male mice were tested twice (with an interval of 4–6 days) for sexual behavior during a 30-min (test 1) or 90-min (test 2) behavioral test with a female mouse (WT females from the αERKO colony maintained in the National Institute of Environmental Health Sciences) in the male's home cage. All females were ovariectomized and s.c.-injected with estradiol benzoate (10 μg and 5 μg at 48 h and 24 h before the tests) and progesterone (500 μg at 4–7 h before the tests) to ensure high sexual receptivity. For each male, the latency and number (expressed as number/10 min) of attempted mounts (including head mounts), mounts, intromissions, and ejaculations were recorded. Ejaculation duration and numbers of mounts and intromissions to the ejaculation (test 2 only) also were recorded for mice that showed ejaculation. Male sexual behaviors were defined as described (13). During test 2, ultrasonic vocalization was monitored with a QMC (London) bat detector (model S200), tuned to a center frequency of 70 kHz, for the first 10 min after the introduction of the female mouse.
Male Aggressive Behavior Test.
Mice were tested for aggressive behaviors four times in a resident-intruder paradigm and once in a homogeneous set paradigm. In the former, each male was tested in his home cage (as a resident) against a group-housed (4–5 mice per cage) olfactory bulbectomized (OBX) male Swiss–Webster (SW) intruder mouse [(SW)fBR purchased from Taconic Farms] for 15 min. Expression of aggression in mice is regulated mainly by olfactory cues, and therefore OBX intruders rarely show aggression. However, because their gonads are intact, they can elicit aggressive behaviors from resident mice. By testing against OBX intruder mice, the aggressive behaviors of resident animals, which were not influenced by any experience of defeat, were measured. For each experimental male, latency to the first aggressive act (see below), cumulative duration of all aggressive bouts, number of lunge/bite aggression bouts (see below), number of offensive attack bouts (see below), and cumulative duration of sexual behavior by resident mice toward intruder mice (chasing with attempted mounts) were recorded. For the homogeneous set aggression tests, pairs of body weight-matched (±3 g) males from the same genotype were tested once, 1–2 days after the fourth resident-intruder aggression tests, in a clean, neutral cage (30 × 20 × 13 cm). They were first placed on either side of the test cage, which was divided in the center by transparent acrylic board. After a 5-min adaptation period, the divider was removed and males were tested for aggression for 15 min. For each pair, numbers of tail rattling (either mouse), numbers of pairs showing aggressive behaviors, latency to the first aggressive act, cumulative duration of all aggressive bouts, number of pairs showing sexual behaviors, and cumulative duration of sexual behavior all were recorded.
Tail rattling, biting, lunge, chasing, boxing, and offensive attack (often accompanied by biting and wrestling) were defined as aggressive behavior acts. An aggressive bout was defined as a continuous series of behavioral interactions including at least one aggressive behavioral act. Three seconds was the maximum amount of time that could elapse between aggressive behavioral acts to be considered part of the same aggressive bout: if intervals between the occurrences of two aggressive behavioral acts exceeded 3 s, the two behavioral acts were scored as two separate aggressive bouts. Because we have previously found that αERKO mice rarely showed male-typical offensive attacks but exhibited female-type lunge and bite aggression (7, 12), we measured numbers of lunge/bite aggression bouts and offensive attack bouts separately.
Immunocytochemistry for ARs.
Mice were deeply anesthetized and perfused transcardially with (i) 100 mM PBS containing 0.1% heparin, pH 7.2, and (ii) 4% paraformaldehyde in 100 mM phosphate buffer (PB), pH 7.2. The brains were removed, postfixed in 4% paraformaldehyde in PB, and stored at 4°C in PB containing 30% sucrose. Brain tissues were cut at 30 μm on a freezing microtome. Free-floating sections were washed in 0.3% hydrogen peroxide in 50 mM Tris-buffered saline (TBS), pH 7.2, for 10 min, and blocked with 4% normal goat serum (Vector Laboratories) in TBS with 0.1% gelatin and 1% Triton X-100 for 2 h. They were then incubated in (i) anti-AR primary antibody (PA1–111A; Affinity BioReagent) in TBS containing 0.5% Triton X-100 and 4% normal goat serum for 48 h at 4°C; (ii) an 1:200 dilution of the biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) in TBS containing 0.5% Triton X-100 and 4% of normal goat serum for 120 min at room temperature; and (iii) the avidin-biotin-complex (Vectastain ABC Elite kit, Vector Laboratories) in TBS containing 0.5% Triton X-100 for 60 min at room temperature. Sections were treated with 0.05% diaminobenzidine and 0.03% hydrogen peroxide in TBS, pH 7.8. Sections were mounted on gelatin-coated slides, dehydrated, and cover-sliped with DePeX mounting medium (BDH Laboratory Supplies, Poole, U.K.). Control conditions involved either preadsorption of antibody with antigen protein or omitting the primary antibody from the staining procedure.
Statistics.
Data were analyzed by a two-way ANOVA for repeated measurements for the main effects of genotype and test day and their interaction, followed by post hoc one-way ANOVAs on each test day if necessary. Data of nonrepeated measurements were analyzed by one-way ANOVAs. Tukey's test was used for post hoc pairwise comparisons at α = 0.05. Differences in the percent of animals showing certain behavior were tested with χ2 test or Fisher exact probability test.
Results and Discussion
Male Sexual Behavior.
Male sexual behaviors were completely disrupted in αβERKO males (Table 1). During 30-min sexual behavior tests with sexually highly receptive female stimulus mice, αβERKO males showed neither mounts, intromissions, nor ejaculations. Instead, half of αβERKO males actually showed aggression toward female mice, even though the females were indifferent. Behavioral patterns of αβERKO male aggression toward females were defensive-type and similar to those of pregnancy-induced and postpartum aggression in female mice (14). The other three genotypes of mice (αERKO, βERKO, and αβWT) exhibited sexual behaviors typical to each genotype, replicating our original findings (7, 8, 12). Similar results were obtained in the second series of test that lasted for 90 min (Table 2). None of the αβERKO males exhibited male sexual behaviors, even a simple mount. βERKO mice, like αβWT, showed all three components of sexual behaviors, including ejaculation, whereas αERKO males showed mounts and intromissions but not ejaculation. These findings suggest that at least one of the ERs is required for the expression of simple mounting in male mice and by implication, that activation of AR alone is not sufficient.
Table 1.
Results of 30-min sexual behavioral tests (test 1)
| Test | αβWT | αβERKO | αERKO | βERKO | |
|---|---|---|---|---|---|
| Aggression | Number of mice | 0/7 | 4/8‡ | 0/2 | 0/9 |
| Mounts | Number of mice | 6/7 | 0/7ठ| 1/2 | 7/9 |
| Mean latency* | 539.17 ± 52.35 | — | 291 | 468.29 ± 36.01 | |
| Mean number/10 min | 2.26 ± 0.27 | 0.00 ± 0.00†‡§ | 1.50 ± 1.06 | 1.94 ± 0.24 | |
| Intromissions | Number of mice | 5/7 | 0/7ठ| 1/2 | 6/9 |
| Mean latency* | 533.80 ± 43.27 | — | 412 | 747.17 ± 94.02 | |
| Mean number/10 min | 3.41 ± 0.54 | 0.00 ± 0.00†‡§ | 3.00 ± 2.12 | 4.01 ± 0.54 | |
| Ejaculation | Number of mice | 1/7 | 0/7 | 0/2 | 1/9 |
| Mean latency* | 934 | — | — | 1,026 | |
| Mean duration* | 33 | — | — | 32 |
Included only the pairs that showed the behavior.
P < 0.05 vs. αERKO.
P < 0.05 vs. βERKO.
P < 0.05 vs. αβWT.
Table 2.
Results of 90-min sexual behavioral tests (test 2)
| Test | αβWT | αβERKO | αERKO | βERKO | |
|---|---|---|---|---|---|
| Ultrasonic vocalization | Number of mice | 7/7 | 1/8ठ| 1/2 | 9/9 |
| Mean latency* | 201.57 ± 10.71 | 231 | 189 | 131.67 ± 3.45§ | |
| Attempted mounts | Number of mice | 7/7 | 0/7ठ| 1/2 | 8/8 |
| Mean latency* | 682.86 ± 90.75 | — | 388 | 998.38 ± 96.49 | |
| Mean number/10 min | 1.89 ± 0.22 | 0.00 ± 0.00†‡§ | 0.78 ± 0.55 | 0.98 ± 0.06 | |
| Mounts | Number of mice | 7/7 | 0/7ठ| 1/2 | 7/8 |
| Mean latency* | 708.86 ± 88.53 | — | 808 | 1,171.43 ± 131.68 | |
| Mean number/10 min | 2.09 ± 0.19 | 0.00 ± 0.00†‡§ | 0.06 ± 0.04§ | 0.79 ± 0.09§ | |
| Intromissions | Number of mice | 6/7 | 0/7ठ| 1/2 | 7/8 |
| Mean latency* | 1,053.83 ± 135.68 | — | 808 | 1,398.71 ± 183.34 | |
| Mean number/10 min | 6.35 ± 0.97 | 0.00 ± 0.00†‡§ | 3.33 ± 2.36 | 4.53 ± 0.52 | |
| Ejaculation | Number of mice | 6/7 | 0/7ठ| 0/2 | 5/8 |
| Mean latency* | 3,336.17 ± 276.63 | — | — | 2,361.20 ± 215.42 | |
| Mean duration* | 27.33 ± 0.60 | — | — | 28.40 ± 1.02 | |
| Mean no. of mount + intromissions to the ejaculation* | 47.17 ± 6.34 | — | — | 26.80 ± 3.56 |
Included only the pairs that showed the behavior.
P < 0.05 vs. αERKO.
P < 0.05 vs. βERKO.
P < 0.05 vs. αβWT.
In the second series of tests, we also examined the presence of ultrasonic vocalization, which normally accompany social investigation (licking of female genital area) or sexual chasing, preceding mounts or intromissions (15). During the first 10 min of sexual behavioral tests, 100% of βERKO and αβWT mice vocalized intensively (regardless of the numbers of mounts/intromissions during the 10-min recording period) whereas only one of eight αβERKO males vocalized, and then very briefly. Although only one of two tested αERKO mice vocalized in the present study, we previously have observed that ultrasonic vocalizations emitted by αERKO mice in response to sexually receptive female mice were not different from those of αWT mice, in both gonadally intact and testosterone-treated gonadectomized males (S.O., R. J. Barfield, and D.W.P., unpublished observation). Because estradiol implants in medial septum and medial preoptic area have effectively restored ultrasonic vocalization in castrated male mice (15), it is assumed that ER activation is responsible for vocalization. Our findings in single and double KO mice suggest that at least one of the two ER gene products also is required for the production of ultrasonic vocalization.
Taken together, these findings show that male sexual responses to receptive females are completely disrupted in αβERKO males. Most of the nonbehavioral reproductive phenotype of αβERKO is similar to that of the αERKO males, including infertility, reduced numbers, and motility of epididymal sperm, yet grossly normal reproductive tract and spematogenesis (9). Behaviorally, however, αERKO and αβERKO resembled each other only in the lack of ejaculation. These findings suggest that an ERβ-dependent action of estradiol (perhaps as an aromatization product of testosterone) in the brain can play a role in the regulation of sexual behavior even though the lack of it (in βERKO mice) can be compensated by ERα. Sexual behaviors of βERKO males were not deficient in comparison to those of αβWT mice. In the 90-min tests, numbers of mountings of βERKO mice were significantly less than those of αβWT mice. These results, however, do not imply that βERKO mice were sexually less active. Rather, they filled a criterion for greater sexual excitement compared with αβWT mice, because their numbers of mounts and intromission to the ejaculation were actually about half of those of αβWT mice (Table 2). These behavioral phenotypes of βERKO mice might be caused by the increase of AR activation in the brain. Although it has not been demonstrated in the brain, higher AR densities have been reported in βERKO prostates (Z. Weihua, M. Warner, and J.-Å.G., unpublished data).
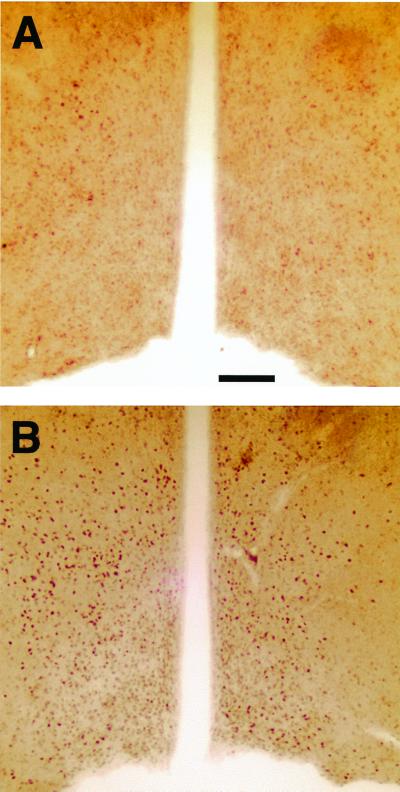
In gonadectomized αERKO male mice, mounts and intromissions were restored to pregonadectomy levels by dihydrotestosterone, a nonaromatizable androgen (12). Therefore, it is possible that sexual behavior in gonadally intact αERKO is partially supported by an AR-mediated action of testosterone in addition to an ERβ-dependent action. In αβERKO mice, however, we believe that AR activation is not sufficient to rescue their impaired sexual behaviors. We found that ligand-bound AR immunoreactivity was not at all reduced in a number of brain regions of αβERKO. Especially, important is the medial preoptic area, which is one of the critical brain areas for the regulation of male sexual behavior by gonadal steroids (Fig. 1). Instead, lack of ER activation during development may be responsible for the severe disruption of sexual behavior in αβERKO males. Furthermore, the almost complete shutdown of male sexual activity in αβERKO mice is contrasted to the behavioral phenotype of aromatase KO mice, which showed only a reduction (10), but not an elimination of components of sexual behaviors including ejaculation (11).
Figure 1.
Photomicrographs showing the AR-immunoreactive (IR) cells (stained with rabbit polyclonal antibody, Affinity Bioreagent) in the medial preoptic area, where gonadal steroids regulate male sexual behaviors. AR-IR cells were not at all reduced and may have been increased in αβERKO mice (B) compared with αβWT mice (A) brains. (Scale bar: 100 μm.)
Male Aggressive Behavior.
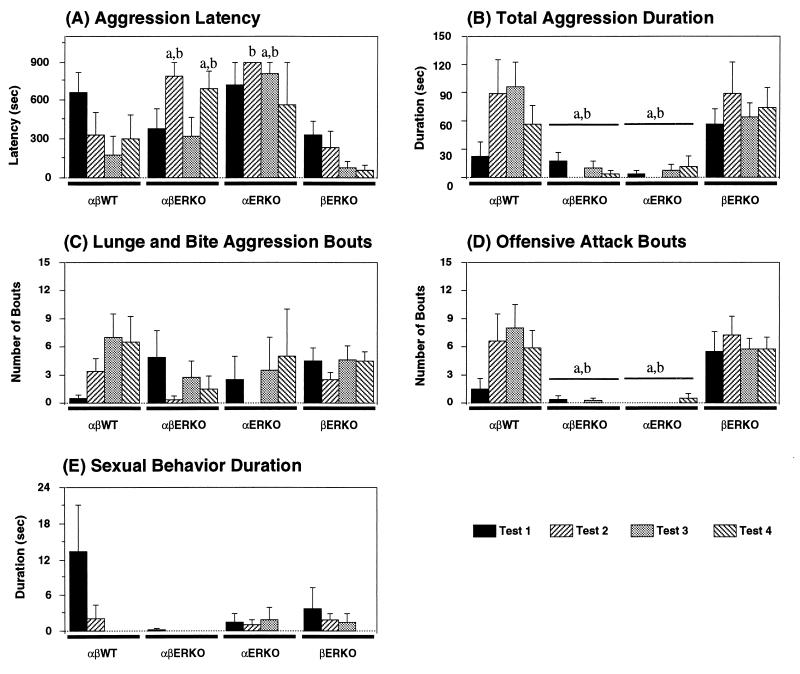
In the resident-intruder paradigm, we tested aggressive behaviors of αβERKO mice toward olfactory-bulbectomized male intruder mouse four times (twice after the first sexual behavior test and twice after the second sexual behavior test). We found that aggressive behaviors were greatly reduced in αβERKO mice (Fig. 2B), very similar to αERKO mice. Strikingly, αβERKO males rarely showed offensive attacks in all four tests (Fig. 2D). Lunge and bite attacks, on the other hand, were present, and some αβERKO showed a few such mild aggression bouts with relatively short latencies especially during the first and third tests (Fig. 2 A and C). In the second and fourth tests, which were done on the day after the first and third tests, αβERKO mice were less aggressive, as clearly demonstrated in the latency measure (Fig. 2A). Based on these characteristics (temporal changes and behavioral pattern), we concluded that these comprised defensive-type aggression, which is more typically seen in pregnant and postpartum female mice (14).
Figure 2.
Effects of ERα and/or ERβ gene disruption on (A) latency to the first aggressive act, (B) cumulative duration of all aggressive bouts, (C) number of lunge and bite aggression bouts, (D) number of offensive attack bouts, and (E) cumulative duration of attempted sexual behaviors toward male intruder mice, during resident-intruder tests. There were significant genotype differences in total aggression duration (B) and number of offensive attack bouts (D) throughout the four tests (P < 0.01) but not in the number of lunge and bite aggression bouts (C). Post hoc comparisons for the main effects of genotype revealed that both αβERKO and αERKO mice were significantly less aggressive compared with αβWT as well as βERKO. On the other hand, there was a significant (P < 0.01) interaction between genotype and test in aggression latency (A). Latencies in αβWT and βERKO males steadily decreased with the repetition of the tests, whereas those of αβERKO were shorter in the first and third tests compared with the second and fourth tests. Post hoc comparisons for genotype differences, therefore, were performed in each test separately for this measurement. Finally, αβWT showed substantial amounts of sexual behaviors in the first test compared with other three genotypes of mice, although overall genotype differences were not significant. a: P < 0.05 vs αβWT, b: P < 0.05 vs βERKO.
In the other two genotypes of males, βERKO and αβWT, substantial numbers of offensive attacks were observed. As previously reported (8), βERKO mice tended to be more aggressive than αβWT controls in the first aggression test, when the αβWT mice showed attempted mounts toward male intruders (Fig. 2E). In subsequent tests, αβWT mice became more aggressive, whereas aggression of βERKO mice remained unchanged.
Abolition of offensive attacks in αβERKO and αERKO mice also was found in the homogeneous set aggression tests, in which pairs of body weight-matched mice from the same genotype were tested in neutral cages (Table 3).
Table 3.
Results of homogeneous set aggression tests
| Test | αβWT | αβERKO | αERKO | βERKO |
|---|---|---|---|---|
| Number of nonaggressive tail rattling | 4.67 ± 4.18 | 0.00 ± 0.00 | 0.00 | 1.50 ± 1.50 |
| Number of aggressive pairs | 2/3 | 0/4 | 0/1 | 3/4 |
| Aggression latency* | 418.50 ± 362.5 | — | — | 131.67 ± 51.95 |
| Total aggression duration | 33.67 ± 23.14 | 0.00 ± 0.00 | 0.00 | 75.75 ± 31.44 |
| Number of pairs showed sexual behaviors | 2/3 | 1/4 | 1/1 | 0/4 |
| Sexual behavior duration | 2.67 ± 1.76 | 3.50 ± 3.50 | 39.00†‡§ | 0.00 ± 0.00 |
Included only the pairs that showed aggression.
P < 0.05 vs. αβERKO.
P < 0.05 vs. βERKO.
P < 0.05 vs. αβWT.
These results suggest that the induction of offensive attacks, unlike male sexual behavior, may be regulated predominantly in a facilitatory manner by the activation of ERα, whether in adulthood or during development. However, this does not necessarily exclude the possibility that the activation of ERβ also may be involved in the normal control of aggressive behavior in male mice. In the present and our previous studies, we found that βERKO mice (4–9 months old) were more aggressive under certain conditions of social experience. Using younger (5–11 weeks old) and naïve (not tested for sexual behavior before aggression tests) mice, we found that βERKO mice were significantly more aggressive than WT controls.∥ These behavioral changes might be because of the lack of an inhibitory action by ERβ on ERα-dependent function in βERKO mice. Such an action could stem from areas of the brain exhibiting coexpression of the two ERs or from independent ERβ- and ERα-regulated pathways that converge on a common neural pathway mediating aggressive behavior responses.
In summary, the present study demonstrates that two types of reproduction-related social behaviors require different patterns of ER gene activation.
Acknowledgments
We are thankful to Dr. C. Pavlides for critical reading of the manuscript. Support for this work was provided by National Science Foundation Grant IBN-9728579 and National Institute of Mental Health Grant MH62147–01 (to S.O.), and National Institutes of Health Grant HD-05751 (to D.W.P.).
Abbreviations
- ER
estrogen receptor
- AR
androgen receptor
- KO
knockout
- WT
wild type
- TBS
Tris-buffered saline
Footnotes
See commentary on page 14038.
Nomura, M., Ueta, Y., Chan, J., Gustafsson, J.-Å., Smithies, O., Korach, K. S., Pfaff, D. W. & Ogawa, S. (2000) Soc. Neurosci. Abstr. 26, 1273 (abstr.).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250473597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250473597
References
- 1.Pfaff D W. Drive. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 2.Shughrue P J, Lane M V, Merchenthaler I. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Kuiper G G J M, Enmark E, Peltohuikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper G G J M, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson J-Å. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 5.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J-Å, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa S, Chan J, Chester A E, Gustafsson J-Å, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couse J F, Hewitt S C, Bunch D O, Sar M, Walker V R, Davis B J, Korach K S. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 10.Honda S, Harada N, Ito S, Takagi Y, Maeda S. Biochem Biophys Res Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- 11.Fisher C R, Graves K H, Parlow A F, Simpson E R. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogawa S, Washburn T F, Taylor J, Lubahn D B, Korach K S, Pfaff D W. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 13.McGill T E. In: Contributions to Behavior-Genetic Analysis: The Mouse as a Prototype. Lindzey G, Thiessen D D, editors. New York: Appleton Century Crofts; 1970. pp. 57–88. [Google Scholar]
- 14.Ogawa S, Makino J. Behav Neural Biol. 1984;40:195–204. doi: 10.1016/s0163-1047(84)90303-0. [DOI] [PubMed] [Google Scholar]
- 15.Nyby J, Matochik J A, Barfield R J. Horm Behav. 1992;26:24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]