Abstract
Objective:
To determine whether patients with Barrett esophagus who undergo antireflux surgery differ from medically treated patients in incidence of esophageal adenocarcinoma and probability of disease regression/progression.
Summary Background Data:
Barrett esophagus is a risk factor for the development of esophageal adenocarcinoma. A question exists as to whether antireflux surgery reduces this risk.
Methods:
Query of PubMed (1966 through October 2005) using predetermined search terms revealed 2011 abstracts, of which 100 full-text articles were reviewed. Twenty-five articles met selection criteria. A review of article references and consultation with experts revealed additional articles for inclusion. Studies that enrolled adults with biopsy-proven Barrett esophagus, specified treatment-type rendered, followed up patients with endoscopic biopsies no less than12 months of instituting therapy, and provided adequate extractable data. The incidence of adenocarcinoma and the proportion of patients developing progression or regression of Barrett esophagus and/or dysplasia were extracted.
Results:
In surgical and medical groups, 700 and 996 patients were followed for a total of 2939 and 3711 patient-years, respectively. The incidence rate of esophageal adenocarcinoma was 2.8 (95% confidence interval, 1.2–5.3) per 1000 patient-years among surgically treated patients and 6.3 (3.6–10.1) among medically treated patients (P = 0.034). Heterogeneity in incidence rates in surgically treated patients was observed between controlled studies and case series (P = 0.014). Among controlled studies, incidence rates were 4.8 (1.7–11.1) and 6.5 (2.6–13.8) per 1000 patient-years in surgical and medical patients, respectively (P = 0.320). Probability of progression was 2.9% (1.2–5.5) in surgical patients and 6.8% (2.6–12.1) in medical patients (P = 0.054). Probability of regression was 15.4% (6.1–31.4) in surgical patients and 1.9% (0.4–7.3) in medical patients (P = 0.004).
Conclusions:
Antireflux surgery is associated with regression of Barrett esophagus and/or dysplasia. However, evidence suggesting that surgery reduces the incidence of adenocarcinoma is largely driven by uncontrolled studies.
A systematic review and meta-analysis of 25 articles of 2011 publications screened supports that antireflux surgery is associated with regression of Barrett esophagus and/or dysplasia. However, evidence suggesting that surgery reduces the incidence of adenocarcinoma is largely driven by uncontrolled studies.
Esophageal adenocarcinoma occurs in an estimated 7000 patients each year in the United States, and its incidence has risen 350% since 1970.1 Although still a relatively rare disease, esophageal adenocarcinoma is associated with a dismal prognosis, with a 5-year overall survival rate of less than 10%.2–4 Furthermore, conventional curative treatment involves esophagectomy, which is associated with an in-hospital mortality rate of 7.5% to 14.5%5 and a correspondingly high morbidity rate.6
Because of the relative rarity of esophageal adenocarcinoma and the associated morbidity of esophagectomy, a preventative strategy should focus on individuals at greatest risk for developing disease. Barrett esophagus, a complication of gastroesophageal reflux disease (GERD) characterized by esophageal mucosa metaplasia, is associated with a 30- to 125-fold increase in risk for the development of esophageal adenocarcinoma7 and therefore represents a marker for patients at risk for disease progression. Barrett's metaplasia may progress from low-grade dysplasia (LGD), to high-grade dysplasia (HGD), and eventually to invasive adenocarcinoma, which may be present in up to 30% of cases of HGD and go unrecognized because of sampling error associated with endoscopic screening and surveillance.8 The presence of HGD is therefore considered an indication for esophagectomy.9
In patients with GERD and Barrett esophagus without dysplasia, however, the appropriate choice of therapy (medical or surgical) is debated. A theoretical advantage of antireflux surgery is the creation of a mechanical valve which prevents all forms of gastroesophageal reflux. In contrast, proton pump inhibitors and histamine receptor antagonists reduce the acidity of gastric secretions but do not prevent nonacidic reflux,10 which has been implicated in carcinogenesis.11 These observations have fueled speculation that surgical antireflux procedures may prevent the development of esophageal adenocarcinoma more effectively than medical antisecretory therapy. At present, however, the indications for antireflux surgery in patients with Barrett esophagus are the same as those for patients without Barrett's, and, with the exception of the addition of endoscopic surveillance for disease progression,12,13 do not extend beyond the goal of controlling symptoms and preventing reflux-related complications.14 It remains unknown whether surgical therapy more effectively prevents progression of Barrett esophagus to cancer.
Several nonrandomized cohort studies have compared the incidence of esophageal cancer after antireflux surgery as compared with treatment with antisecretory medications in the setting of Barrett esophagus. To date, these studies have yielded inconsistent results, which may reflect insufficient study power due to the low incidence of disease progression. Indeed, the relatively low incidence of esophageal cancer makes it unlikely that an adequately powered controlled prospective trial will ever be possible. To synthesize the published data, we performed a systematic review of the literature to determine whether a significant conclusion can be drawn as to whether antireflux surgery is associated with a lower incidence of esophageal adenocarcinoma compared with antisecretory therapy alone.
METHODS
Search Strategy
The MEDLINE database was searched for articles from 1966 to October 2005 (Fig. 1), using the following search terms: Barrett esophagus AND (Nissen fundoplication OR antireflux surgery OR antireflux procedure OR proton pump inhibitor OR adenocarcinoma OR esophageal neoplasm) AND English[la]. Studies were identified that evaluated the incidence of adenocarcinoma in patients with Barrett esophagus treated specifically with medical or surgical therapy. The full text of these relevant articles was reviewed. The reference lists of these articles were also reviewed to find additional candidate studies. Experts (B.A.J., J.G.H.) were asked to list any additional articles that met inclusion criteria. Data extracted for this study were taken from the published reports; authors were not contacted to obtain additional information. Non-English language studies were not included.
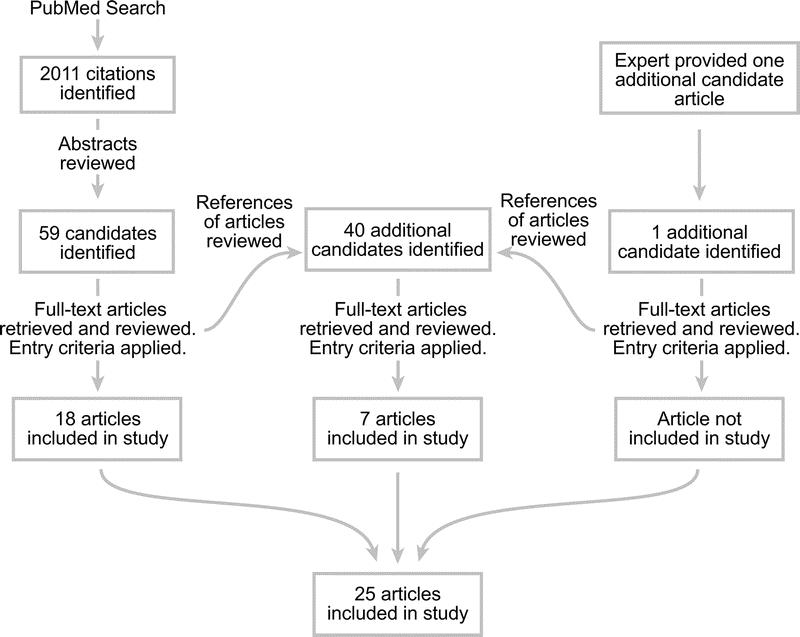
FIGURE 1. Search strategies used in systematic review and numbers of studies included at each stage.
Study Criteria
To be included, a study must have provided a description of the medical or surgical treatment rendered. The investigation must have presented sufficient data to determine the incidence of cancer (expressed in patient-years) separately for medical and surgical groups. Patients who underwent surgical treatment and were additionally treated with medical therapy were included in the surgical group. The goal of this systematic review was to evaluate the role of fundoplication in preventing progression to cancer; as such, studies that used predominantly nonstandard surgical procedures (eg, biliopancreatic diversion, Angelchik prostheses, and endoluminal therapies) were not included. All subjects must have had histologically proven Barrett esophagus and undergone surveillance endoscopy no less than 12 months after the institution of therapy. Barrett esophagus was defined as the presence of intestinal metaplasia in the distal esophagus. The length of intestinal metaplasia was not used as a criterion for inclusion in the analysis. Studies that were composed of mostly pediatric subjects (<18 years of age) were excluded. Studies were categorized into randomized controlled trials (RCTs), cohort studies, and case series. Cohort studies were defined as controlled observational studies comparing medical and surgical treatments for the study population. Case series are reports of outcomes in patients in one treatment arm (medical or surgical), without appropriate controls in a comparison arm. Some reports of controlled studies included data in which adenocarcinoma incidence rate could be extracted for a subset of reported patients, even though the study was not designed to examine the question of this systematic review; these patients were included as a case series. If a cohort or case series from a group appeared to be published more than once with significant temporal overlap, only the report that included the largest number of patients was used in the analysis.
For each treatment group within a given study, we recorded the number of patients, median and range of patient-years of follow-up, and the number of subjects who developed esophageal adenocarcinoma. If available, additional data were collected on the outcomes of Barrett esophagus patients with no dysplasia, LGD and HGD, and progression or regression of disease. For this analysis, progression and regression refer to changes between the following states: HGD, LGD, nondysplastic Barrett esophagus, and squamous epithelium. Only initial and final biopsy results were considered for determination of progression and regression. Changes in the length of Barrett esophagus and the development of squamous islands were not considered in defining progression or regression.
Statistical Analysis
Data from all included studies were pooled to calculate the incidence rates of esophageal adenocarcinoma for medical and surgical treatment arms. Pooled estimates of patient age in each treatment group were calculated by taking the weighted mean ages reported in each study. For studies that reported only median ages, this value was used to approximate the mean age. Adenocarcinoma incidence rates and probability of progression or regression were compared between the 2 treatment arms. Pooled estimates and 95% credible intervals for adenocarcinoma incidence rates and progression and regression probabilities were computed under a Bayesian modeling framework.15 Meta-regression models including a random effect for between-study variance were fitted with WinBUGS software.16 Indicator variables for treatment arm and study design were included in the meta-regression models, which allowed for testing for differences. To assess for heterogeneity within a treatment arm, comparisons were made between controlled studies (RCTs and cohort studies) and uncontrolled studies (case series) for that treatment. Cumulative estimates and confidence intervals of adenocarcinoma incidence rates were calculated for the addition of each successive study using the Byar approximation to the Poisson.17
To assess the feasibility of a larger study to detect a difference in the incidence rate and probabilities of progression and regression, a simulated analysis of hypothetical studies reporting a total of 100,000 patient-years of follow up in each arm was conducted. Pooled estimates of cancer incidence and probability of progression and regression were used as inputs for this simulation. Such a larger study was considered feasible if the differences could be demonstrated to a statistically significant level in the simulation.
RESULTS
Individual Studies
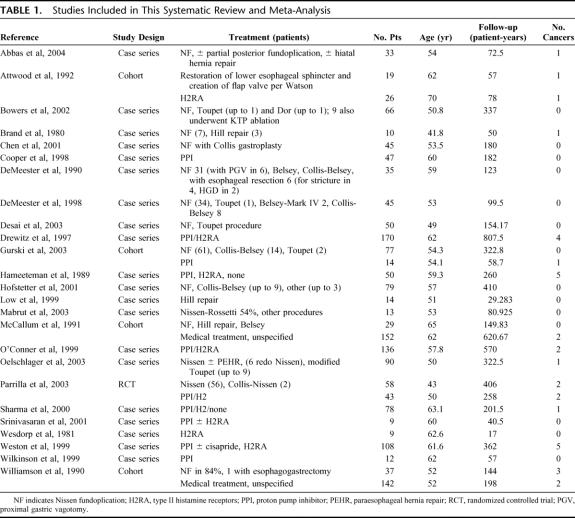
A PubMed search using the specified terms yielded 2011 English-language entries (Fig. 1). Upon abstract review, 100 articles appeared to evaluate the incidence of adenocarcinoma in patients with Barrett esophagus treated surgically or medically. The full-text articles were retrieved and the inclusion criteria were reapplied. Twenty-five articles met criteria and were included. Of these studies, one was an RCT, and 4 were cohort studies. Twenty studies were either uncontrolled case series or subsets of patients from larger studies designed to investigate a different question (Table 1).
TABLE 1. Studies Included in This Systematic Review and Meta-Analysis
Excluded Studies
A total of 78 studies were excluded. One study was a case report of a single patient.18 Four studies were excluded because they included patients that overlapped with another study included in this review.19–22 Eight studies did not specify the length of follow-up.23–30 In 3 studies, the length of follow-up was less than 12 months.31–33 Six studies included patients with nonmetaplastic GERD and did not provide sufficient data regarding the subset of patients with Barrett esophagus.34–39 Twenty studies did not differentiate between patients treated medically from those treated surgically.26,27,40–57 Eight studies did not evaluate the incidence of adenocarcinoma.58–65 Nine articles were reviews, which provided no novel clinical data.66–74 Two articles did not document the use of endoscopic follow-up with biopsies.23,75 Ten reports did not include enough data to express the incidence of adenocarcinoma in cases per patient-year.76–89 One study was excluded because it did not present a mathematically consistent incidence rate of adenocarcinoma.90 Two studies were restricted to patients with HGD or LGD.86,91 Three studies used nonstandard surgical procedures.29,92,93
Pooled Data
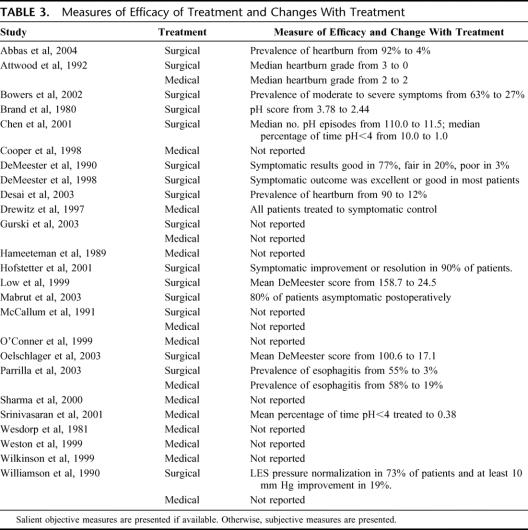
We included 25 studies with an aggregate total of 1696 patients with Barrett esophagus. Of these patients, 700 were treated only with medical therapy (referred to as “the medically treated group”) and were followed up for 3711 patient-years. The remaining 996 patients underwent antireflux surgery (referred to as “the surgically treated group”) and were followed up for 2939 patient-years after therapy (Table 2). The mean age was 52.8 years in the surgical group and 59.4 years in the medical groups. Twenty-three studies reported the prevalence of LGD and HGD on initial endoscopy, although the probability of progression or regression could be extracted from only 21 of these reports.94–116 At the baseline period for the cohorts, the prevalence of LGD was 8% in the medically treated group and 15.5% in the surgically treated group. The prevalence of HGD was 2% in the medically treated group and 0.3% in the surgically treated group. In most studies, the effectiveness of the therapy was assessed on the basis of objective testing and/or symptomatic improvement (Table 3).
TABLE 2. Characteristics of Medically and Surgically Treated Groups
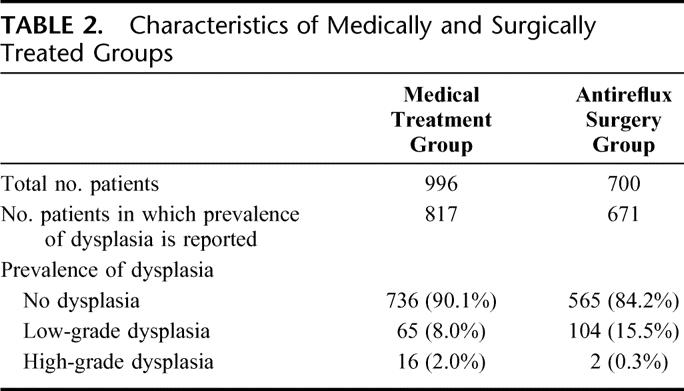
TABLE 3. Measures of Efficacy of Treatment and Changes With Treatment
Pooled Estimates of Adenocarcinoma Incidence Rate
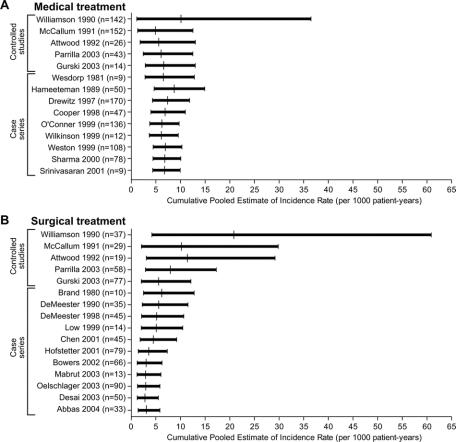
Because some studies reported an incidence of zero and therefore yielded an undefined confidence interval for the incidence rate of adenocarcinoma, it is not feasible to present confidence intervals of the cancer incidence rates for each study individually. Instead, graphs were constructed to show the cumulative estimates of the cancer incidence rate based on each study as it is pooled with those temporally preceding it for medically treated patients (Fig. 2a) and surgically treated patients (Fig. 2b).
FIGURE 2. Cumulative pooled estimates of incidence of adenocarcinoma (A) for medically treated patients and (B) for surgically treated patients. Each successive row shows the cancer incidence rate and 95% confidence interval when data are pooled from that study and all studies preceding it in chronological order.
When data from all included studies were pooled (Fig. 3), the median incidence of adenocarcinoma was 2.8 cases per 1000 patient-years among surgically treated patients (95% confidence interval [CI], 1.2–5.3), and 6.3 per 1000 patient-years among medically treated patients (95% CI, 3.6–10.1) (P = 0.034). Heterogeneity in incidence rates for surgically treated patients was observed between the controlled studies, which reported an incidence of 4.8 cases per 1000 patient-years (95% CI, 1.7–11.1), and case series, which reported an incidence rate of 1.4 cases per 1000 patient-years (95% CI, 0.3–3.9) (P = 0.048). When data from only the controlled studies (RCT and cohort study) were pooled, the median incidence of adenocarcinoma in the surgically treated group did not differ significantly from that of the medically treated group: 4.8 cases per 1000 patient-years (95% CI, 1.7–11.1) versus 6.5 per 1000 patient-years (95% CI, 2.6–13.8), respectively (P = 0.32). Among the uncontrolled studies, the median incidence of adenocarcinoma in the surgically treated group was significantly lower than that of the medically treated group: 1.4 cases per 1000 patient-years (95% CI, 0.3–3.9) versus 6.1 per 1000 patient-years (95% CI, 2.3–11.0), respectively (P = 0.014).
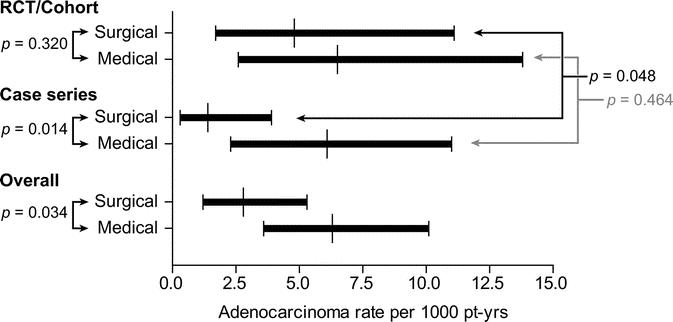
FIGURE 3. Comparison of pooled incidence rates of esophageal adenocarcinoma between surgically and medically treated patients. This comparison was repeated using only controlled studies and again using only case series. To test for heterogeneity, cancer incidence rates among each treatment group were also compared between case series and controlled studies.
In 2 case series, which examined the effect of medical therapy in Barrett esophagus patients,104,112 an unknown proportion of patients did not receive any antisecretory therapy throughout the study period. When these studies were excluded from analysis, the overall median incidence of esophageal adenocarcinoma was 2.8 per 1000 patient-years in the surgically treated group (95% CI, 1.2–5.3) and 5.8 in the medically treated group (95% CI, 3.1–9.1) (P = 0.042).
A widely cited cohort study by McCallum et al was included in this review,108 which has been published only in abstract form. When the analysis was repeated excluding this study, the overall difference in cancer incidence rate between medical and surgical therapy remained statistically different (3.0 cases per 1000 patient-years among surgically treated patients versus 7.0 per 1000 patient-years among medically treated patients, P = 0.024).
The feasibility of a larger study to detect a difference was assessed by simulating a larger hypothetical study in which each treatment arm contained a follow-up of 100,000 patient-years. Under the conditions found in the pooled analysis of controlled studies, the predictive incidence rate difference is 1.6 more cancers per 1000 person-years in the medical arm than in the surgical arm with a 95% confidence interval of −15.1 to 23.1 per 1000 person-years (P = 0.366).
Regression of Barrett Esophagus
Studies were pooled to estimate the proportion of patients who developed progression or regression of disease for each of the 2 groups during the follow-up period (Figs. 4 and 5). None of the included studies provided the follow-up period stratified by the initial dysplastic grade. Consequently, incidence rates of progression and regression according to initial grade of dysplasia could not be determined.
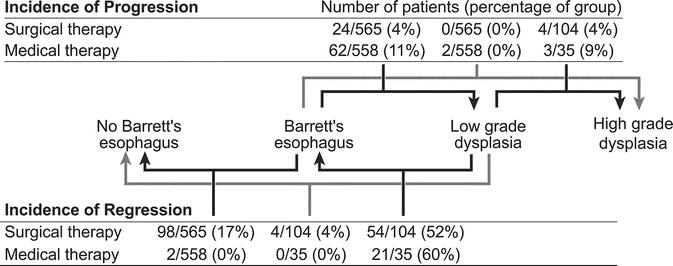
FIGURE 4. Proportions of patients with progression or regression of dysplasia and regression to squamous epithelium.
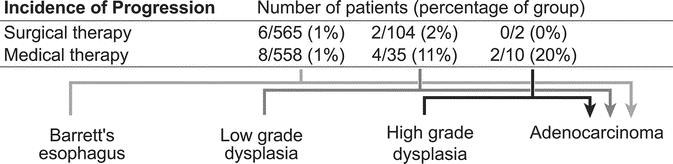
FIGURE 5. Proportions of patients progressing from each grade of dysplasia to esophageal adenocarcinoma.
When data from all studies were pooled (Fig. 6), the probability of regression was 15.4% in surgically treated patients (95% CI, 6.1%–31.4%) and 1.9% in medically treated patients (95% CI, 0.4%–7.4%) (P = 0.004). Among the controlled studies, 6.4% of surgically treated patients (95% CI, 1.1%–30.1%) and 0.5% of medically treated patients (95% CI, 0.0%–3.3%) demonstrated regression (P = 0.024). Among the uncontrolled studies, 21.9% of patients in the surgical group (95% CI, 8.7–46.3) and 5.6% of those in the medical group (95% CI, 0.7%–24.9%) demonstrated regression (P = 0.054). Heterogeneity was detected between the medically treated patients in controlled studies and those in case series (P = 0.032).
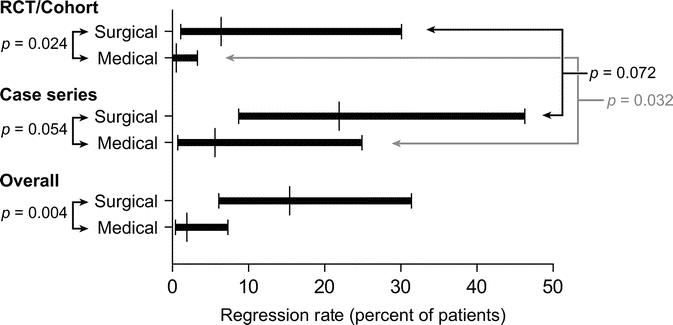
FIGURE 6. Comparison of probability of regression to lower grades of dysplasia, nondysplastic, or nonmetaplastic tissue, between surgically and medically treated patients. This comparison was repeated using only controlled studies and again using only case series. To test for heterogeneity, probability of regression was also compared between case series and controlled studies within each treatment group.
One case series by DeMeester et al101 met the inclusion criteria for this review but did not report progression or regression for all patients in the study. Also, this study enrolled a small number of patients with HGD, who underwent fundoplication after resection of the dysplastic portion of esophagus. Therefore, any regression was likely the result of resection rather than the antireflux procedure. With the exclusion of this study, the pooled rate of regression in the case series was 25.3% (95% CI, 11.9%–44.1%) in the surgical group and 6.6% (95% CI, 1.5%–11.7%) in the medical group (P = 0.018). Similarly, the overall pooled regression rate was 18.6% (95% CI, 7.4%–34.7%) in the surgical group and 2.2% (95% CI, 1.5%–18.7%) for the medically treated patients (P = 0.004).
In 2 case series, an unknown number of patients were not treated with antisecretory medication.104,112 When only these reports were excluded, the overall probability of regression was 15.6% (95% CI, 6.7%–30.4%) in surgically treated patients and 2.7% (95% CI, 0.5%–7.9%) in medically treated patients (P = 0.006).
Progression of Barrett Esophagus
When data from all studies were pooled, the probability of progression to LGD or HGD was 2.9% in surgically treated patients (95% CI, 1.2–5.5) and 6.8% in those treated medically (95% CI, 2.6–12.1) (P = 0.054) (Fig. 7). Among the RCT and cohort studies, probability of progression was 3.6% in the surgically treated group (95% CI, 0.9–9.0) and 9.5% in patients in the medically treated group (95% CI, 3.0–22.2) (P = 0.088). Among the case series, probability of progression was 2.2% in the surgically treated group (95% CI, 0.7–5.0) and 4.0% in the medically treated group (95% CI, 0.7–11.0) (P = 0.226).
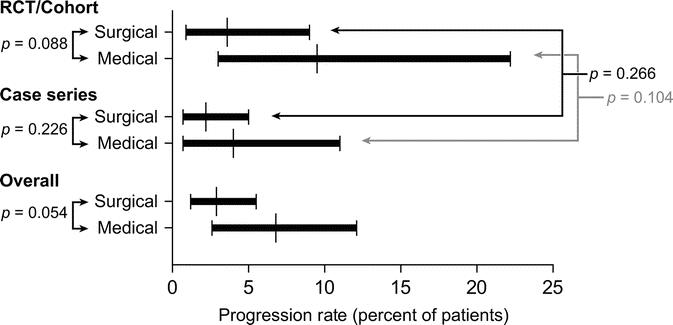
FIGURE 7. Comparison of probability of progression to more advanced grades of dysplasia, between surgically and medically treated patients. This comparison was repeated using only controlled studies and again using only case series. To test for heterogeneity, probability of progression was also compared between case series and controlled studies, within each treatment group.
DISCUSSION
This systematic review compares the incidence rates of esophageal adenocarcinoma in patients with Barrett esophagus treated with medical therapy versus antireflux surgery. When differences in study design are ignored, the results suggest that antireflux surgery is associated with a significantly reduced incidence rate of esophageal adenocarcinoma when compared with medically treated patients. This difference in incidence rates was maintained even after exclusion of studies in which some subjects in the medically treated arm received no antisecretory therapy.
However, heterogeneity was observed between case series and controlled studies in cancer incidence rates among patients undergoing antireflux surgery. Whereas the controlled studies reported a median incidence of 4.8 cases per 1000 patient-years for surgically treated patients, the case series demonstrated a median incidence of 1.4 cases per 1000 patient-years, a statistically significant difference. This heterogeneity suggests that the pooled estimates of cancer incidence in surgically treated patients may be artificially lowered due to publication or inclusion biases for case series. No such heterogeneity was observed among medically treated patients.
Analyzing only RCTs and cohort studies, a statistically significant difference in adenocarcinoma incidence rates could not be demonstrated between surgical and medical therapy. This lack of a detectable difference may be attributed in part to the difficulty in designing an adequately powered controlled prospective study to address such a rare disease. Under the conditions found in this systematic review, over 100,000 patient-years of follow-up in each arm would still not be sufficient to demonstrate a significant difference in observed incidence rates. Therefore, an RCT is probably infeasible.
The cumulative estimates of the incidence of adenocarcinoma among surgically treated patients demonstrate a downward shift of the incidence rate among more recent case series. A similar trend was not seen among the medically treated patients. Although this observation may be attributed to publication bias, an alternative explanation is that adenocarcinoma incidence rates may have improved with recent advances in antireflux surgery, such as the introduction of minimally invasive surgery, development of fellowship training programs, and an endemic familiarity with the procedure associated with its widespread acceptance as an effective therapy for GERD.117–119 This hypothesis would be supported by demonstrating a temporal improvement in the efficacy of surgical therapy with respect to symptom control and normalization of distal esophageal pH. However, because the studies included in this systematic review used widely varying techniques to evaluate adequacy of therapy (Table 3), this hypothesis would be difficult to test in a retrospective fashion.
The present study calculated the incidence rate of adenocarcinoma based on the number of cases per 1000 patient-years. A limitation of this method is that no distinction is made between the incidence rate of a small number of patients followed for a long period of time and a large number of patients followed for a short length of time. Thus, this method carries the assumption that cancer risk does not vary with length of time from diagnosis of Barrett's or initiation of treatment. Because of the limitations of individual case reports, however, a more rigorous method of aggregating the data (such as a time-to-event analysis) cannot be performed.
Patients in the medically treated group were both older and had a higher prevalence of HGD when compared with the surgically treated group. Because increasing age and the presence of HGD are both risk factors for the development of esophageal adenocarcinoma,120 one would expect a higher cancer incidence in the medically treated patients compared with surgery patients. Although this was not the case, it should be noted that, in practice, patients offered surgical therapy generally have more severe symptoms and potentially more esophageal exposure to carcinogenic refluxate. The possibility that these 2 patient populations (medically treated and surgically treated) may not be directly comparable underscores the need to account for study design when analyzing the literature.
Despite the lack of difference in disease progression, surgically treated patients demonstrated a higher incidence of disease regression, which was observed in 15.4% of surgically treated patients compared with 1.9% of medically treated patients. Even when only controlled studies were analyzed, the probability of developing regression was greater in surgically treated patients than in medically treated patients (6.5% vs. 0.5%, P = 0.024). Of note, the largest difference between surgical and medical therapy was demonstrated in the probability of regression from nondysplastic Barrett esophagus to normal squamous epithelium (17% vs. 0.4%). Regression from LGD to normal epithelium occurred in 4% of surgically treated patients and 0% of medically treated patients.
The dramatic difference in regression rates is difficult to reconcile in the face of similar cancer incidences among the 2 treatment groups in this systematic review. It is possible that the confounding effects of esophageal inflammation on making a diagnosis of dysplasia played a role in the tendency to “over-call” LGD in the surgically treated patients.121 “Regression” in these patients may have represented the resolution of inflammation associated with surgical therapy, rather than an actual reversal of the metaplasia-dysplasia-carcinoma sequence. Indeed, the pretreatment prevalence of LGD was greater in the surgically treated group than in the medically treated group, supporting the hypothesis that patients selected for surgery may have more severe reflux disease. Alternatively, fundoplication itself may create anatomic changes, which hinder adequate esophageal sampling during surveillance endoscopy, in effect, “hiding” dysplasia from post-treatment surveillance and thus artificially lowering dysplasia rates. Arguing against this hypothesis, however, are data from DeMeester et al who reported that complete esophageal endoscopic sampling to the level of the cardia was possible after fundoplication.100
This systematic review demonstrates that, among controlled studies, antireflux surgery in patients with Barrett esophagus does not prevent the development of esophageal adenocarcinoma appreciably more than medical therapy. The lower pooled incidence rate of esophageal cancer after antireflux surgery is predominantly driven by case series and not controlled studies, a finding that likely reflects publication bias. The estimated reduction in incidence rate of esophageal adenocarcinoma associated with antireflux surgery when compared with medical therapy is small: 1.7 cases per 1000 patient-years, making a randomized controlled trial infeasible. Even though antireflux surgery promotes regression of Barrett esophagus, it has not demonstrably reduced the incidence rate of esophageal adenocarcinoma and therefore cannot currently be recommended as an antineoplastic procedure. A registry with carefully defined endpoints to follow the outcomes of patients with Barrett esophagus would be necessary to establish the antineoplastic effect of antireflux surgery in comparison to medical therapy.
Footnotes
Supported in part by National Institutes of Health Grant Nos. K23 DK066165-01 (to B.A.J.) and 9K30RR022506-06 (to C.D.M.).
Reprints: Blair A. Jobe, MD, Portland VA Medical Center, Surgical Service–P3Surg, PO Box 1034, Portland, OR 97207. E-mail: jobeb@ohsu.edu.
REFERENCES
- 1.Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 2.Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78:1820–1828. [DOI] [PubMed] [Google Scholar]
- 3.Ide H, Nakamura T, Hayashi K, et al. Esophageal squamous cell carcinoma: pathology and prognosis. World J Surg. 1994;18:321–330. [DOI] [PubMed] [Google Scholar]
- 4.Torres C, Turner JR, Wang HH, et al. Pathologic prognostic factors in Barrett's associated adenocarcinoma: a follow-up study of 96 patients. Cancer. 1999;85:520–528. [PubMed] [Google Scholar]
- 5.Dimick JB, Wainess RM, Upchurch GR Jr, et al. National trends in outcomes for esophageal resection. Ann Thorac Surg. 2005;79:212–216; discussion 217–218. [DOI] [PubMed]
- 6.Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg. 2004;198:536–541; discussion 541–542. [DOI] [PubMed]
- 7.Dent J, Bremner CG, Collen MJ, et al. Barrett's oesophagus. J Gastroenterol Hepatol. 1991;6:1–22. [DOI] [PubMed] [Google Scholar]
- 8.Spechler SJ. Disputing dysplasia. Gastroenterology. 2001;120:1864–1868. [DOI] [PubMed] [Google Scholar]
- 9.Spechler SJ. Dysplasia in Barrett's esophagus: limitations of current management strategies. Am J Gastroenterol. 2005;100:927–935. [DOI] [PubMed] [Google Scholar]
- 10.Vela MF, Camacho-Lobato L, Srinivasan R, et al. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599–1606. [DOI] [PubMed] [Google Scholar]
- 11.Kauer WK, Peters JH, DeMeester TR, et al. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone: the need for surgical therapy re-emphasized. Ann Surg. 1995;222:525–531; discussion 531–533. [DOI] [PMC free article] [PubMed]
- 12.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett's esophagus: the Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–1032. [DOI] [PubMed] [Google Scholar]
- 13.Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol. 2002;97:1888–1895. [DOI] [PubMed] [Google Scholar]
- 14.Fass R, Sampliner RE. Barrett's oesophagus: optimal strategies for prevention and treatment. Drugs. 2003;63:555–564. [DOI] [PubMed] [Google Scholar]
- 15.Sutton AJ. Methods for Meta-Analysis in Medical Research. New York: John Wiley, 2000. [Google Scholar]
- 16.Spiegelhalter D, Thomas A, Best N. WinBUGS, version 1.2. User Manual. Cambridge: MRC Biostatistics Unit, 1999. [Google Scholar]
- 17.Rothman K, Boice J Jr. Epidemiologic Analysis With a Programmable Calculator. Boston: Epidemiology Resources, 1982. [Google Scholar]
- 18.Sampliner RE, Fass R. Partial regression of Barrett's esophagus: an inadequate endpoint. Am J Gastroenterol. 1993;88:2092–2094. [PubMed] [Google Scholar]
- 19.Chen LQ, Nastos D, Hu CY, et al. Results of the Collis-Nissen gastroplasty in patients with Barrett's esophagus. Ann Thorac Surg. 1999;68:1014–1020; discussion 1021. [DOI] [PubMed]
- 20.Ortiz A, Martinez de Haro LF, Parrilla P, et al. Conservative treatment versus antireflux surgery in Barrett's oesophagus: long-term results of a prospective study. Br J Surg. 1996;83:274–278. [PubMed] [Google Scholar]
- 21.Sharma P, Morales TG, Bhattacharyya A, et al. Dysplasia in short-segment Barrett's esophagus: a prospective 3-year follow-up. Am J Gastroenterol. 1997;92:2012–2016. [PubMed] [Google Scholar]
- 22.Weston AP, Krmpotich PT, Cherian R, et al. Prospective long-term endoscopic and histological follow-up of short segment Barrett's esophagus: comparison with traditional long segment Barrett's esophagus. Am J Gastroenterol. 1997;92:407–413. [PubMed] [Google Scholar]
- 23.Bammer T, Hinder RA, Klaus A, et al. Rationale for surgical therapy of Barrett esophagus. Mayo Clin Proc. 2001;76:335–342. [DOI] [PubMed] [Google Scholar]
- 24.Hillman LC, Chiragakis L, Shadbolt B, et al. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett's oesophagus. Med J Aust. 2004;180:387–391. [DOI] [PubMed] [Google Scholar]
- 25.Klinkenberg-Knol EC, Nelis F, Dent J, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and influence on gastric mucosa. Gastroenterology. 2000;118:661–669. [DOI] [PubMed] [Google Scholar]
- 26.Luostarinen ME, Mattila JJ, Auvinen OL, et al. Histological improvement of oesophagitis after Nissen fundoplication. Ann Med. 1998;30:547–552. [DOI] [PubMed] [Google Scholar]
- 27.Naef AP, Savary M. Conservative operations for peptic esophagitis with stenosis in columnar-lined lower esophagus. Ann Thorac Surg. 1972;13:543–551. [DOI] [PubMed] [Google Scholar]
- 28.Naef AP, Savary M, Ozzello L. Columnar-lined lower esophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett's esophagus with 12 adenocarcinomas. J Thorac Cardiovasc Surg. 1975;70:826–835. [PubMed] [Google Scholar]
- 29.Sagar PM, Ackroyd R, Hosie KB, et al. Regression and progression of Barrett's oesophagus after antireflux surgery. Br J Surg. 1995;82:806–810. [DOI] [PubMed] [Google Scholar]
- 30.Starnes VA, Adkins RB, Ballinger JF, et al. Barrett's esophagus: a surgical entity. Arch Surg. 1984;119:563–567. [DOI] [PubMed] [Google Scholar]
- 31.Collen MJ, Lewis JH, Benjamin SB. Gastric acid hypersecretion in refractory gastroesophageal reflux disease. Gastroenterology. 1990;98:654–661. [DOI] [PubMed] [Google Scholar]
- 32.Mann NS, Tsai MF, Nair PK. Barrett's esophagus in patients with symptomatic reflux esophagitis. Am J Gastroenterol. 1989;84:1494–1496. [PubMed] [Google Scholar]
- 33.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett's esophagus and the effects of acid suppression. Gastroenterology. 1999;117:327–335. [DOI] [PubMed] [Google Scholar]
- 34.Lundell L, Miettinen P, Myrvold HE, et al. Long-term management of gastro-oesophageal reflux disease with omeprazole or open antireflux surgery: results of a prospective, randomized clinical trial. The Nordic GORD Study Group. Eur J Gastroenterol Hepatol. 2000;12:879–887. [DOI] [PubMed] [Google Scholar]
- 35.Oberg S, Johansson J, Wenner J, et al. Endoscopic surveillance of columnar-lined esophagus: frequency of intestinal metaplasia detection and impact of antireflux surgery. Ann Surg. 2001;234:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patti MG, Arcerito M, Feo CV, et al. Barrett's esophagus: a surgical disease. J Gastrointest Surg. 1999;3:397–403; discussion 403–404. [DOI] [PubMed]
- 37.Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285:2331–2338. [DOI] [PubMed] [Google Scholar]
- 38.Spechler SJ. Comparison of medical and surgical therapy for complicated gastroesophageal reflux disease in veterans: the Department of Veterans Affairs Gastroesophageal Reflux Disease Study Group. N Engl J Med. 1992;326:786–792. [DOI] [PubMed] [Google Scholar]
- 39.Wetscher GJ, Gadenstaetter M, Klingler PJ, et al. Efficacy of medical therapy and antireflux surgery to prevent Barrett's metaplasia in patients with gastroesophageal reflux disease. Ann Surg. 2001;234:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonelli L. Barrett's esophagus: results of a multicentric survey. G.O.S.P.E. (Gruppo Operativo per lo Studio delle Precancerosi Esofagee. Endoscopy. 1993;25:652–654. [DOI] [PubMed] [Google Scholar]
- 41.Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett's) esophagus. N Engl J Med. 1985;313:857–859. [DOI] [PubMed] [Google Scholar]
- 42.Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett's esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–1939. [DOI] [PubMed] [Google Scholar]
- 43.Csendes A, Smok G, Quiroz J, et al. Clinical, endoscopic, and functional studies in 408 patients with Barrett's esophagus, compared to 174 cases of intestinal metaplasia of the cardia. Am J Gastroenterol. 2002;97:554–560. [DOI] [PubMed] [Google Scholar]
- 44.Dulai GS, Shekelle PG, Jensen DM, et al. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett's cohort. Am J Gastroenterol. 2005;100:775–783. [DOI] [PubMed] [Google Scholar]
- 45.Eckardt VF, Kanzler G, Bernhard G. Life expectancy and cancer risk in patients with Barrett's esophagus: a prospective controlled investigation. Am J Med. 2001;111:33–37. [DOI] [PubMed] [Google Scholar]
- 46.Hage M, Siersema PD, van Dekken H, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett's oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39:1175–1179. [DOI] [PubMed] [Google Scholar]
- 47.Horwhat JD, Baroni D, Maydonovitch C, et al. The incidence of regression and normalization of intestinal metaplasia in a cohort of patients with EGJSIM, SSBE, and LSBE followed over 3 years. Gastroenterology. 2000;A224. [Google Scholar]
- 48.Iftikhar SY, James PD, Steele RJ, et al. Length of Barrett's oesophagus: an important factor in the development of dysplasia and adenocarcinoma. Gut. 1992;33:1155–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz D, Rothstein R, Schned A, et al. The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett's esophagus. Am J Gastroenterol. 1998;93:536–541. [DOI] [PubMed] [Google Scholar]
- 50.Klinkenberg-Knol EC, Festen HP, Jansen JB, et al. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med. 1994;121:161–167. [DOI] [PubMed] [Google Scholar]
- 51.Macdonald CE, Wicks AC, Playford RJ. Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ. 2000;321:1252–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macdonald CE, Wicks AC, Playford RJ. Ten years' experience of screening patients with Barrett's oesophagus in a university teaching hospital. Gut. 1997;41:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miros M, Kerlin P, Walker N. Only patients with dysplasia progress to adenocarcinoma in Barrett's oesophagus. Gut. 1991;32:1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ovaska J, Miettinen M, Kivilaakso E. Adenocarcinoma arising in Barrett's esophagus. Dig Dis Sci. 1989;34:1336–1339. [DOI] [PubMed] [Google Scholar]
- 55.Robertson CS, Mayberry JF, Nicholson DA, et al. Value of endoscopic surveillance in the detection of neoplastic change in Barrett's oesophagus. Br J Surg. 1988;75:760–763. [DOI] [PubMed] [Google Scholar]
- 56.Spechler SJ, Robbins AH, Rubins HB, et al. Adenocarcinoma and Barrett's esophagus: an overrated risk? Gastroenterology. 1984;87:927–933. [PubMed] [Google Scholar]
- 57.van Blankenstein M, Bohmer CJ, Hop WC. The incidence of adenocarcinoma in Barrett's esophagus in an institutionalized population. Eur J Gastroenterol Hepatol. 2004;16:903–909. [DOI] [PubMed] [Google Scholar]
- 58.Braghetto I, Csendes A, Smok G, et al. Histological inflammatory changes after surgery at the epithelium of the distal esophagus in patients with Barrett's esophagus: a comparison of two surgical procedures. Dis Esophagus. 2004;17:235–242. [DOI] [PubMed] [Google Scholar]
- 59.Malesci A, Savarino V, Zentilin P, et al. Partial regression of Barrett's esophagus by long-term therapy with high-dose omeprazole. Gastrointest Endosc. 1996;44:700–705. [DOI] [PubMed] [Google Scholar]
- 60.Peters FT, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett's oesophagus during omeprazole treatment: a randomised double blind study. Gut. 1999;45:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ransom JM, Patel GK, Clift SA, et al. Extended and limited types of Barrett's esophagus in the adult. Ann Thorac Surg. 1982;33:19–27. [DOI] [PubMed] [Google Scholar]
- 62.Sampliner RE. Effect of up to 3 years of high-dose lansoprazole on Barrett's esophagus. Am J Gastroenterol. 1994;89:1844–1848. [PubMed] [Google Scholar]
- 63.Sampliner RE, Garewal HS, Fennerty MB, et al. Lack of impact of therapy on extent of Barrett's esophagus in 67 patients. Dig Dis Sci. 1990;35:93–96. [DOI] [PubMed] [Google Scholar]
- 64.Sharma P, Sampliner RE, Camargo E. Normalization of esophageal pH with high-dose proton pump inhibitor therapy does not result in regression of Barrett's esophagus. Am J Gastroenterol. 1997;92:582–585. [PubMed] [Google Scholar]
- 65.Weston AP, Badr AS, Hassanein RS. Prospective multivariate analysis of factors predictive of complete regression of Barrett's esophagus. Am J Gastroenterol. 1999;94:3420–3426. [DOI] [PubMed] [Google Scholar]
- 66.Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett's esophagus? A meta-analysis. Am J Gastroenterol. 2003;98:2390–2394. [DOI] [PubMed] [Google Scholar]
- 67.Csendes A. Surgical treatment of Barrett's esophagus: 1980–2003. World J Surg. 2004;28:225–231. [DOI] [PubMed] [Google Scholar]
- 68.Csendes A, Braghetto I, Burdiles P, et al. A new physiologic approach for the surgical treatment of patients with Barrett's esophagus: technical considerations and results in 65 patients. Ann Surg. 1997;226:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fitzgerald RC. Barrett metaplasia: reassessment of treatment and follow-up. Curr Opin Oncol. 2004;16:372–377. [DOI] [PubMed] [Google Scholar]
- 70.Gutschow CA, Schroder W, Prenzel K, et al. Impact of antireflux surgery on Barrett's esophagus. Langenbecks Arch Surg. 2002;387:138–145. [DOI] [PubMed] [Google Scholar]
- 71.Harle IA, Finley RJ, Belsheim M, et al. Management of adenocarcinoma in a columnar-lined esophagus. Ann Thorac Surg. 1985;40:330–336. [DOI] [PubMed] [Google Scholar]
- 72.Peters FT, Ganesh S, Kuipers EJ, et al. Effect of elimination of acid reflux on epithelial cell proliferative activity of Barrett esophagus. Scand J Gastroenterol. 2000;35:1238–1244. [DOI] [PubMed] [Google Scholar]
- 73.Radigan LR, Glover JL, Shipley FE, Shoemaker RE. Barrett esophagus. Arch Surg. 1977;112:486–491. [DOI] [PubMed] [Google Scholar]
- 74.Shaheen NJ. Does fundoplication change the risk of esophageal cancer in the setting of GERD? Am J Gastroenterol. 2005;100:1009–1011. [DOI] [PubMed] [Google Scholar]
- 75.McDonald ML, Trastek VF, Allen MS, et al. Barrett's esophagus: does an antireflux procedure reduce the need for endoscopic surveillance? J Thorac Cardiovasc Surg. 1996;111:1135–1138; discussion 1139–1140. [DOI] [PubMed]
- 76.Burnett HF, Read RC, Morris WD, et al. Management of complications of fundoplication and Barrett's esophagus. Surgery. 1977;82:521–530. [PubMed] [Google Scholar]
- 77.Cooper BT, Barbezat GO. Barrett's oesophagus: a clinical study of 52 patients. Q J Med. 1987;62:97–108. [PubMed] [Google Scholar]
- 78.Csendes A, Burdiles P, Braghetto I, et al. Dysplasia and adenocarcinoma after classic antireflux surgery in patients with Barrett's esophagus: the need for long-term subjective and objective follow-up. Ann Surg. 2002;235:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeMeester TR. Antireflux surgery in the management of Barrett's esophagus. J Gastrointest Surg. 2000;4:124–128. [DOI] [PubMed] [Google Scholar]
- 80.Deviere J, Buset M, Dumonceau JM, et al. Regression of Barrett's epithelium with omeprazole. N Engl J Med. 1989;320:1497–1498. [DOI] [PubMed] [Google Scholar]
- 81.El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett's esophagus. Am J Gastroenterol. 2004;99:1877–1883. [DOI] [PubMed] [Google Scholar]
- 82.Farrell TM, Smith CD, Metreveli RE, et al. Fundoplication provides effective and durable symptom relief in patients with Barrett's esophagus. Am J Surg. 1999;178:18–21. [DOI] [PubMed] [Google Scholar]
- 83.Iascone C, DeMeester TR, Little AG, et al. Barrett's esophagus: functional assessment, proposed pathogenesis, and surgical therapy. Arch Surg. 1983;118:543–549. [DOI] [PubMed] [Google Scholar]
- 84.O'Riordan JM, Byrne PJ, Ravi N, et al. Long-term clinical and pathologic response of Barrett's esophagus after antireflux surgery. Am J Surg. 2004;188:27–33. [DOI] [PubMed] [Google Scholar]
- 85.Skinner DB, Walther BC, Riddell RH, et al. Barrett's esophagus: comparison of benign and malignant cases. Ann Surg. 1983;198:554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weston AP, Sharma P, Topalovski M, et al. Long-term follow-up of Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:1888–1893. [DOI] [PubMed] [Google Scholar]
- 87.Yau P, Watson DI, Devitt PG, et al. Laparoscopic antireflux surgery in the treatment of gastroesophageal reflux in patients with Barrett esophagus. Arch Surg. 2000;135:801–805. [DOI] [PubMed] [Google Scholar]
- 88.Ye W, Chow WH, Lagergren J, et al. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology. 2001;121:1286–1293. [DOI] [PubMed] [Google Scholar]
- 89.Oberg S, Wenner J, Johansson J, et al. Barrett esophagus: risk factors for progression to dysplasia and adenocarcinoma. Ann Surg. 2005;242:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Csendes A, Braghetto I, Burdiles P, et al. Long-term results of classic antireflux surgery in 152 patients with Barrett's esophagus: clinical, radiologic, endoscopic, manometric, and acid reflux test analysis before and late after operation. Surgery. 1998;123:645–657. [PubMed] [Google Scholar]
- 91.Sharma P, Falk GW, Weston AP, et al. Natural history of low grade dysplasia: an infrequent finding which usually regresses: preliminary results from the Barrett's esophagus study. Gastroenterology. 2002;A20. [Google Scholar]
- 92.Csendes A, Burdiles P, Korn O, et al. Late results of a randomized clinical trial comparing total fundoplication versus calibration of the cardia with posterior gastropexy. Br J Surg. 2000;87:289–297. [DOI] [PubMed] [Google Scholar]
- 93.Salo JA, Salminen JT, Kiviluoto TA, et al. Treatment of Barrett's esophagus by endoscopic laser ablation and antireflux surgery. Ann Surg. 1998;227:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abbas AE, Deschamps C, Cassivi SD, et al. Barrett's esophagus: the role of laparoscopic fundoplication. Ann Thorac Surg. 2004;77:393–396. [DOI] [PubMed] [Google Scholar]
- 95.Attwood SE, Barlow AP, Norris TL, et al. Barrett's oesophagus: effect of antireflux surgery on symptom control and development of complications. Br J Surg. 1992;79:1050–1053. [DOI] [PubMed] [Google Scholar]
- 96.Bowers SP, Mattar SG, Smith CD, et al. Clinical and histologic follow-up after antireflux surgery for Barrett's esophagus. J Gastrointest Surg. 2002;6:532–538; discussion 539. [DOI] [PubMed]
- 97.Brand DL, Ylvisaker JT, Gelfand M, et al. Regression of columnar esophageal (Barrett's) epithelium after anti-reflux surgery. N Engl J Med. 1980;302:844–848. [DOI] [PubMed] [Google Scholar]
- 98.Chen LQ, Hu CY, Gaboury L, et al. Proliferative activity in Barrett's esophagus before and after antireflux surgery. Ann Surg. 2001;234:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cooper BT, Neumann CS, Cox MA, et al. Continuous treatment with omeprazole 20 mg daily for up to 6 years in Barrett's oesophagus. Aliment Pharmacol Ther. 1998;12:893–897. [DOI] [PubMed] [Google Scholar]
- 100.DeMeester SR, Campos GM, DeMeester TR, et al. The impact of an antireflux procedure on intestinal metaplasia of the cardia. Ann Surg. 1998;228:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DeMeester TR, Attwood SE, Smyrk TC, et al. Surgical therapy in Barrett's esophagus. Ann Surg. 1990;212:528–540; discussion 540–542. [DOI] [PMC free article] [PubMed]
- 102.Desai KM, Soper NJ, Frisella MM, et al. Efficacy of laparoscopic antireflux surgery in patients with Barrett's esophagus. Am J Surg. 2003;186:652–659. [DOI] [PubMed] [Google Scholar]
- 103.Gurski RR, Peters JH, Hagen JA, et al. Barrett's esophagus can and does regress after antireflux surgery: a study of prevalence and predictive features. J Am Coll Surg. 2003;196:706–712; discussion 712–713. [DOI] [PubMed]
- 104.Hameeteman W, Tytgat GN, Houthoff HJ, et al. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. [DOI] [PubMed] [Google Scholar]
- 105.Hofstetter WL, Peters JH, DeMeester TR, et al. Long-term outcome of antireflux surgery in patients with Barrett's esophagus. Ann Surg. 2001;234:532–538; discussion 538–539. [DOI] [PMC free article] [PubMed]
- 106.Low DE, Levine DS, Dail DH, et al. Histological and anatomic changes in Barrett's esophagus after antireflux surgery. Am J Gastroenterol. 1999;94:80–85. [DOI] [PubMed] [Google Scholar]
- 107.Mabrut JY, Baulieux J, Adham M, et al. Impact of antireflux operation on columnar-lined esophagus. J Am Coll Surg. 2003;196:60–67. [DOI] [PubMed] [Google Scholar]
- 108.McCallum RW, Polepalle S, Davenport K, et al. Role of anti-reflux surgery against dysplasia in Barrett's esophagus. Gastroenterology. 1991;100:A121. [Google Scholar]
- 109.O'Connor JB, Falk GW, Richter JE. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. [DOI] [PubMed] [Google Scholar]
- 110.Oelschlager BK, Barreca M, Chang L, et al. Clinical and pathologic response of Barrett's esophagus to laparoscopic antireflux surgery. Ann Surg. 2003;238:458–464; discussion 464–466. [DOI] [PMC free article] [PubMed]
- 111.Parrilla P, Martinez de Haro LF, Ortiz A, et al. Long-term results of a randomized prospective study comparing medical and surgical treatment of Barrett's esophagus. Ann Surg. 2003;237:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharma P, Weston AP, Morales T, et al. Relative risk of dysplasia for patients with intestinal metaplasia in the distal oesophagus and in the gastric cardia. Gut. 2000;46:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Srinivasan R, Katz PO, Ramakrishnan A, et al. Maximal acid reflux control for Barrett's oesophagus: feasible and effective. Aliment Pharmacol Ther. 2001;15:519–524. [DOI] [PubMed] [Google Scholar]
- 114.Weston AP, Badr AS, Hassanein RS. Prospective multivariate analysis of clinical, endoscopic, and histological factors predictive of the development of Barrett's multifocal high-grade dysplasia or adenocarcinoma. Am J Gastroenterol. 1999;94:3413–3419. [DOI] [PubMed] [Google Scholar]
- 115.Wilkinson SP, Biddlestone L, Gore S, et al. Regression of columnar-lined (Barrett's) oesophagus with omeprazole 40 mg daily: results of 5 years of continuous therapy. Aliment Pharmacol Ther. 1999;13:1205–1209. [DOI] [PubMed] [Google Scholar]
- 116.Williamson WA, Ellis FH Jr, Gibb SP, et al. Effect of antireflux operation on Barrett's mucosa. Ann Thorac Surg. 1990;49:537–541; discussion 541–542. [DOI] [PubMed]
- 117.Hagen JA, Peters JH. Minimally invasive approaches to antireflux surgery. Semin Thorac Cardiovasc Surg. 2000;12:157–172. [DOI] [PubMed] [Google Scholar]
- 118.Jones SB, Jones DB. Surgical aspects and future developments of laparoscopy. Anesthesiol Clin North Am. 2001;19:107–124. [DOI] [PubMed] [Google Scholar]
- 119.Martin RC 2nd, Kehdy FJ, Allen JW. Formal training in advanced surgical technologies enhances the surgical residency. Am J Surg. 2005;190:244–248. [DOI] [PubMed] [Google Scholar]
- 120.Gopal DV, Lieberman DA, Magaret N, et al. Risk factors for dysplasia in patients with Barrett's esophagus (BE): results from a multicenter consortium. Dig Dis Sci. 2003;48:1537–1541. [DOI] [PubMed] [Google Scholar]
- 121.Goldblum JR, Lauwers GY. Dysplasia arising in Barrett's esophagus: diagnostic pitfalls and natural history. Semin Diagn Pathol. 2002;19:12–19. [PubMed] [Google Scholar]