Abstract
Magnesium-dependent induction of Vibrio fischeri flagellar (Mif) biogenesis depends upon two diguanylate cyclases, suggesting an inhibitory role for cyclic di-GMP. Here, we report that cells defective for the sugar phosphotransferase system (PTS) exhibited a magnesium-independent phenotype similar to that of mutants of the Mif pathway. Unlike Mif mutants, PTS mutants also were hyperbioluminescent.
The second messenger, cyclic AMP (cAMP), is synthesized by adenylate cyclase (AC). In bacteria of the family Enterobacteriaceae (e.g., Escherichia coli), the activity of AC becomes enhanced by its interaction with the phosphorylated version of EIIAGlc (phospho-EIIAGlc), the glucose-specific IIA component of the phosphoenolpyruvate (PEP):carbohydrate phosphotransferase system (PTS) (6, 17, 18, 20). The glucose-specific PTS is composed of three soluble, cytoplasmic proteins (EI, HPr, and EIIAGlc) and one integral cytoplasmic membrane protein (EIICBGlc). These proteins sequentially transfer a phosphoryl group from PEP to glucose, with the concomitant transport of the sugar across the membrane (Fig. 1). The presence of glucose in the environment pulls phosphoryl groups through the PTS, ensuring that the system's components remain essentially unphosphorylated; depletion of that glucose results in the accumulation of phospho-EIIAGlc, which activates AC to synthesize cAMP. This cAMP binds cAMP receptor protein (CRP, also known as CAP), which then regulates the transcription of hundreds of genes, including flhDC, the master regulator of the Enterobacteriaceae flagellar regulon (for reviews, see references 6a, 10, 11, 17, and 30).
FIG. 1.
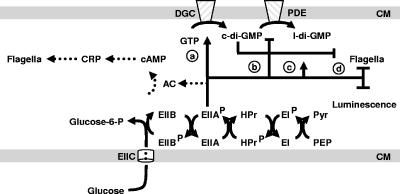
The PTS and the proposed Mif pathways. CM, cytoplasmic membrane; l-di-GMP, linear di-GMP; EIIC and EIIB, subunits of the glucose-specific PTS transporter; EIIA, HPr, and EI, signaling components of the PTS pathway; Pyr, pyruvate. Dotted lines represent a pathway known to function in E. coli. See the text for the proposed interaction between the PTS and Mif pathways (a through d) and their effects upon flagellar biogenesis and on bioluminescence.
Cyclic di-GMP (c-di-GMP) is a newly appreciated second messenger, apparently unique to bacteria, that modulates diverse cellular processes (for a recent review, see reference 21). First identified as a positive effector of cellulose synthase in Gluconoacetobacter xylinus (reviewed in reference 22), c-di-GMP regulates transition from the motile, planktonic state to sessile, community-based behaviors, such as biofilm development. It tends to inhibit motility, both flagellar and twitching, while enhancing the biosynthesis of capsular components required by developing biofilms. c-di-GMP is synthesized by diguanylate cyclases (DGC) and degraded by phosphodiesterases (PDE) (21). Together these activities maintain the steady-state concentration of c-di-GMP (22). DGC activity has been associated with the highly conserved GGDEF domain. One PDE activity (to linear di-GMP) has been associated with the highly conserved EAL domain, while a second PDE activity (to two GMPs) has been associated with the HD-GYP domain (21). Finally, a c-di-GMP-binding domain (termed PilZ) was recently reported (2) and shown to inhibit flagellar assembly in E. coli (25).
Insights into the control of c-di-GMP production and its targets have come from our investigations of motility in the marine bacterium Vibrio fischeri. This bacterium, found as free-living, motile individuals or as a sessile community in association with the Hawaiian squid Euprymna scolopes, regulates the biogenesis of its flagella in response to the concentration of environmental magnesium (Mg2+). In the presence of abundant Mg2+, such as that found in seawater, V. fischeri cells possess flagella; when Mg2+ becomes limiting, they do not (14). This Mg2+-dependent induction of flagellar (Mif) biogenesis depends upon at least two DGCs, one confirmed (MifA) and one putative (MifB) (Fig. 1). Unlike wild-type (WT) cells, mutants that lack MifA or MifB or both synthesize flagellin proteins and become motile even when Mg2+ is limiting (15). Since MifA (and likely also MifB) catalyzes the production of c-di-GMP, these observations are consistent with a model in which c-di-GMP inhibits flagellar biogenesis, while Mg2+ interferes with or overcomes that inhibition. Since overexpression of mifA did not exert a significant effect upon the transcription of flagellin genes, we have proposed that c-di-GMP acts posttranscriptionally (15).
Disruption of both mifA and mifB under Mg2+-limiting conditions failed to promote the same level of migration that WT cells achieve in the presence of Mg2+; this result suggests the existence of additional Mif pathway components. Therefore, during a recent study (15), we searched for those additional components using a screen for transposon (Tn) mutants that migrated in the absence of Mg2+. The majority of the putative mutations mapped to mifA; however, several did not. Here, we report the characterization of one of the non-mifA mutants.
This non-mifA mutant (strain KV2657) exhibited a phenotype similar to those of mifA and mifB mutants: in the absence of Mg2+, like the mifA vector integration (Campbell) mutant (mifA::pKV217; strain KV2672) or the mifB Campbell mutant (mifB::pTMO125; strain KV2532), it migrated sooner and more rapidly than the positive control, KV1421 (Fig. 2A and B). In the presence of Mg2+, the migration rate of KV2657, like that of mutant mifA or mifB, was similar to that of the KV1421 control (Fig. 2A and C). Furthermore, even with Mg2+, the control strain left a dense mass of poorly motile and nonmotile cells at the site of inoculation. The Tn mutant, in contrast, did not, consistent with the hypothesis that a larger proportion of its cells became motile sooner than those of the control (Fig. 2A and data not shown), a behavior reminiscent of mif mutants (15).
FIG. 2.
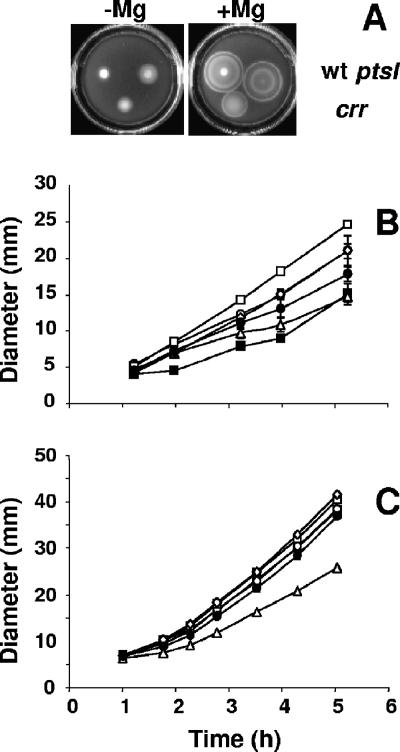
Disruption of the ptsHI-crr operon permits migration in the absence of added Mg2+. (A) Cells were grown overnight at 28°C in tryptone broth-saline (TBS) (5), inoculated onto the surface of TBS motility plates containing either no added Mg2+ (−Mg) or containing 35 mM MgSO4 (TBS-Mg2+) (+Mg) (14), incubated at 28°C for 5 hours, and photographed. wt refers to strain KV1421 and ptsI refers to strain KV2801 (ptsI::pTMO151), while crr refers to strain KV2850 (crr::pTMO152). (B and C) Displacement of the outer band of strains: KV1421 (WT; solid squares), KV2672 (mifA::pKV217; open squares), KV2532 (mifB::pTMO125; open diamonds), KV2657 (ptsI::Tn5; closed circles), KV2801 (ptsI::pTMO151; open circles), and KV2850 (crr::pTMO152; open triangles) in TBS (B) or in TBS-Mg2+ (C).
Sequence analysis revealed that the Tn in strain KV2657 inserted (along with its delivery vector) into VF1896 (Fig. 3A), an open reading frame (ORF) annotated to encode the C-terminal two-thirds of the PTS component EI; the N terminus of EI was annotated as VF1895 (23). We hypothesized that the annotation of separate ORFs resulted from a sequencing error. We thus amplified, cloned, and sequenced the annotated junction of VF1895 and VF1896 and determined that VF1895/VF1896 represents a single ORF (data not shown).
FIG. 3.
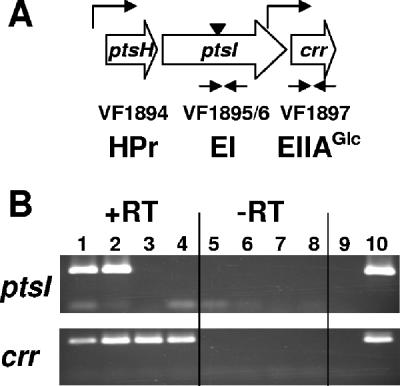
Arrangement of PTS genes. (A) Schematic shows the putative operon structure, the gene products, and the approximate locations of the Tn5 (inverted triangle) and the primers (inverted arrows) used for both the Campbell insertions and the RT-PCR shown in panel B. Bent arrows indicate putative promoters. (B) RT-PCR, performed as described previously (15), shows that the Campbell insertion allele ptsI::pTMO151 (strain KV2801) is not polar on the downstream crr gene. The parent strain KV1421 is shown as a control. ptsI, primers specific for ptsI; crr, primers specific for crr. Lanes 1, 2, 5, 6, strain KV1421; lanes 3, 4, 7, 8, strain KV2801; lane 9, negative control (distilled water); lane 10, positive control (ESR1 genomic DNA).
VF1895/VF1896 resides on chromosome 1 of V. fischeri, within a locus (VF1894 to -1897) (23) that bears a strong resemblance to the ptsHI-crr locus of E. coli K-12 (10, 24). The proteins encoded by VF1894, VF1895/VF1896, and VF1897 share extensive identity, respectively, to those encoded by ptsH (HPr, 79%), ptsI (EI, 73%), and crr (EIIAGlc, 83%) (1, 27). On the basis of this identity and the data presented below, we designated the VF1894 to -1897 locus as the V. fischeri ortholog of the E. coli ptsHI-crr operon. To test this designation, we constructed Campbell mutations in both the VF1895/VF1896 (ptsI::pTMO151) and the downstream VF1897 (crr::pTMO152) genes, verifying their construction by Southern blotting analysis. Although the resulting strains (KV2801 and KV2850, respectively) grew as well as their WT parent in rich medium, they grew poorly or not at all in minimal medium supplemented with several sugars, including glucose, glucosamine, and N-acetylglucosamine (data not shown).
To confirm the role of ptsI in Mg2+-dependent motility, we compared the migration behavior of strain KV2801 (the Campbell mutant) to that of strain KV2657, the original ptsI Tn insertion mutant (Fig. 2B). The Campbell mutant exhibited a similar behavior: in the absence of Mg2+, it migrated sooner and faster than the control strain KV1421 (Fig. 2A and B); in the presence of Mg2+, it behaved much like KV1421. Disruption of ptsI should preclude the phosphorylation of downstream components of the PTS pathways, including those specific for glucose, e.g., EIIAGlc (Fig. 1). To determine whether EIIAGlc also plays a role in controlling motility, we tested the crr Campbell mutant (strain KV2850): in the absence of Mg2+, it began migrating considerably sooner than its WT parent (Fig. 2A and B). In the presence of Mg2+, however, the crr mutant migrated quite a bit more slowly than either the WT or the mifA mutant (Fig. 2A and C). The reason for this behavior remains unknown but is unlikely to be due to differences in growth rate (data not shown).
The behavior of the ptsI mutant could be due either to an inability to phosphorylate downstream components or to a polar effect on the transcription of the downstream crr gene. In E. coli, the ptsHI-crr locus contains two minor promoters positioned upstream of crr within ptsI (10, 17, 26). Thus, a polar mutation in the E. coli ptsI gene does not eliminate transcription from crr (17). To determine whether this is also true of the V. fischeri ptsHI-crr locus, we performed reverse transcription (RT)-PCR (15) using primer pairs specific for ptsI and crr (Fig. 3A). In contrast to the control cells (strain KV1421), which possessed transcripts for both ptsI and crr, the Tn insertion mutant (strain KV2657) showed a transcript for only crr (Fig. 3B). These data demonstrate that the Tn insertion into ptsI does not exert polarity on the downstream crr. Thus, like its E. coli ortholog, the V. fischeri crr gene likely possesses its own promoter, and the effect of a ptsI deficiency likely results from an inability to phosphorylate EIIAGlc and/or other downstream components.
In E. coli, phospho-EIIAGlc activates flagellar biogenesis by enhancing AC activity. The resultant cAMP binds CRP, which activates transcription of flhDC, the operon that encodes the master activator of the E. coli flagellar regulon (11, 28). In contrast, the data for V. fischeri (which uses a regulatory system that does not include flhDC) are consistent with a negative role for phospho-EIIAGlc, much like that for MifA, which appears to act subsequent to transcription (15). To determine whether EI and/or EIIAGlc also act posttranscriptionally, we performed a semiquantitative RT-PCR analysis with flaA, flaC, and flaE, which encode major V. fischeri flagellins (12), and with fliF, predicted to encode the M ring, whose insertion into the cytoplasmic membrane constitutes the first step in building flagella (19). The cDNA levels were not substantially affected by the status of mifB (strain KV2825), ptsI (KV2801), or crr (KV2850) (data not shown). In contrast, the cDNA levels were substantially diminished but not eliminated by the disruption of rpoN (data not shown), which encodes σ54, proposed to sit near or at the top of the hierarchical V. fischeri flagellar regulon (strain KV1513) (19, 29). We conclude that both the PTS and the Mif pathway impact flagellar biogenesis at similar levels.
Thus, we propose that phospho-EIIAGlc interacts with the Mif system to regulate flagellar biogenesis, most likely at a posttranscriptional level (Fig. 1). This model is supported by the following observations: (i) ptsI and crr mutants of V. fischeri exhibited similar behavior, i.e., increased migration in the absence of Mg2+ (Fig. 2), suggesting that phospho-EIIAGlc is a prerequisite for the inhibition of flagellar biogenesis; (ii) disruption of either ptsI or crr exerted no significant effect upon the transcription of flagellin genes; and (iii) these behaviors resemble those of mif mutants (15). We do not yet know how the Mif and PTS pathways interact, although we can imagine an interaction between phospho-EIIAGlc and the Mif pathway at any one of a number of levels (Fig. 1): phospho-EIIAGlc could (i) activate a DGC; (ii) inhibit a PDE composed of an EAL domain and a nonconsensus “regulatory” GGDEF domain (21); (iii) interfere with the ability of c-di-GMP to interact with its target; or (iv) act independently. EIIAGlc, either in its phosphorylated or nonphosphorylated form, tends to interact with a variety of proteins, including not only EIICBGlc and AC (5a, 17) but also FrsA, which controls the fermentation-respiration switch (9). Alternatively, the effect could be mediated by another PTS component, e.g., EI or HPr.
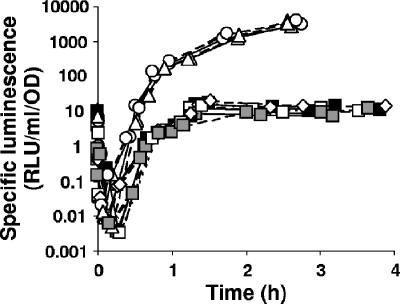
To further our understanding of the relationship between the PTS and the Mif pathway, we also considered the following published observations: (i) in E. coli, phospho-EIIAGlc activates AC to produce cAMP (6, 17, 20); (ii) cAMP binds and activates the transcriptional regulator CRP (30); and (iii) CRP of V. fischeri has been linked to the control of bioluminescence (J. L. Bose, U. Kim, W. Bartkowski, R. P. Gunsalus, A. M. Overley, K. L. Visick, and E. V. Stabb, submitted for publication; 7, 8, 13). We therefore asked whether a mutation in mifA, mifB, ptsI, or crr impacted bioluminescence. We grew WT and mutant cells in a tryptone-based seawater medium (which contains Mg2+) and measured bioluminescence over time. To account for any small differences in growth, we plotted the specific luminescence (relative light units/ml/optical density unit) versus optical density (Fig. 4). Cells defective for mifA (KV2672) or mifB (KV2532) or both (KV2826) displayed bioluminescence patterns comparable to that of the WT strain (ES114). Surprisingly, however, mutants defective for either ptsI (KV2801) or crr (KV2850) exhibited substantially elevated levels of bioluminescence. Specifically, these mutations promoted an increase of over 300-fold in bioluminescence, allowing this naturally nonvisibly bioluminescent strain of V. fischeri to produce visibly detectable light. Given the clear differences in the bioluminescence patterns of these strains, these data support the conclusion that Mif and the PTS function in distinct pathways, at least with respect to bioluminescence.
FIG. 4.
The ptsHI-crr operon regulates bioluminescence. Cells were grown at 28°C in SWT (3) supplemented with extra NaCl (for a final concentration of 552 mM), and bioluminescence was measured over time. To account for any small differences in growth, specific luminescence (relative light units/ml/optical density unit [OD]) was plotted versus optical density. The experiment was performed with two independent isolates (denoted as solid and dotted lines) of the following strains: ES114 (WT, closed squares), KV2532 (mifB::pTMO125, open diamonds), KV2672 (mifA::pKV217, open squares), KV2801 (ptsI::pTMO151, open circles), KV2850 (crr::pTMO152, open triangles), and KV2826 (mifA::pKV217 ΔmifB, gray squares).
At first glance, the observation that the PTS controls bioluminescence is not surprising: both cAMP and CRP have been reported to activate bioluminescence in V. fischeri (J. L. Bose et al., submitted; 7, 8, 13), and it is well known that phospho-EIIAGlc activates AC in E. coli (17). However, if phospho-EIIAGlc activated AC in V. fischeri as it does in E. coli, then one would predict that the PTS would activate bioluminescence. Instead, the PTS appears to inhibit bioluminescence (Fig. 4). Similarly, phospho-EIIAGlc, cAMP, and CRP activate transcription of the flagellar regulon in E. coli (11, 28); yet, phospho-EIIAGlc appears to inhibit flagellar biogenesis in V. fischeri (Fig. 2). These parallel observations concerning the effect of the PTS on distinct cellular processes suggest that the influence of the V. fischeri PTS might exhibit some novel properties relative to those of its better-studied distant relative E. coli.
Acknowledgments
This work was supported by the estate of William G. Potts in support of medical research at the Stritch School of Medicine at Loyola Univeristy Chicago and by NIH grant GM59690, awarded to K.L.V., and NIH grant GM066130, awarded to A.J.W.
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amikam, D., and M. Y. Galperin. 2006. PilZ domain is part of the bacterial c-di-GMP binding protein. Bioinformatics 22:3-6. [DOI] [PubMed] [Google Scholar]
- 3.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 172:3701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.DeLoney-Marino, C. R., A. J. Wolfe, and K. L. Visick. 2003. Chemoattraction of Vibrio fischeri to serine, nucleosides, and N-acetylneuraminic acid, a component of squid light-organ mucus. Appl. Environ. Microbiol. 69:7527-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Blaauwen, J. L., and P. W. Postma. 1985. Regulation of cyclic AMP synthesis by enzyme IIIGlc of the phosphoenolpyruvate:sugar phosphotransferase system in crp strains of Salmonella typhimurium. J. Bacteriol. 164:477-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunlap, P. V., and E. P. Greenberg. 1985. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J. Bacteriol. 164:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlap, P. V., and E. P. Greenberg. 1988. Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-LuxR protein regulatory circuit. J. Bacteriol. 170:4040-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo, B. M., M. J. Yoon, C. R. Lee, T. W. Nam, Y. J. Choe, H. Jaffe, A. Peterkofsky, and Y. J. Seok. 2004. A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J. Biol. Chem. 279:31613-31621. [DOI] [PubMed] [Google Scholar]
- 10.Kotrba, P., M. Inui, and H. Yukawa. 2001. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J. Biosci. Bioeng. 92:502-517. [DOI] [PubMed] [Google Scholar]
- 11.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 12.Millikan, D. S., and E. G. Ruby. 2004. Vibrio fischeri flagellin A is essential for normal motility and for symbiotic competence during initial squid light organ colonization. J. Bacteriol. 186:4315-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nealson, K. H., A. Eberhard, and J. W. Hastings. 1972. Catabolite repression of bacterial bioluminescence: functional implications. Proc. Natl. Acad. Sci. USA 69:1073-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Shea, T. M., C. R. DeLoney-Marino, S. Shibata, S.-I. Aizawa, A. J. Wolfe, and K. L. Visick. 2005. Magnesium promotes flagellation of Vibrio fischeri. J. Bacteriol. 187:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Shea, T. M., A. H. Klein, K. Geszvain, A. J. Wolfe, and K. L. Visick. 2006. Diguanylate cyclases control magnesium-dependent motility of Vibrio fischeri. J. Bacteriol. 188:8196-8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reference deleted.
- 17.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1996. Phosphoenolpyruvate:carbohydrate phosphotransferase systems, p. 1149-1174. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 18.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prüss, B. M., D.-J. Kim, S. Forst, R. T. Fleming, K. L. Visick, and A. J. Wolfe. 2005. Genomics of flagella, p. 1-12. In B. M. Prüss (ed.), Global regulatory networks in enteric bacteria. Research Signpost, Kerala, India.
- 20.Reddy, P., and M. Kamireddi. 1998. Modulation of Escherichia coli adenylyl cyclase activity by catalytic-site mutants of protein IIAGlc of the phosphoenolpyruvate:sugar phosphotransferase system. J. Bacteriol. 180:732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romling, U., M. Gomelsky, and M. Y. Galperin. 2005. C-di-GMP: the dawning of a novel bacterial signaling system. Mol. Microbiol. 57:629-639. [DOI] [PubMed] [Google Scholar]
- 22.Ross, P., R. Mayer, and M. Benziman. 1991. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 55:35-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruby, E. G., M. Urbanowski, J. Campbell, A. Dunn, M. Faini, R. Gunsalus, P. Lostroh, C. Lupp, J. McCann, D. Millikan, A. Schaefer, E. Stabb, A. Stevens, K. Visick, C. Whistler, and E. P. Greenberg. 2005. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. USA 102:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudd, K. E. 2000. EcoGene: a genome sequence database for Escherichia coli K-12. Nucleic Acids Res. 28:60-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryjenkov, D. A., R. Simm, U. Romling, and M. Gomelsky. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP. The PilZ domain protein YcgR controls motility in enterobacteria. J. Biol. Chem. 281:30310-30314. [DOI] [PubMed] [Google Scholar]
- 26.Salgado, H., S. Gama-Castro, M. Peralta-Gil, E. Díaz-Peredo, F. Sánchez-Solano, A. Santos-Zavaleta, I. Martínez-Flores, V. Jiménez-Jacinto, C. Bonavides-Martínez, J. Segura-Salazar, A. Martínez-Antonio, and J. Collado-Vides. 2006. RegulonDB (version 5.0): Escherichia coli K-12 transcriptional regulatory network, operon organization, and growth conditions. Nucleic Acids Res. 34:D394-D397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schäffer, A. A., L. Aravind, T. L. Madden, S. Shavirin, J. L. Spouge, Y. I. Wolf, E. V. Koonin, and S. F. Altschul. 2001. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 29:2994-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soutourina, O., A. Kolb, E. Krin, C. Laurent-Winter, S. Rimsky, A. Danchin, and P. Bertin. 1999. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J. Bacteriol. 181:7500-7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe, A. J., D. S. Millikan, J. M. Campbell, and K. L. Visick. 2004. Vibrio fischeri σ54 controls motility, biofilm formation, luminescence, and colonization. Appl. Environ. Microbiol. 70:2520-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng, D., C. Constantinidou, J. L. Hobman, and S. D. Minchin. 2004. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 32:5874-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]