Abstract
Little is known about the behavior of the ovarian surface epithelium (OSE), which plays a central role in ovarian cancer etiology. It has been suggested that incessant ovulation causes OSE changes leading to transformation and that high gonadotropin levels during postmenopause activate OSE receptors, inducing proliferation. We examined the chronology of OSE changes, including tumor appearance, in a mouse model where ovulation never occurs due to deletion of follitropin receptor. Changes in epithelial cells were marked by pan-cytokeratin (CK) staining. Histologic changes and CK staining in the OSE increased from postnatal day 2. CK staining was observed inside the ovary by 24 days and increased thereafter in tumor-bearing animals. Ovaries from a third of aged (1 year) mutant mice showed CK deep inside, indicating cell migration. These tumors resembled serous papillary adenoma of human ovaries. Weak expression of GATA-4 and elevation of PCNA, cyclooxygenase-1, cyclooxygenase-2, and platelet-derived growth factor receptors α and β in mutants indicated differences in cell proliferation, differentiation, and inflammation. Thus, we report that OSE changes occur long before epithelial tumors appear in FORKO mice. Our results suggest that neither incessant ovulation nor follicle-stimulating hormone receptor presence in the OSE is required for inducing ovarian tumors; thus, other mechanisms must contribute to ovarian tumorigenesis.
Keywords: Chronology, cytokeratin, FORKO mouse, ovarian tumor, surface epithelium
Introduction
The cell type of ovarian cancer is age-dependent. Aggressive hormone-secreting granulosa cell tumors are more frequent in young women [1], but epithelial ovarian neoplasia predominates in older and menopausal women [2]. The ovarian surface epithelium (OSE) is a modified pelvic mesothelium that covers the ovary [3]. OSE cells make up a single layer, varying in type from simple squamous to cuboidal to low pseudostratified columnar epithelial cells, which participate actively in the mechanism of gonadotropin-induced ovulatory follicular rupture [4,5]. Although the majority of ovarian cancers [epithelial ovarian cancer (EOC)] in aging women are thought to arise from the OSE [4,6,7], there are some arguments as to their origin [7,8]. EOC is classified into four main subtypes: serous, mucinous, endometrioid, and clear cell, based mainly on histologic differences. EOC is the fourth most common cause of cancer death among women and has the highest mortality rate among gynecologic cancers [9]. Despite improved knowledge of etiology, aggressive cytoreductive surgery, and modern combination chemotherapy, the 5-year survival rate is < 40% [9]. Lack of adequate diagnostic screening test for early disease detection and rapid progression to chemoresistance have been major stumbling blocks in securing appreciable improvements. Experimental models for human diseases are critical, not only to understand the biologic and genetic factors that influence the disease process but also to develop strategies for treatment. In particular, experimental models of ovarian tumor development, which mimic perimenopausal and postmenopausal states in women, could enhance efforts to understand molecular changes that occur during development and progression to ovarian carcinoma.
Recent literature reveals that the proliferation and migration of the OSE are regulated by hormones, growth factors, and cytokines. Gonadotropins, including follicle-stimulating hormone (FSH) and luteinizing hormone (LH), have been implicated in OSE proliferation, migration, and protection from apoptosis in humans, mice, rats, and cows in vivo and in vitro [10–14]. Steroid hormones such as estrogen, progesterone, and androgen also modulate the OSE [3,4]. Besides these, other regulators of the OSE include epidermal growth factor (EGF) [15] and platelet-derived growth factor (PDGF) [16].
We have previously reported that in our follitropin receptor knockout (FORKO) mouse model, pituitary gonadotropins (FSH and LH) and ovarian androgen levels are significantly increased, whereas estrogen levels remain very low [17]—an endocrine profile that is similar, in many respects, to postmenopausal conditions and other hormone-related disorders in women; furthermore, by 12 months, > 90% of FORKO mice developed various kinds of ovarian pathology, including neoplasms of sex cord-stromal type, as well as cysts [17]. In addition, our recent findings indicate that FORKO mice have a thicker OSE at an early age [18] and increase the expression of tight junction proteins exclusively in these cells [19]. The presence of platelet-derived growth factor receptors (PDGFRs) and hormonal regulation in the OSE, as well as different expression patterns between wildtype (WT) and FORKO [20] mice, prompted us to examine the postnatal chronology of the OSE in mutants. We hypothesized that the OSE of FORKO ovaries undergoes early and progressive changes culminating in tumors. In testing this hypothesis, our objectives were: 1) to determine how the OSE changes during development in an aberrant hormonal environment; and 2) to determine the presence of ovarian epithelial tumor in aged FORKO mice. Herein we report that ovarian epithelial tumors also occur in FORKO mice that are sterile and never ovulate. Thus, our results challenge the absolute requirement of incessant ovulation for precipitating EOC and suggest that other conditions also significantly contribute to the disease process.
Materials and Methods
Animals
The studies described in this report were performed according to accepted and approved guidelines of the institutional animal care and ethics committee. FORKO mice were established as previously described [17]. Animals were housed under controlled temperature and constant light (12 hours of light, 12 hours of darkness), with food and water provided ad libitum. The female mice used in this experiment were derived by breeding F2 generation heterozygotes of sv129 background. They were genotyped by polymerase chain reaction according to methods we have described recently [21]. Age-matched mutants and WT mice were compared in each experiment.
Histologic Analysis
Animals were sacrificed by CO2 inhalation, and all internal organs were examined for visual signs of abnormalities. The ovaries were cleaned of extraneous tissues and then fixed in 10% formalin at room temperature for 16 to 20 hours. All tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), according to standard histologic procedures that we have used in previous studies [21]. The classification of ovarian pathology, including tumor type, was performed according to the descriptions provided in the atlas on basic histopathology [22] and pathology of the female genital tract, with special reference to the mouse [23,24].
Confocal Microscopy Immunofluorescence Study of Cytokeratin
Immunofluorescence was performed on paraffin-embedded sections. Briefly, formaldehyde-fixed ovaries were embedded in paraffin and sectioned at 5 mm. Following deparaffinization, tissue sections were microwaved in citric acid solution (0.01M citric acid solution containing 0.01 M sodium citrate, pH 6.0) to unmask antigenic sites. Sections were treated with 3% hydrogen peroxide for 5 minutes to block endogenous peroxidase. After blocking in 5% nonimmune serum for 1 hour at room temperature, sections were incubated overnight at 4°C with pan-cytokeratin (CK) antibody (1:150; Sigma, St. Louis, MO). Fluorescein-conjugated goat anti-mouse secondary antibody was used at a dilution of 1:200. Images were captured following confocal microscopy.
Immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded sections using the Dako Cytomation Liquid DAB Substrate Chromogen Staining System (Dako, Carpinteria, CA). Antigen retrieval procedure was performed for localization of PCNA, PDGFR-α, PDGFR-β, inhibin-a, GATA-4, cyclooxygenase-1 (COX-1), and cyclooxygenase-2 (COX-2). Sections were treated with 3% hydrogen peroxide for 5 minutes to block endogenous peroxidase. Rabbit antiserum to the N-terminal peptide of inhibin-α subunit (from Dr. B. D. Schanbacher, formerly of the USDA, Clay Center, NE) was used at a dilution of 1:1000. As this reacts with the free α-subunit and inhibin dimer, we assume that all forms of inhibin are revealed. 3β-Hydroxysteroid dehydrogenase (3β-HSD) antibody was provided by Dr. A. H. Payne (Stanford University, Palo Alto, CA). This rabbit antiserum produced against the recombinant mouse 3β-HSD1 protein was used at a dilution of 1:1000. Antibodies to COX-1 (1:500) and COX-2 (1:750) were a gift of Dr. S Kargman (Merck Frosst, Kirkland, QC). For PCNA, after antigen retrieval and quenching of endogenous peroxidase, sections were treated with 0.3% Triton X-100 for 10 minutes and incubated overnight at 4°C with monoclonal anti-mouse PCNA (1:300). Goat antibodies to the transcription factor GATA-4 were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and used at 1:400 dilution. PDGFR-α and PDGFR-β antibodies (kindly provided by Dr. Carl-Henrik Heldin, Ludwig Institute for Cancer Research, Uppsala, Sweden) were diluted in a blocking serum solution at 1:400 and 1:300, respectively. In negative controls, normal serum was substituted for primary antibody in the first reaction. The corresponding rabbit secondary antibody (1:200) was used for subsequent processing. Signals were amplified with avidin-biotinylated horseradish peroxidase developed with diaminobenzidine, counterstained with hematoxylin, and dehydrated again. Sections were analyzed under a light microscope.
Results
Chronology of the Alteration of OSE Cells as Early as 2 Days after Birth
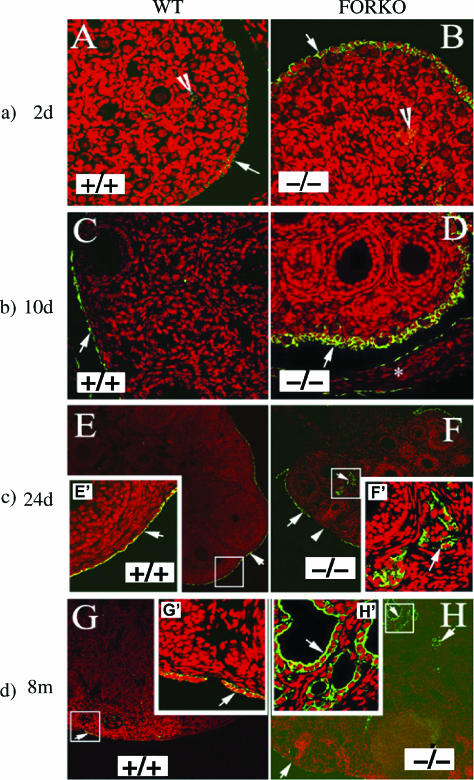
As most FORKO mice acquire ovarian tumors by 12 to 15 months [17], we performed immunofluorescence analysis using the cytokeratin antibody of ovaries as early as 2 days to 8 months to determine the progressive alteration of the OSE in FORKO mice. As cytokeratin expression is an acceptable marker for the identification of epithelial cells, we used a pan-cytokeratin antibody to reveal the OSE [25]. The OSE in FORKO ovaries is thicker than that in WT mice. In 2-and 10-day ovaries, CK expression was evident only in the OSE of both WT and FORKO, but the expression of CK in FORKO ovaries was higher (Figure 1). As early as 24 days, epithelial cells were found to migrate inside some FORKO ovaries. In contrast, none of the WT 24-day ovaries was stained by CK inside the ovary. By 8 months of age, more epithelial cells had migrated inside the FORKO ovaries and cysts had also been found (data not shown). These results confirm that: 1) abrogating follicle-stimulating hormone receptor (FSH-R) signaling affects ovarian development as early as 2 days, including effects on the OSE [18]; and 2) hormonal imbalances in young FORKO mice might induce the migration and proliferation of the OSE at an early stage.
Figure 1.
Epithelial cell immunofluorescence staining (arrows) with CK antibody in ovaries at different ages. Green (fluorescein isothiocyanate) indicates a positive signal, and red shows DAPI staining in the nucleus. (a) Immunofluorescence staining of the 2-day-old ovaries of WT and FORKO mice. Note that CK immunofluorescence staining is just located in the OSE and that positive signal is much higher in FORKO OSE than in WT. Original magnification, x40. (b) Immunostaining of CK in the 10-day-old ovaries of WT and FORKO mice. Note that there is also no positive staining in WT ovary except the OSE and that positive staining in FORKO ovary is higher than that in WT ovary (arrow). Star represents ovarian bursa. Original magnification, x40. (c) Immunofluorescence staining of the 24-day-old ovaries of WT and FORKO mice. Note that CK immunofluorescence staining is located in WT OSE and that positive signal is higher in FORKO OSE; furthermore, there is a positive signal (green) appearing within the FORKO ovary. Inserts (E′ and F′; original magnification, x40) are an enlargement of the white boxed area in each (original magnification, x10). (d) Immunofluorescence staining result of the 8-month-old ovaries of WT and FORKO mice. Note that there is no positive staining in WT ovary except the OSE, whereas positive staining appears in some parts within the FORKO ovary. Inserts (G′ and H′; original magnification, x40) are an enlargement of the white boxed area in each (original magnification, x10).
CK Expression Inside the FORKO Ovary and Aging Effects
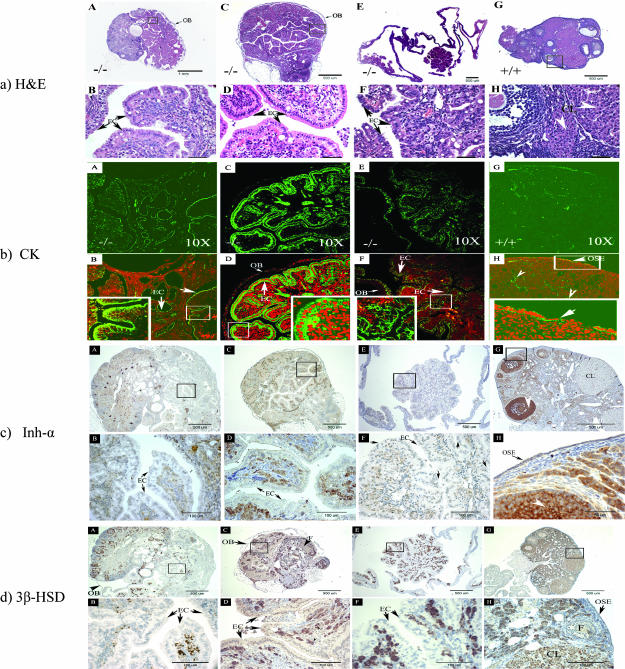
To investigate whether alteration of the OSE and migration of the OSE inside the ovary could be connected to the induction of late EOC tumors, we also assessed CK expression and histopathology in FORKO and WT ovaries at 12 to 15 months of age (n = 84 for FORKO; n = 23 for WT). In this study, we did not check the differences of tumor incidence between the right and left ovaries of FORKO and WT mice, as we pooled ovaries from respective groups to get a general idea of OSE pathology. Typical examples are shown in Figure 2, and the extent of OSE penetration we found in the ovarian interior is summarized in Table 1. Pathological changes attributable to the OSE (Figure 2a) were consistent with immunofluorescence staining for cytokeratin. In such a comparison, nearly 30% of aged FORKO ovaries showed staining deep inside (Figure 2b). On the contrary, < 10% of WT age-matched ovaries were found to have cysts, which were lined with only one layer of epithelial cells.
Figure 2.
Ovarian histopathology and immunostaining characteristics of the appearance of ovarian serous papillary cystadenoma in 12- to 15-month-old FORKO females. Note that epithelial-lined structures in FORKO ovaries mimic human serous ovarian adenocarcinomas. OB = ovarian bursa; EC = epithelial cell. (a) H&E ovarian histopathology of the appearance of FORKO (A–F) and WT mice (G–H) in 12- to 15-month-old females. (A–F) Ovaries from FORKO females with ovarian serous papillary cystadenoma. (B, D, and F) Higher magnifications of (A), (C), and (E), respectively. (A) Representative ovary from null mutant showing serous tumor. Note that the remnant of the ovary is small, with few identifiable follicular structures compared with the large tumor. (C) The common feature of serous tumors is the presence of a tall, columnar, ciliated epithelial cell lining and clear serous fluid filling the cystic space. (E) Another kind of serous tumor full of clear fluid and no identifiable follicle structure. (G and H; higher magnification of G) WT ovary from a 13-month-old female mouse containing antral follicles and corpora lutea. (b) Immunofluorescence staining (arrows) of CK in serial sections from 12-month-old FORKO serous tumor (A–F) and WT ovary (G–H). Note the strong CK staining in FORKO serous tumors, in contrast to absent specific positive signal in WT ovary, except in OSE cells. Bottom panels (B, D, and F) are the overlay of the (green) color of immunofluorescence signal and the (red) color of PI for the nucleus. The insets in each are the enlargement of the white boxed area. Arrowhead shows unspecific staining. Original magnification, x10. (c) Immunostaining (arrows) of inhibin-α in serial sections from 12 (plus)-month-old FORKO serous tumors (A–F) and WT ovary (G–H). Note that there is absent staining or very weak staining of inhibin-α in cells expressing CK, indicating their epithelial nature. Bottom panels (B, D, and F) are the enlargement of the black boxed area (A, C, and E). (d) Immunostaining (arrows) of 3β-HSD in sections from 12 (plus)-month-old FORKO serous tumors (A–F) and WT ovary (G–H). Note absent staining or very weak staining of 3β-HSD in cells expressing CK. Bottom panels (B, D, and F) are the enlargement of the black boxed area (A, C, and E).
Table 1.
The Extent of OSE Penetration into the Ovarian Interior as Revealed by Cytokeratins.
| WT Ovaries |
FORKO Ovaries |
|||
| OSE | Inside | OSE | Inside | |
| 2 days | + (15) | - (15) | ++ (9) | - (19) |
| + (10) | ||||
| 10 days | + (16) | - (16) | ++ (6) | - (18) |
| + (12) | ||||
| 24 days | + (12) | - (12) | + (12) | + 8.3% (12) |
| 8 months | + (12) | - (12) | + (12) | + 16.7% (12) |
| 12 months | + (23) | + 8.7% (23) | + (84) | + 27.4% (84) |
(-) No signal; (+) strong signal; (++) very strong signal.
The number of ovaries examined is indicated inside the parentheses.
FORKO Ovarian Tumor Pathology
Majority of the CK-positive FORKO ovaries were identified as having ovarian serous papillary cystadenoma or cystadenocarcinoma, an observation that is also consistent with the findings of another group studying similar mutants [26]. Figure 2a shows typical examples of ovarian serous papillary cystadenocarcinoma by H&E. They are composed of fronds and branching papillary projections of cuboidal to columnar cilia epithelia with occasional vacuoles and eosinophilic cytoplasm resembling human ovarian serous papillary cystadenocarcinoma. Figure 3 further reveals that there are two more kinds of tumors in these groups of FORKO mutants: serous papillary cystadenoma and granulosa cell tumor (arrowhead), except ovarian Sertoli-Leydig cell tumor, as previously reported [17].
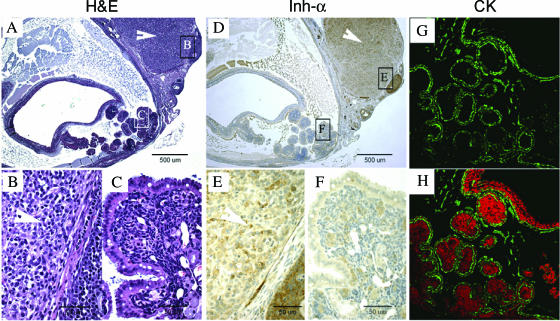
Figure 3.
Example of the histopathology and immunostaining characteristics of a serous tumor and an ovarian granulosa cell tumor in one FORKO ovary. (A) H&E results. (D) Inhibin-α immunostaining. (G) CK immunofluorescence. (B and C; E and F) Enlargement of the boxed area of (A) and (D), respectively. (H) The overlay of CK-positive color and PI for the nucleus (green = positive signal; red = nucleus). (A) A representative FORKO aged ovary with two clear parts: granulosa cell tumor (B) and cyst papillary tumor (C). (D) Inhibin-α immunostaining of the FORKO ovary. Note that serous papillary tumor stained very weakly (F), in contrast to the strong staining of granulosa cell tumor (GCT; E). No CK immunofluorescence positive signal was detected on the part of the GCT, whereas strong CK staining was found on the part of serous papillary tumor.
Characterization of Tumor Properties
To further confirm the types of ovarian tumors, additional cell markers for epithelial cell, granulosa cell, and theca cell tumors were selected and used in serial sections. First, we selected a CK antibody that recognizes cytokeratins 1, 5, 6, and 8. It is a broad-spectrum antibody that reacts specifically with a variety of normal, reactive, and neoplastic epithelial tissues. The antibody reacts with simple cornifying and noncornifying squamous epithelia and pseudostratified epithelia and is not reactive in granulosa cell tumors [27]. Thus, we could distinguish OSE-derived tumors from granulosa cell tumors that also occur in our mutants. Cytokeratin staining was confined to normal OSE in WT mice (Figure 2b), as previously reported by others [25] and in all ages. In mutant ovaries attributed an OSE-type pathology, strong CK staining was detected in tumor cells inside the FORKO ovary (Figure 2b).
Interestingly, absent or weak inhibin-α (Figure 2c) staining was detectable in neoplastic cells that were stained by CK in mutant ovaries, in contrast to the staining of granulosa cells in the remaining follicles of the same ovaries. Inhibin-α subunit is expressed mainly in granulosa cells, but is also detected in normal WT OSE and weakly in corpus luteum (CL) (Figure 2c). Expression of the enzyme 3β-HSD is characteristic of steroidogenic cells that produce androgen in ovaries. In WT ovary, 3b-HSD (Figure 2d) was confined to stromal and theca cells, CL, and the OSE. In FORKO tumors, 3b-HSD staining was not detected in cells stained by CK (Figure 2d), whereas there were some cells that strongly stained inside papillary structures. The nature of 3b-HSD expression in these tumors is different from that in Sertoli-Leydig tumors, as reported previously [17]. Expression of the marker of epithelial cell differentiation and the absence of typical markers of granulosa cell (α-inhibin) and Sertoli-Leydig tumors (3α-HSD) strongly indicate their surface epithelial origin [28], as opposed to a granulosa or a sex cord-stromal cell origin.
mmunohistochemical Analysis of PCNA in Tumor
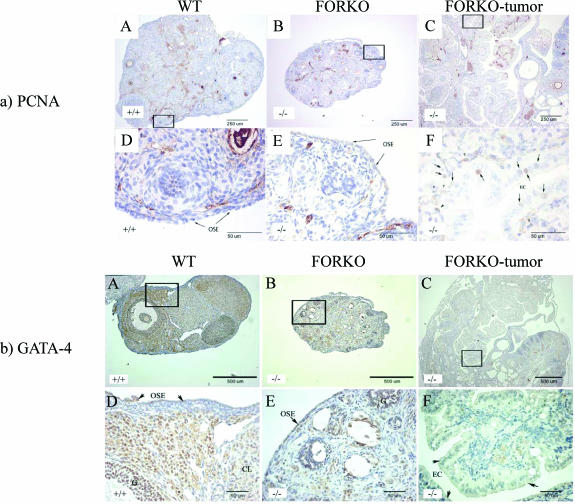
Cell proliferation in tissue sections was evaluated by PCNA. Nuclear staining for PCNA was detected in three kinds of ovaries, including WT, FORKO, and FORKO tumor ovaries. Figure 4a shows representative images of PCNA staining. Visually, the number of cells positive for PCNA in the epithelial cells of FORKO tumor (Figure 4a) was high. A small number of OSE cells positive for PCNA were seen in aged WTand nontumor FORKO ovaries. These data indicate that epithelial cells in FORKO tumor continue to undergo active proliferation, whereas the OSE of aged WT and non-tumor FORKO ovaries remains almost quiescent (Table 2).
Figure 4.
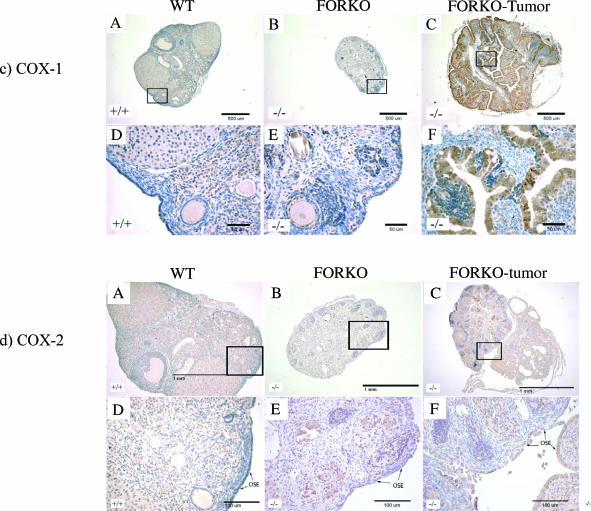
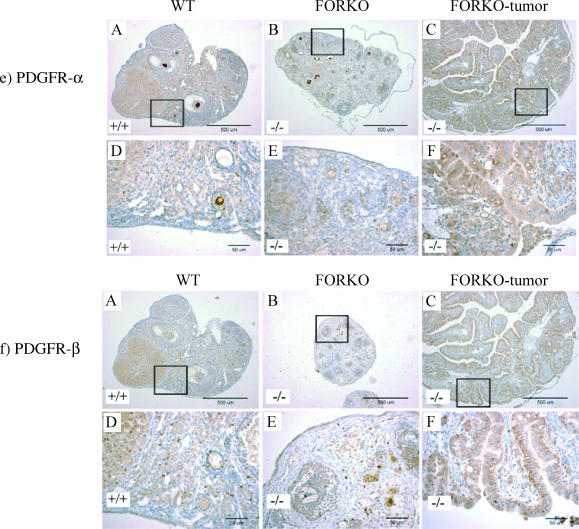
Expression of PCNA (a), GATA (b), COX-1 (c), COX-2 (d), PDGFR-α (e), and PDGFR-β (f) in WT (+/+), FORKO (-/-), and FORKO tumor ovaries. (A, B, and C) WT, unaffected FORKO, and FORKO tumor ovaries, respectively. (D, E, and F) Higher magnifications of (A), (B), and (C), respectively. (a) Expression of PCNA in WT (+/+), FORKO (-/-), and FORKO tumor ovaries. Note that no nucleus of the OSE from WT and unaffected FORKO ovaries was stained by PCNA antibody, except for immunostaining shown in granulosa cells and some stromal cells, whereas immunostaining was present in the nucleus of some epithelial cells (arrows) in FORKO tumor ovaries. Arrowheads pointed to nonepithelial cells. (b) Expression of GATA-4 in WT (+/+), FORKO (-/-), and FORKO tumor ovaries. (A) Strong GATA-4 staining in WT ovary was confined to the OSE, granulosa cell compartment, and some stromal and thecal cells surrounding the follicles. (B) In unaffected FORKO ovaries, weak GATA-4 staining was confined to the OSE, granulosa cell compartment, and some stromal and thecal cells surrounding the follicles. (C) In FORKO ovaries with serous tumor, no GATA-4- immunopositive epithelial cells are seen in the ovary. Note that cells inside the papillary structure (arrows) indicate immunostaining. For clarity, we have not labeled all positive and negative cells. (c) Expression of COX-1 in WT (+/+), FORKO (-/-), and FORKO tumor ovaries. (A) In WT ovaries, COX-1 expression is observed in stromal cells and granulosa cells of some follicles and in CL, with weak immunopositive cells observed in the OSE. (B) In the unaffected FORKO ovary, weak COX-1-immunopositive cells are observed in some stromal cells and in the OSE. (C) In tumorbearing FORKO ovaries, a very strong expression of COX-1 is confined to epithelial cells of a tumor section. (d) Expression of COX-2 in WT (+/+), FORKO (-/-), and FORKO tumor ovaries. (A) In WT ovaries, COX-2 expression occurs in stromal cells and granulosa cells of some follicles and in CL cells, with weakly immunopositive cells in the OSE. (B) In the unaffected FORKO ovary, COX-2 expression is observed in granulosa cells and is strongly observed in the OSE. (C) In FORKO tumor ovaries, a very strong expression of COX-2 is confined to epithelial cells of a tumor section. Note that COX-2 expression is higher in epithelial cells and granulosa cells of FORKO ovary than in those of WT ovary. (e and f) Expression of PDGFR-α (e) and PDGFR-β (f) in WT (+/+), FORKO (-/-), and FORKO tumor ovaries. Strong positive immunoreactivity to PDGFR-α and PDGFR-β is detected in CL, and less staining in granulosa cells and OSE cells in WT and unaffected FORKO ovaries but very intense staining in epithelial cells of FORKO ovarian tumor section.
Table 2.
Expression of PCNA, GATA-4, COX-1, COX-2, PDGFR-α, and PDGFR-β in Epithelial Cells of 12 (Plus)-Month-Old WT, FORKO, and FORKO Tumor Ovaries.
| WT | FORKO | FORKO Tumor | |
| PCNA | +/- | +/- | ++ |
| GATA-4 | ++ | + | +/- |
| COX-1 | +/- | +/- | ++ |
| COX-2 | +/- | + | ++ |
| PDGFR-α | + | +/- | ++ |
| PDGFR-β | + | +/- | ++ |
Microscopic evaluation by two independent observers not connected to the study.
(+/-) Weak signal; (+) strong signal; (++) very strong signal.
Immunohistochemical Analysis of GATA-4 in Tumor
As expression of the transcription factor GATA-4 is reportedly lost in most human ovarian serous carcinomas [29], immunohistochemical analysis of FORKO tumor was of interest. Our previous work showed that GATA-4 expression in granulosa cells was decreased in FORKO ovary compared with age-matched WT ovary in immature mice [18]. Here, we demonstrate that GATA-4 is strongly expressed in the nucleus of epithelial cells of morphologically normal OSE of WT ovary (Figure 4b). All OSE are intensely positive for GATA-4 staining in the nucleus. Some cells scattered in the ovarian stroma are also GATA-4-positive. In FORKO ovary, GATA-4 staining in the nucleus of the OSE, granulosa cells, and some stromal cells was weaker than that in WT. In contrast to the staining of the OSE in WT and FORKO ovaries, GATA-4 protein expression is lost in FORKO serous ovarian tumors (Table 2). This indicates that loss of GATA factors or their cognate regulatory pathways leads to de-differentiation of epithelial cells, which perhaps contributes to tumorigenicity.
COX-1 and COX-2 Expression in OSE Tumor
COX-1 and COX-2 are two distinct isoforms that catalyze the conversion of arachidonic acid to prostaglandins. COX-1 expression is constitutive, whereas COX-2 is expressed in inflammatory cells and is highly induced by various stimuli (growth factors, UV, and so on) in a wide variety of cells. Overexpression of COX-1 has been recently reported in another mouse model of EOC [30]. Figure 4, c and d, shows representative images of COX-1 and COX-2 staining. High expressions of both COX-1 and COX-2 were observed in FORKO ovarian tumor epithelial cells that were inside the ovary, whereas there was no staining in the OSE of WTand FORKO ovaries (Table 2). This indicates that cellular overexpression of COX-1 and COX-2 enzymes might enhance their tumorigenic potential in FORKO mutants.
Expression of PDGFR-α and PDGFR-β
Our previous work revealed that both PDGFR-α and PDGFR-β are located in the OSE, and their expression is subject to hormonal regulation [20]. We hypothesized that the PDGF signal pathway might play an important role in FORKO ovarian tumorigenesis. In this study, a high expression of both PDGFR-β (Figure 4e) and PDGFR-β (Figure 4f) was evident in FORKO ovarian serous tumor (Table 2).
Discussion
FSH-R signaling plays a vital role in ovarian development and function. To understand the biology of FSH-R-dependent processes in the ovary, we first produced mice lacking FSH-R(s) [31]. FORKO mice are sterile despite very high levels of FSH and LH; by 12 months, the majority of these animals had developed various kinds of ovarian pathology, including neoplasms of sex cord-stromal type, as well as cysts [17,18,31]. Subsequently, in 2-day-old mutant neonates, faster follicle recruitment was also noted [18]. In continuing our investigations on these mutants, for the first time, we have now found alteration in the OSE as early as 2 days after birth and that ovarian epithelial tumors occurred in the complete absence of ovulation. This finding contrasts with the theory of incessant ovulation being responsible for inducing EOC in women [32]. Our observations in the FORKO ovary of thicker OSE from 2 days onward until 24 days compared with that of age-matched WT, and evidence of the inward migration of epithelial cells beginning in young mutants is consistent with other changes occurring within the ovary [18].
The factors responsible for predisposing the OSE to a tumorigenic state are not fully known. Many agents (including gonadotropins; steroid hormones estrogen, androgen, and progesterone; and growth factors) could regulate OSE proliferation and migration. Several reports claim that FSH and LH receptors are located in human OSE [11,13,33]. FSH and LH apparently increase the cell proliferation of normal rabbit, rat, and mouse OSE cells in vivo and in vitro [10,12,34]. Other studies report FSH and LH stimulation of cell migration with no effect on proliferation [14]. Receptors for estrogen, progesterone, and androgen were found at the mRNA and/or protein level in humans [35] and rats [36] OSE. Elevated androgen could stimulate the proliferation of ovarian epithelial cells [3]. EGF and PDGF also stimulate OSE growth significantly [4]. Interestingly, as LH and androgen levels are elevated early, estrogen level remains consistently low in female FORKO [17], and as some growth factors such as PDGF and receptors are located in mouse OSE with alterations in FORKO mutants [20], it is likely that the hormonal imbalances that occur very early could have contributed to the alterations we have noted. These factors influenced the expression of regulatory genes in a manner that induces the proliferation and migration of FORKO OSE cells. Although this is a plausible scenario, the occurrence of full-blown OSE tumors in only a certain percentage of mutants and not in all mice experiencing hormonal imbalances is rather intriguing. Such a pattern is also reminiscent of aging women. Although every woman will undergo menopause and experience high circulating FSH and LH levels along with some form of hormonal imbalance, not all will develop ovarian tumors and not all will acquire the OSE type on aging. This has been rationalized as being due to the contribution of genetic factors or other epigenetic influences that have not been clearly sorted out. We should also note that in other mouse models of ovarian tumors, including those derived from different transgenic approaches, only up to 50% of the animals developed tumors (30). However, it is not known if hormonal imbalances occur in such mutants. How and in what manner regulatory factors could influence the strain of mutant mice that we and others have studied to induce tumors in select mice remains an enigma at this time. Thus, to fully understand the origin of disease process, it will be highly relevant to establish early patterns of change in the group of select cells that emerge to produce late tumors. Such maneuvers are feasible only in experimental mutant models.
Based on our previous [17] and current studies, we can infer that aging FORKO mutants develop a mixture of ovarian tumor types that also include epithelial ovarian tumors (Figures 2 and 3). Our findings of two kinds of tumors within the same ovary (Figure 3) suggest a complex mode of cellular interactions. In our aged FORKO female mouse, there are at least three distinct characteristics that are similar to those of perimenopausal and postmenopausal women: 1) a significant increase in the production of FSH and LH (although circulating FSH remains high at all times, this hormone could not function in FORKO mice, as all receptors have been ablated); and 2) estrogen is virtually absent and progesterone is reduced, but androgen level is higher throughout life, and, finally, ovaries are depleted of oocytes.
The surface epithelium is involved in follicular rupture and subsequent repair of the follicle wall in reproductive periods. Although controversy remains regarding the cellular origin of ovarian cancers [8,37], most investigators believe that ovarian cancers develop from epithelial cells that cover the ovarian surface or those that line inclusion cysts within the cortical stroma. Although a significant increase in gonadotropic hormones and other hormonal aberrations occurred as early as 24 days, signs of ovarian tumors were not apparent until 8 months or much later in FORKO females. Although we have no precise explanation at the present time for this observation, the long latency is reminiscent of ovarian cancer in women that occurs in later decades of life. Perhaps in addition to hormonal imbalances that occur in our mice, the loss of negatively regulating factors accompanying oocyte disappearance that only occurs later in life could be an additional contributor. Oocyte loss/destruction, in combination with overproduction of pituitary gonadotropins (particularly LH), leads to follicular atresia, stromal hypertrophy, and ovarian epithelial adenomas [38]. In addition, androgen contribution in generating tumors in FORKO mutants assumes significance because of its sustained high level. Thus, AR expression in > 80% of ovarian tumors [39] and an increased risk of ovarian cancer in women with elevated circulating levels of androgens [40] support androgen involvement. Androgen stimulates the growth of the OSE in guinea pigs, inducing the formation of benign cysts, small adenomas in the ovarian parenchyma, and papillomas on the ovarian surface [41].
Our PCNA data showing increased cell proliferation of epithelial cells in tumor sections, but not in the OSE (in both WT and atretic unaffected FORKO ovaries), are interesting. Very little proliferation of the OSE is detectable in adult mice [10]. In adult tissues, GATA transcription factors likely function to maintain cells in a differentiated state [42]. Loss of GATA factors or their cognate regulatory pathways could lead to dedifferentiation of epithelial cells, contributing to tumorigenicity. GATA-4 is expressed in sex cord-derived ovarian and gonad tumors [43], but is lost in some ovarian epithelial cancers [44]. Selective loss of GATA-4 in FORKO ovarian epithelial tumor, but not in normal OSE, suggests that affected cells undergo dedifferentiation. A higher expression of COX-1 and COX-2 in FORKO ovarian epithelial tumors is also consistent with recent findings. It has been shown that COX-2 is expressed in a wide variety of epithelial cancers and that COX-1 overexpression is common to EOC, rendering them as the primary target for various chemoprevention studies employing specific COX-2 inhibitors or nonsteroidal anti-inflammatory drugs that inhibit both COX-1 and COX-2 [45]. COX-1 serves as a potential marker of EOC [27], and COX-2 has been implicated as a tumor promoter because it stimulates angiogenesis [46] and promotes metastasis [47], suggesting that overexpression of both COX-1 and COX-2 may have contributed to tumorigenic potential. As both PDGFR-α and PDGFR-β are expressed strongly in ovarian serous tumors, our work implicates the PDGF family in this process, and their inhibitors could be potential candidates for reducing tumor burden. We propose that further mechanistic studies in this direction be accelerated by securing OSE-type cells from different stages in affected FORKO mutant mice either by culturing them or by selective capture for gene expressions.
In conclusion, we have observed that the loss of FSH-R signaling results in alteration of the OSE in early life and that ovarian epithelial tumor development occurs only on aging in mutant mice. Our results provide the first in vivo evidence that the complete elimination of FSH-R also contributes to the initiation of OSE changes and the induction of gonadal epithelial tumorigenesis.
Acknowledgements
We thank Chunyan Hu and Shan Liu for assistance with histopathologic diagnosis. We are very grateful to Carl-Henrik Heldin (Ludwig Institute for Cancer Research) for providing the rabbit polyclonal antisera to PDGFR-α and PDGFR-β. We also thank B. D. Schanbacher and A. H. Payne for providing us with the various reagents used in this study.
Footnotes
This investigation was supported by the Canadian Cancer Society.
References
- 1.Zanagnolo V, Sartori E, Trussardi E, Pasinetti B, Maggino T. Preservation of ovarian function, reproductive ability and emotional attitudes in patients with malignant ovarian tumors. Eur J Obstet Gynecol Reprod Biol. 2005;123:235–243. doi: 10.1016/j.ejogrb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 4.Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch WJ, McDonnel AC. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 2002;123:743–750. doi: 10.1530/rep.0.1230743. [DOI] [PubMed] [Google Scholar]
- 6.Auersperg N, Edelson MI, Mok SC, Johnson SW, Hamilton TC. The biology of ovarian cancer. Semin Oncol. 1998;25:281–304. [PubMed] [Google Scholar]
- 7.Fleming JS, Beaugie CR, Haviv I, Chenevix-Trench G, Tan OL. Incessant ovulation, inflammation and epithelial ovarian carcino-genesis: revisiting old hypotheses. Mol Cell Endocrinol. 2006;247:4–21. doi: 10.1016/j.mce.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Tan OL, Hurst PR, Fleming JS. Location of inclusion cysts in mouse ovaries in relation to age, pregnancy, and total ovulation number: implications for ovarian cancer? J Pathol. 2005;205:483–490. doi: 10.1002/path.1719. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 10.Davies BR, Finnigan DS, Smith SK, Ponder BA. Administration of gonadotropins stimulates proliferation of normal mouse ovarian surface epithelium. Gynecol Endocrinol. 1999;13:75–81. doi: 10.3109/09513599909167536. [DOI] [PubMed] [Google Scholar]
- 11.Parrott JA, Doraiswamy V, Kim G, Mosher R, Skinner MK. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol Cell Endocrinol. 2001;172:213–222. doi: 10.1016/s0303-7207(00)00340-3. [DOI] [PubMed] [Google Scholar]
- 12.Stewart SL, Querec TD, Gruver BN, O'Hare B, Babb JS, Patriotis C. Gonadotropin and steroid hormones stimulate proliferation of the rat ovarian surface epithelium. J Cell Physiol. 2004;198:119–124. doi: 10.1002/jcp.10401. [DOI] [PubMed] [Google Scholar]
- 13.Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61:6768–6776. [PubMed] [Google Scholar]
- 14.Choi JH, Choi KC, Auersperg N, Leung PC. Gonadotropins activate proteolysis and increase invasion through protein kinase A and phosphatidylinositol 3-kinase pathways in human epithelial ovarian cancer cells. Cancer Res. 2006;66:3912–3920. doi: 10.1158/0008-5472.CAN-05-1785. [DOI] [PubMed] [Google Scholar]
- 15.Siemens CH, Auersperg N. Serial propagation of human ovarian surface epithelium in tissue culture. J Cell Physiol. 1988;134:347–356. doi: 10.1002/jcp.1041340305. [DOI] [PubMed] [Google Scholar]
- 16.Dabrow MB, Francesco MR, McBrearty FX, Caradonna S. The effects of platelet-derived growth factor and receptor on normal and neoplastic human ovarian surface epithelium. Gynecol Oncol. 1998;71:29–37. doi: 10.1006/gyno.1998.5121. [DOI] [PubMed] [Google Scholar]
- 17.Danilovich N, Roy I, Sairam MR. Ovarian pathology and high incidence of sex cord tumors in follitropin receptor knockout (FORKO) mice. Endocrinology. 2001;142:3673–3684. doi: 10.1210/endo.142.8.8320. [DOI] [PubMed] [Google Scholar]
- 18.Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of ovarian development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biol Reprod. 2003;69:1281–1293. doi: 10.1095/biolreprod.103.015552. [DOI] [PubMed] [Google Scholar]
- 19.Aravindakshan J, Chen X, Sairam MR. Differential expression of claudin family proteins in mouse ovarian serous papillary epithelial adenoma in aging FSH receptor deficient mutants. Neoplasia. 2006;8:984–994. doi: 10.1593/neo.06529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Aravindakshan J, Yang Y, Tiwari-Pandey R, Sairam MR. Aberrant expression of PDGF ligands and receptors in the tumor prone ovary of follitropin receptor knockout (FORKO) mouse. Carcinogenesis. 2006;27:903–915. doi: 10.1093/carcin/bgi305. [DOI] [PubMed] [Google Scholar]
- 21.Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141:4295–4308. doi: 10.1210/endo.141.11.7765. [DOI] [PubMed] [Google Scholar]
- 22.Milikowski CBI. Color Atlas of Basic Histopathology Appleton and Lange, Stamford. 1997. [Google Scholar]
- 23.Liebelt AG, Sass B, Lombard LS. Mouse ovarian tumors—a review including classification and induction of neoplastic lesions and description of several previously unreported types. J Exp Pathol. 1987;3:115–145. [PubMed] [Google Scholar]
- 24.Maronpot RR. Pathology of Mouse. Clearwater, FL: Cache River; 1999. [Google Scholar]
- 25.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, Persons DL, Smith PG, Terranova PF. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 26.Abel MH, Huhtaniemi I, Pakarinen P, Kumar TR, Charlton HM. Age-related uterine and ovarian hypertrophy in FSH receptor knockout and FSHbeta subunit knockout mice. Reproduction. 2003;125:165–173. doi: 10.1530/rep.0.1250165. [DOI] [PubMed] [Google Scholar]
- 27.Crist KA, Zhang Z, You M, Gunning WT, Conran PB, Steele VE, Lubet RA. Characterization of rat ovarian adenocarcinomas developed in response to direct instillation of 7,12-dimethylbenz[a]anthracene (DMBA) coated suture. Carcinogenesis. 2005;26:951–957. doi: 10.1093/carcin/bgi039. [DOI] [PubMed] [Google Scholar]
- 28.Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 2003;63:1389–1397. [PubMed] [Google Scholar]
- 29.Lassus H, Laitinen MP, Anttonen M, Heikinheimo M, Aaltonen LA, Ritvos O, Butzow R. Comparison of serous and mucinous ovarian carcinomas: distinct pattern of allelic loss at distal 8p and expression of transcription factor GATA-4. Lab Invest. 2001;81:517–526. doi: 10.1038/labinvest.3780260. [DOI] [PubMed] [Google Scholar]
- 30.Daikoku T, Tranguch S, Trofimova IN, Dinulescu DM, Jacks T, Nikitin AY, Connolly DC, Dey SK. Cyclooxygenase-1 is overexpressed in multiple genetically engineered mouse models of epithelial ovarian cancer. Cancer Res. 2006;66:2527–2531. doi: 10.1158/0008-5472.CAN-05-4063. [DOI] [PubMed] [Google Scholar]
- 31.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 33.Zheng W, Magid MS, Kramer EE, Chen YT. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am J Pathol. 1996;148:47–53. [PMC free article] [PubMed] [Google Scholar]
- 34.Osterholzer HO, Streibel EJ, Nicosia SV. Growth effects of protein hormones on cultured rabbit ovarian surface epithelial cells. Biol Reprod. 1985;33:247–258. doi: 10.1095/biolreprod33.1.247. [DOI] [PubMed] [Google Scholar]
- 35.Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc Natl Acad Sci USA. 1999;96:5722–5727. doi: 10.1073/pnas.96.10.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams AT, Auersperg N. Autoradiographic investigation of estrogen binding in cultured rat ovarian surface epithelial cells. J Histochem Cytochem. 1983;31:1321–1325. doi: 10.1177/31.11.6619537. [DOI] [PubMed] [Google Scholar]
- 37.Dubeau L. The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gynecol Oncol. 1999;72:437–442. doi: 10.1006/gyno.1998.5275. [DOI] [PubMed] [Google Scholar]
- 38.Vanderhyden BC. Loss of ovarian function and the risk of ovarian cancer. Cell Tissue Res. 2005;322:117–124. doi: 10.1007/s00441-005-1100-1. [DOI] [PubMed] [Google Scholar]
- 39.Ilekis JV, Connor JP, Prins GS, Ferrer K, Niederberger C, Scoccia B. Expression of epidermal growth factor and androgen receptors in ovarian cancer. Gynecol Oncol. 1997;66:250–254. doi: 10.1006/gyno.1997.4764. [DOI] [PubMed] [Google Scholar]
- 40.Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, Comstock GW. Serum gonadotropins and steroid hormones and the development of ovarian cancer. JAMA. 1995;274:1926–1930. [PubMed] [Google Scholar]
- 41.Silva EG, Tornos C, Fritsche HA, Jr, el Naggar A, Gray K, Ordonez NG, Luna M, Gershenson D. The induction of benign epithelial neoplasms of the ovaries of guinea pigs by testosterone stimulation: a potential animal model. Mod Pathol. 1997;10:879–883. [PubMed] [Google Scholar]
- 42.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 43.Laitinen MP, Anttonen M, Ketola I, Wilson DB, Ritvos O, Butzow R, Heikinheimo M. Transcription factors GATA-4 and GATA-6 and a GATA family cofactor, FOG-2, are expressed in human ovary and sex cord-derived ovarian tumors. J Clin Endocrinol Metab. 2000;85:3476–3483. doi: 10.1210/jcem.85.9.6828. [DOI] [PubMed] [Google Scholar]
- 44.Capo-chichi CD, Roland IH, Vanderveer L, Bao R, Yamagata T, Hirai H, Cohen C, Hamilton TC, Godwin AK, Xu XX. Anomalous expression of epithelial differentiation—determining GATA factors in ovarian tumorigenesis. Cancer Res. 2003;63:4967–4977. [PubMed] [Google Scholar]
- 45.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 46.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 47.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]