Abstract
Diagnosis platforms incorporating low-cost microfluidic chips enable sensitive, rapid, and accurate genetic analysis that could facilitate customized therapies tailored to match the vulnerabilities of any types of cancer. Using ex vivo cancer cells, we have detected the unique molecular signature and a chromosomal translocation in multiple myeloma. Multiple myeloma is characterized by IgH rearrangements and translocations that enable unequivocal identification of malignant cells, detected here with integrated microfluidic chips incorporating genetic amplification via reverse transcriptase-polymerase chain reaction and capillary electrophoresis. On microfluidic chips, we demonstrated accurate and versatile detection of molecular signatures in individual cancer cells, with value for monitoring response to therapy, detecting residual cancer cells that mediate relapse, and evaluating prognosis. Thus, testing for two clinically important molecular biomarkers, the IgH VDJ signature and hybrid transcripts signaling the t(4;14) chro-mosomal translocation, with predictive value in diagnosis, treatment decisions, and monitoring has been efficiently implemented on a miniaturized microfluidic system.
In multiple myeloma (MM), a cancer characterized by extensive, complex chromosomal abnormalities, recurrent translocations involving the immunoglobulin heavy chain gene on chromosome 14 are among the most frequent translocations, being found in about 60% of myeloma patients1 and many myeloma cell lines.2 The t(4;14)(p16;q32) reciprocal translocation, which results in aberrant regulation of genes on both chromosomes 4 and 14, has been particularly well studied.3,4 As a result of the t(4;14) translocation, the fibroblast growth factor receptor 3 (FGFR3) gene in chromosome 14 is overexpressed, whereas on chromosome 4, theMMSET gene is overexpressed.4 Multiple breakpoints on chromosome 4 have been identified within the MMSET gene,3 with REII-BP as a likely target gene.1 In addition, each MM patient is characterized by unique molecular signature, the IgH VDJ gene rearrangement, which provides a unique marker to identify all malignant cells in each patient.
Patients having the t(4;14) translocation have reduced survival.1,5,6,7 t(4,14) myeloma, characterized by drug resistance and rapid relapse,8 has been shown to predict for poor response to conventional chemotherapy and to high-dose chemotherapy followed by stem cell rescue.7 The clinical significance of the t(4;14) translocation suggests that monitoring of all MM patients would enable more informed treatment decisions. Because of the cost and technical complexity of molecular diagnostics, they are not routinely used in most hospitals.
Although fluorescence in situ hybridization readily detects the t(4;14) translocation,9 fluorescence in situ hybridization in its current form is time-consuming, uses expensive probes, is labor-intensive, and requires highly skilled personnel to operate and interpret the results. The t(4;14) translocation can be identified using reverse transcriptase-polymerase chain reaction (RT-PCR) to detect hybrid IgH-MMSET transcripts from the derivative4 chromosome.1,3,6,10,11 The integrated RT-PCR and capillary electrophoresis (CE)-based microchip detection method reported here is well suited for automated patient monitoring and could lead to routine usage at, possibly, every clinic visit. We believe this is the first demonstration of an inexpensive microfluidic system for biomarker screening of cancer patients.
Microfluidic chips consist of networks of reservoirs (microliter and submicroliter volumes) and channels (micrometer scale) within which biomolecules can be manipulated in an automated manner using small volume samples and reduced reagent consumption, leading to lower costs and faster testing, for point-of-care patient monitoring. Currently, few microfluidic systems have extensive integration of functionality or validation using clinical samples. Recently, microchip-based clinically relevant assays12,13,14,15,16,17,18 on microfluidic platforms have been demonstrated. Although many bioanalytical processes have been ported onto the microchip platform,12,19,20,21,22,23,24,25,26,27,28 widespread clinical implementation will require effective and appropriate integration of sample processing, genetic amplification, detection, and fluid-handling systems. Ramsey and colleagues,29,30 Mathies and collea-gues,31,32 Landers and colleagues,12,33 and other groups23,34 have demonstrated impressive integrations of PCR-CE systems. However, there are few reports on the integration of reverse transcription onto a microchip PCR platform16,28,35 and none that include chip-based CE detection. This perhaps reflects the use of a two-step RT-PCR approach that requires considerable manipulation of reagents and reaction products and increases the possibility of contamination, thereby increasing the complexity of the system and the challenges to integration of CE for product detection. Here, we have used a single-step RT-PCR approach, facilitating the integration of RT-PCR and CE on the same chip. Furthermore, testing strategies published to date do not address the need to monitor cancer at the single-cell level,36,37 a critical issue in clinical management of cancer heterogeneity.
In this work, microfluidic testing shows strong potential for facilitating the design of customized therapies tailored to each cancer, as well as for monitoring the response to therapy and the detection of residual cancer cells that ultimately may lead to relapse. With progress in miniaturization, these devices could become inexpensive tools for routine testing of molecular biomarkers at every clinic visit. With further development, such testing could be completed in minutes. In contrast, fluorescence in situ hybridization, the only other molecular approach in clinical practice, requires days. Our microfluidic platforms have been used for PCR thermal cycling and product analysis on-chip,38,39 detection of gene polymorphisms,40,41,42,43 and detection of viral titers in unprocessed urine from renal transplant recipients18 (with integrated sample preparation). Overall, on-chip PCR uses small reaction volumes,38 with analysis of picoliters of PCR product during each microchip CE run at a sensitivity comparable with that of conventional technology requiring 10 to 200 times more sample.39 Here, we report on the sensitive detection of IgH MMSET hybrid transcripts and transcripts encoding clonotypic IgH VDJ gene rearrangements in bone marrow (BM) and blood cells from patients with t(4;14) MM, in aggregate populations and in individual cells. We have successfully screened patient samples for these clinically important cancer biomarkers on an RT-PCR-CE microfluidic chip, with validation against a conventional “gold standard.”
Materials and Methods
Patients
After Institutional Review Board approval and informed consent, blood and BM samples were obtained at diagnosis or relapse from 12 patients with multiple myeloma, including four with the MB4-1, four with the MB4-2, and four with the MB4-3 breakpoints. Blood and BM were processed as previously described.36 All patients were confirmed as having the t(4;14) translocation as previously described.1,5
Microfluidic Chips and Platform
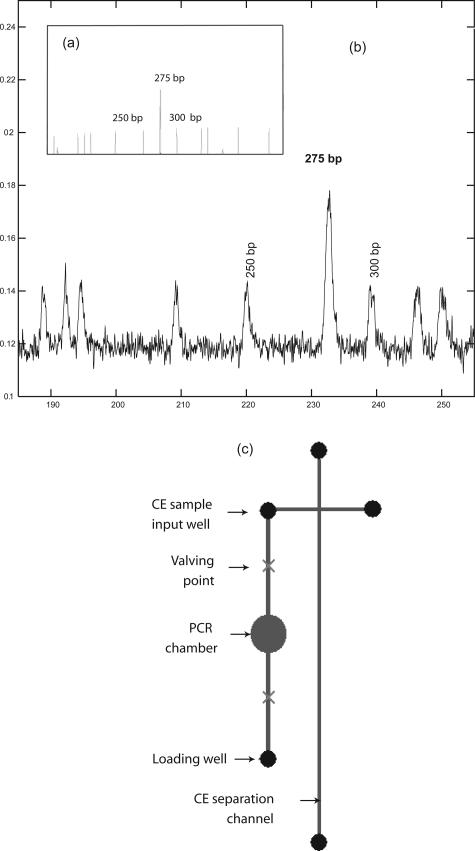
The chips used here are patterned poly(dimethyl)siloxane (PDMS) irreversibly bonded to a glass substrate as previously described.18 For the initial phase of this study, a two-chip system was used incorporating a three-reservoir chip to perform RT-PCR and analysis using the glass CE chip. The CE chip is a two-layer glass chip from Micralyne (Edmonton, AB, Canada) as described previously.39 The two-chip approach increased the speed of protocol development and throughput in analysis. Where indicated, an integrated PDMS/glass chip incorporating the architecture of both of these chips (a PCR-CE chip) was used. Glass CE chips were fully reusable,44 and consistency was monitored using a standard calibration procedure.45 Thermal cycling was performed using a custom-designed dual-Peltier system (G. Kaigala, J. Jiang, C.J. Backhouse, and H.J. Marquez, manuscript under revision) and other physical subsystems. This chip thermal cycler is controlled using a custom-built software controller that is resident on a microcontroller of the system. Precision in control of temperature in the range of ±0.1°C at set points is achieved, and temperature ramp rates of ∼6°C/second are achieved. On-chip diaphragm micro-pumping and micro-pinch-off valving using robotic arms that takes advantage of the deforming capability of PDMS are used here as previously described,38 thus ensuring a system that is reusable with no cross-contamination between runs. Dead volume using this micropumping approach is less than 10% of the reaction chamber volume. For the integrated PCR-CE chips, using the diaphragm pumps, fluid from the reaction chamber (after thermal cycling is completed) is dispensed into the output chamber, which is also the input well to the CE section of this integrated chip.
Microchip PCR
All reaction mixes were prepared in a total volume of 25 μl for running multiple replicates of chip-based PCR and a control using the conventional thermal cycler. This 25-μl mix contained 2.5 μl of 10× PCR buffer [20 mmol/L Tris-HCl, pH 8.4, and 50 mmol/L (final concentration) KCl], 1 μl of MgCl2 (2 mmol/L), 1 μl of dNTP (0.2 mmol/L), 1.5 μl of each of the forward and reverse primers (0.2 μmol/L), 0.5 μl of Platinum Taq (0.5 U) (Invitrogen Life Technology, Burlington, ON, Canada), 2 μl of cDNA template, 2.5 μl of bovine serum albumin (1 mg/ml) (NEB, Pickering, ON, Canada) (10 mg/ml), and 13.5 μl of double distilled water. Two microliters of this mixture was introduced to each on-chip PCR chamber. This volume was chosen to ensure that sufficient template was available for the amplification reaction; smaller volumes lead to limiting dilution of templates. Peltier-based thermocycling was at 94°C for 5 minutes; 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds; and a final extension of 72°C for 10 minutes.
Forward and reverse primer sequences to amplify hybrid transcripts with the MB4-2 (438 bp) and MB4-3 (275 bp) breakpoints5,11 were as follows: Iu1, 5′-AGCCCTTGTTAATGGACTTG-3′; and ms6r, 5′-CCTCAATTTCCCTGAAATTGGTT-3′. To detect a 343-bp product for MB4-1, the Iu1 primer was paired with the ME3 primer in MMSET exon 3: 5′-AGCTTGTCGGCTGGAATAAA-3′. The Iu1 primer was tagged with VIC (ABI, Foster City, CA). Products from representative on-chip reactions were sequenced to confirm the identity of the PCR products detected by on microchip-based CE.
Single-Cell PCR On-Chip
Individual BM plasma cells from t(4;14) myeloma were sorted into PCR tubes containing 8 μl of direct lysis buffer as previously described,36 followed by reverse transcription, precipitation of the cDNA, redissolving the DNA pellet in 3 μl of PCR mixture, and transferring 2 μl to the sample reservoir of the PCR chip for 35 thermal cycles. The product was then mixed with a size standard (GS500) off-chip and transferred to a glass chip for CE in a denaturing separation matrix.
Reverse Transcription On-Chip
The RT protocol for two-step RT-PCR involved mixing 1 μg of RNA to 1 μl of dT15 primer (10 μmol/L) and adding water to reach a 12-μl volume. This mixture was held at 70°C for 10 minutes for primer annealing and then held at 4°C. To this mixture, 4 μl of 5× buffer, 2 μl of dithiothreitol (0.1 mol/L), 1 μl of SuperScript II enzyme (Invitrogen Life Technology), and 1 μl of dNTP (10 mmol/L) were added to make up a total volume of 20 μl. Two microliters of this mixture was introduced to each on-chip RT well. The thermal cycling conditions were 42°C for 60 minutes and 94°C for 3 minutes.
Single-step RT-PCR reactions were prepared in a total volume of 50 μl. The mixture included 25 μl of 2× reaction mixture (a buffer containing 0.4 mmol/L of each dNTP and 2.4 mmol/L MgSO4) and 1 μl of the enzyme mixture comprising SuperScript III RT and high-fidelity Platinum Taq polymerase (Invitrogen Life Technology). One microliter of each forward and reverse primer (10 μmol/L), 1 μg of RNA template, and double-distilled water were added to a final volume of 50 μl. Two microliters of this mixture was introduced into each RT-PCR well. Thermal cycling conditions were 45°C for 30 minutes; 94°C for 2 minutes; 38 cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 68°C for 30 seconds; and a final extension time of 7 minutes at 68°C.
Electrophoretic Detection of Fluorescently Tagged PCR Products On-Chip
PCR products were identified and sized using microchip capillary electrophoresis39 as described previously.18 On-chip PCR products were also analyzed using DNA fragment analysis in a 16-capillary ABI3100 DNA analysis system (Applied Biosystems, Foster City, CA) as the conventional standard46,47 using different dilutions of fluorescent product (none, 1/4, 1/10, and 1/100) as indicated in Results.
The microchip CE channels were filled with POP6 polymer, a denaturing sieving matrix. Polymer was heated for 10 minutes at 67°C before being loaded into the microchip to reduce viscosity to facilitate loading on the chip.48 In the sample loading well of the CE chip (volume of 3 μl) or the CE section of the PCR-CE chip, the on-chip PCR product was denatured for 4 minutes at 96°C, rapidly cooled to ∼4°C, and mixed with 1.2 μl of HiDi formamide (ABI), 1 μl of size standard (GS500), and 0.8 μl of 1× genetic analysis buffer with ethylenediamine tetraacetic acid to a total volume of 3 μl. A mix of 1 μl of POP6 and 2 μl of 1× genetic analysis buffer with ethylenediamine tetraacetic acid was loaded in the separation wells. The injection voltage of 400 V was applied, and separation voltage was 6 kV.
Results
On-Chip PCR Consistently Detects IgH-MMSET Hybrid Transcripts
For the first stage of this work, PCR was performed using cDNA from BM of myeloma patients having the t(4;14) translocation. The use of cellular cDNA, compared with our previous work using high-copy viral DNA,18 required modification of PCR conditions. With only moderate copy numbers, reaction efficiency is reduced because of potential surface adsorption of template and PCR reagents.49 Bovine serum albumin was added at an optimal concentration (1 mg/ml) to reduce the surface-related effects and was coupled with an increased concentration of Taq polymerase and dNTPs. With these modifications, the on-chip PCR reaction was consistently positive, and negative controls were consistently negative.
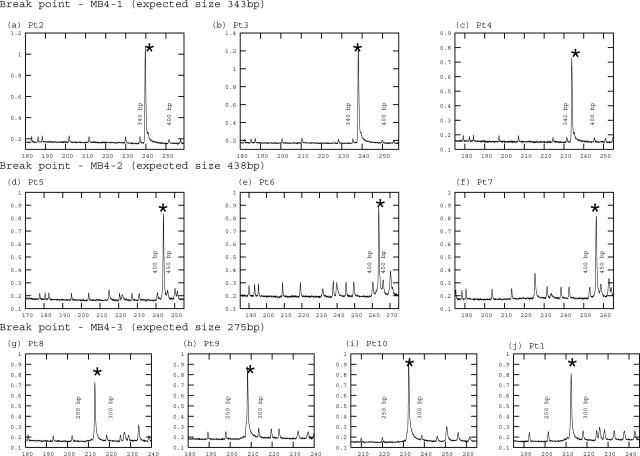
BM from 10 patients with t(4;14) MM, including the three different translocation breakpoints (MB4-1, MB4-2, and MB4-3), were tested on-chip (Figure 1, a–j) in three or more independent PCR reactions. Detectable product was amplified from cDNA of ex vivo MM BM cells for all of the patients. For all 10 patients, the on-chip PCR on PDMS/glass chips was reproducible from run to run in a total of more than 40 runs. PCR product was sized after mixing with fluorescent size standard (GS500), with separation using glass CE chips within the microfluidic tool kit (μTK) and by DNA fragment analysis on the ABI3100 (the standard), with parallel aliquots of the same PCR product from individual on-chip wells. The presence of product was further confirmed by acrylamide gel electrophoresis using pooled PCR product from two to four chips (data not shown). Sequencing of the on-chip PCR product confirmed that the correct product had been amplified.
Figure 1.
On-chip amplification of t(4;14) IgH-MMSET hybrid transcripts from patients with different translocation breakpoints. Both PCR product and the size standards (GS500) were fluorescently labeled (VIC). PCR using cDNA was performed in a three-well PCR chip. CE was performed after manually transferring the PCR product to a glass CE chip. Electropherograms were generated in a μTK. The PCR product is indicated by an asterisk. a–c: On-chip PCR of MB4-1 breakpoints (343 bp): cDNA from different patients having MB4-1 breakpoints were amplified on-chip, and a 1:4 dilution of the product was analyzed by microchip CE. d–f: On-chip PCR of MB4-2 breakpoints (438 bp): cDNA from three patients having MB4-2 breakpoints were amplified on-chip, and a 1:4 dilution of the product was analyzed on a μTK. g–j: On-chip PCR of MB4-3 breakpoints (275 bp): cDNA from four patients having MB4-2 breakpoints were amplified on-chip, and a 1:4 dilution of the product was analyzed on a μTK. The on-chip PCR products from reactions included in a though c were also detected at the appropriate size on the ABI3100 capillary analysis system (not shown).
On-Chip CE to Detect PCR Product from Individual t(4;14)-Positive Myeloma Cells
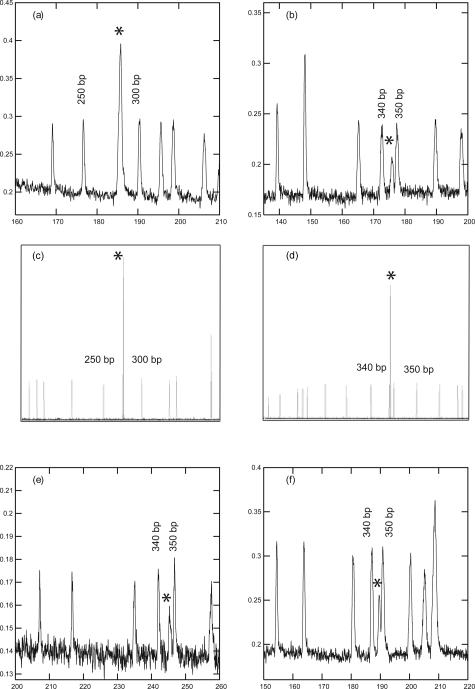
A series of individual t(4;14)-positive myeloma plasma cells (PCs) were sorted into PCR tubes containing direct lysis buffer and subjected to RT-PCR using a conventional thermocycler. Approximately 50 pl of the amplified PCR product localized in the intersection of a glass CE chip was then analyzed and compared with detection of the same product using the ABI3100. For one set of RT-PCR reactions, the PCR product was first concentrated by precipitation, followed by CE using a glass microfluidic CE chip (Figure 2a). For another series of individual cells, 50 pl of the conventionally amplified, unconcentrated RT-PCR product was directly analyzed using on-chip CE (without the precipitation step) (Figure 2b). Representative separations of products amplified from individual cells are shown. Both strategies show a microchip detection sensitivity comparable with that observed for clonotypic IgH VDJ transcripts from individual MM PCs39 and comparable with that seen for conventional DNA fragment analysis as measured using the ABI3100 (Figure 2, c and d).
Figure 2.
On-chip CE and on-chip PCR/CE of cDNA detects hybrid transcripts from individual t(4;14) plasma cells. The PCR product is indicated by an asterisk. a and b: PCR product from cDNA of an individual plasma cell after amplification using a conventional in-tube system and analysis using a glass CE chip. The electropherogram in a is from an MB4-3 patient (275 bp) with PCR products that have been precipitated and concentrated before analysis. The electropherogram in b is from an MB4-1 patient (343 bp) with direct analysis of unconcentrated PCR product. c represents an aliquot of concentrated PCR product from PCR reaction a and d represents an aliquot of unconcentrated PCR product from PCR reaction b, both run on the ABI3100. e and f: On-chip PCR of cDNA from a single plasma cell, with manual transfer of PCR product to a glass CE chip after mixing with size standard and electropherograms generated on a μTK. This representative plasma cell had an MB4-1 breakpoint. e: Analysis of a 50-pl injection plug. f: Analysis of a 250-pl injection plug. The plug volume is defined by the injection time, before the separation.
On-Chip PCR of cDNA from Individual t(4;14)-Positive MM PCs
Any given cancer, including MM, is highly heterogeneous. However, single-cell analysis using a conventional approach36,46 is too difficult for routine clinical use. Amplification of hybrid transcripts from the cDNA of single PCs was performed on-chip, using individual plasma cells that had been sorted into PCR tubes and subjected to off-chip reverse transcription with precipitation of the cDNA to a 1-μl volume. Figure 2, e and f, shows the PCR product amplified on-chip with 35 cycles of PCR. To perform sample (DNA) pre-concentration and signal strength enhancement during CE, two injection protocols were followed for the product detection, in which either a single injection plug consisting of ∼50 pl (Figure 2e) or a second analysis of a longer injection plug (5 additional seconds, translating to ∼250-pl injection plug) (Figure 2f), were analyzed by CE, as previously described.39 In both cases, a PCR product of the correct size was amplified on-chip from the cDNA of one cell.
On-Chip RT-PCR Detects Hybrid Transcripts in MM PCs
To realize a complete analysis system, during the development toward integrated RT-PCR-CE, we first integrated PCR with CE on a single microchip. Using cDNA prepared by conventional means followed by on-chip PCR and detection, tests were performed in duplicate for two different patients with different breakpoints (data not shown). PCR was in a 2-μl reaction volume, appropriately miniaturized to ensure that transcript copy numbers in ex vivo cells would be detectable. This volume could be reduced considerably by including a nucleic acid purification step (in preparation). PCR product was efficiently amplified and detected using conditions that had been earlier established for the two-chip system described in Figure 1, indicating that the integrated chip is able to match the conventional standard of PCR amplification in a ABI9700 thermocycler with detection on the ABI3100 capillary system. The detection of product by CE on the integrated PDMS/glass chip was comparable with that on a separate glass-based CE chip.
To achieve higher levels of integration and to minimize potential for contamination and reduce the total analysis time, RT was subsequently integrated on the PCR-CE platform. On-chip RT-PCR was performed initially as a two-step process with reverse transcription of total RNA to cDNA in the first well and PCR in the second well. Once optimized, the RT-PCR protocol was adapted to seamlessly integrate the functionality of RT and PCR as a single-step reaction, without the need to change reagents. Two-microliter reactions were used for detection of these moderate copy number transcripts. This one-step RT-PCR protocol was then transferred onto the PCR-CE integrated microchip (Figure 3c)to detect t(4;14) hybrid transcripts. On-chip RT-PCR was performed on total RNA from BM of a patient having the MB4-3 breakpoint, yielding a product of 275 bp (Figure 3b). The predicted product sizing was verified on the ABI3100 system (Figure 3a).
Figure 3.
Integrated on-chip reverse transcription/PCR/CE detects hybrid transcripts from RNA isolated from t(4;14) plasma cells. RNA was purified from bone marrow cells of a patient with t(4;14) myeloma (MB4-3 breakpoint) for on-chip RT-PCR-CE (b). The results are representative of those from two different patients. RNA was mixed with the required reagents, applied to the injection well of a microfluidic chip, and followed by a reverse transcription step, PCR amplification, and product detection using CE, with all three steps integrated on the same chip (c). After thermal cycling, the PCR product was moved into the sample input well of the CE section of the chip using the external micropumps, and size standard was introduced. Approximately 50 pl of PCR product and size standard were then electrokinetically injected followed by product/size standard electrophoretic separation. Before initiating the CE, an aliquot of the on-chip product was removed from the PCR exit well, and the product size confirmed using the ABI3100 (a). The top panel shows the on-chip RT-PCR-CE, and the bottom panel shows an aliquot of the same on-chip RT-PCR reaction run on the ABI3100.
Sensitivity of the Chip-Based Assay for Detection of Hybrid Transcripts
For clinical usage, it is essential to determine the sensitivity of the on-chip t(4;14) detection assay relative to the conventional thermocycler-based PCR and product detection techniques. Sorted t(4;14)-positive KMS-18 cells were spiked into normal patient blood samples at known concentrations as indicated in Table 1. Using the cDNA from this mixture, on-chip PCR and CE were performed (in duplicate, unless otherwise indicated) with analysis on the μTK. The chip PCR results were compared with PCR product amplified by conventional thermal cycling and detected using a 2% agarose gel or the ABI3100. Two independently prepared KMS-18 dilution series were tested with all three detection methods. The ABI3100 consistently detected PCR product in with as few as 0.001% spiked KMS-18 cells (1/100,000). Gel electrophoresis consistently detected as few as 0.01% KMS-18 (1/10,000). For the chip-based assay, PCR product was consistently detected with as few as 0.1% KMS-18 cells (1/1000). For dilutions between 1/2000 and 1/10,000, the chip-based assay scored positive for one dilution series but not for the other. For dilutions of less than in 1/10,000, the chip-based assay was unable to detect hybrid transcripts from the spiked t(4;14)-positive cells. This is likely to reflect adsorption of reaction components (PCR template, reagents, and/or PCR product) on the chip surfaces, suggesting that appropriate surface coatings are needed to minimize potential surface interactions.
Table 1.
Sensitivity of the On-Chip t(4;14) PCR
| Thermal cycling | Analysis | Trial | 100% | 10% | 1% | 0.1% | 0.05% | 0.01% | 0.005% | 0.001% | 0.0005% | 0.0001% | Negative control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional thermal cycler | 2% Agarose gel | a | Y | Y | Y | Y | Y | Y | W | N | N | N | N |
| b | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | ||
| ABI 3100 | a | Y | Y | Y | Y | Y | Y | Y | Y | N | N | N | |
| b | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | N | ||
| On-chip | μTK | a | Y | Y | Y | Y | Y | Y | N | N | ND | ND | N |
| b | Y | Y | Y | Y | N | N | N | N | ND | ND | N |
KMS-18 cell lines, which express t(4;14) hybrid transcripts, were spiked into normal blood samples at the percentages indicated to prepare two independently constructed dilution series (a and b). On-chip PCR was performed in duplicate, using cDNA in a PCR chip followed by transfer to a glass CE chip for CE. The results were compared with the amplification performed by conventional thermal cycling with analysis using a 2% agarose gel or using capillary separation on the ABI3100.
Y, product detected; N, no product detected; W, weak product; ND, not done.
Sequential Monitoring of t(4;14) over the Course of Treatment
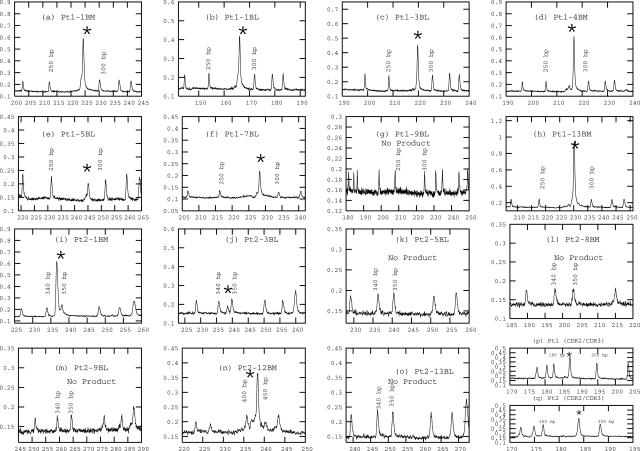
Two patients (1 and 2) were monitored over a 13-month period that included samples taken at diagnosis and throughout treatment. BM samples were taken at diagnosis and relapse; blood samples were collected on monthly clinic visits. The malignant MM clone in blood and BM was monitored using the chip-based system (Figure 4) and additionally using the gel electrophoresis and ABI3100 detection methods (Table 2). As a second biomarker to identify malignant cells, the clonotypic IgH VDJ rearrangement, a unique molecular signature for the MM clone in each individual patient,36 was detected with primers specific to the complementarity determining regions (CDR2 and CDR3) of the clonotypic IgH VDJ for each individual patient (representative electropherogram shown in Figure 4, p and q, for patients 1 and 2, as previously described).40 All samples were tested for both clonotypic and t(4;14) hybrid transcripts (Table 2). Negative control reactions were consistently negative for both conventional and chip reactions. The same pattern of detection was seen conventionally and on-chip with the sole exception of sample 3BL for patient 2, where hybrid transcript was consistently detected on-chip but not using conventional systems. Although the results for Table 1 show a lesser sensitivity for the chip-based test in detecting hybrid transcripts in a dilution series made using a cell line, the experiment of Table 2 uses ex vivo patient cells, which may have higher transcript copy numbers, possibly explaining the detection of t(4;14) on-chip but not using conventional methods for 3BL of patient 2. Because the negative controls were negative, thus excluding contaminants, and the product was reproducibly detected in replicate runs, this observation may indicate the potential of the chip to be more sensitive under appropriate conditions. For both systems, the testing revealed an interesting but unexplained change in the nature of the t(4;14) translocation in patient 2, confirming the ability of the chip system to detect unexpected events. For both the ABI3100 and on-chip testing of patient 2 12-BM, a verifiable MB4-1 product was absent, but MB4-2 primers amplified a product with the predicted size for an MB4-2 hybrid transcript (Table 2). The identity of the product as MB4-2 was confirmed by sequencing (not shown). Thus, testing on-chips was able to clearly detect an important biological change in the chromosomal abnormalities of this malignant clone. These experiments confirm that on-chip analysis has specificity and versatility comparable with that of conventional platforms, even though the current chip platform requires further optimization to match or exceed the sensitivity of conventional approaches.
Figure 4.
Sequential monitoring of t(4;14) hybrid transcripts over the course of treatment. For two patients, sequential samples were analyzed on-chip at diagnosis and during the course of treatment, including BM and blood (BL) samples, as indicated in the figure (a–o), showing a pattern of detection that is concordant with conventional testing. The PCR product is indicated by an asterisk. Note the change in breakpoint shown in n. cDNA from each sample was amplified on a PCR chip, followed by CE using glass CE chips in a μTK. Representative analyses of the patient-specific IgH signature, amplified by primers specific for the CDR2 and CDR3 of the myeloma clone in each patient, are shown in p and q. This analysis was done for every sample as detailed in Table 2.
Table 2.
Sequential Monitoring of Patient Samples to Detect Cancer Cells with the t(4;14) Translocation and the Clonotypic IgH VDJ Signature and Detection of Molecular Changes in Breakpoint Type
| Patient | Patient visit to the clinic | Analysis methods
|
|||||
|---|---|---|---|---|---|---|---|
| 2% Agarose gel
|
ABI 3100
|
On-chip
|
|||||
| Iu1/ms6r | CDR2/CDR3 | Iu1/ms6r | CDR2/CDR3 | Iu1/ms6r | CDR2/3 | ||
| 1 | 1-BM | + (MB4-3) | + (177 bp) | + (MB4-3) | + (177 bp) | + (MB4-3) | + (177 bp) |
| 1-BL | + | + | + | + | + | + | |
| 3-BL | + | + | + | + | + | + | |
| 4-BM | + | + | + | + | + | + | |
| 5-BL | + | + | + | + | + | + | |
| 7-BL | + | + | + | + | + | + | |
| 9-BL | − | − | − | − | − | − | |
| 13-BM | + | + | + | + | + | + | |
| 13-BL | + | + | + | + | + | + | |
| 2 | 1-BM | + (MB4-1) | + (168 bp) | +(MB4-1) | +(168 bp) | + (MB4-1) | + (168 bp) |
| 3-BL | − | + | − | + | + | + | |
| 5-BL | − | + | − | + | − | + | |
| 8-BM | − | + | − | + | − | + | |
| 9-BL | − | Faint | − | − | − | ||
| 12-BM | + (MB4-2) | + | + (MB4-2) | + | + (MB4-2) | + | |
| 13-BL | − | + | − | + | − | + | |
Column headings indicate the primer pairs used for each amplification reaction. The primers for CDR2/CDR3 region were specific for the clonotypic IgH VDJ of each patient. Their specificity has been verified elsewhere. BM, bone marrow sample. BL, peripheral blood sample. If product was amplified, the result is recorded in this table as +, and if negative, as −. Values in parentheses indicate product size and, where applicable, chromosomal breakpoint type.
Discussion
The microfluidic platforms developed here use novel technology suitable for personalized biomarker screening of MM patients, enabling predictions of risk and stratification for treatment, based on the genetic signature of each cancer. This inexpensive and multifunctional characterization tool that integrates PCR/RT-PCR with detection of product is likely to enable widespread screening of patients, to improve prognostic predictions, and to inform therapeutic decision-making. As a tool for providing a yes/no assessment of the t(4;14) translocation status of a given patient, this miniaturized microfluidic platform already matches conventional gold standard methods in accuracy and specificity. Even with the prototype systems described here, chip-based testing uses reduced reagent levels and, for testing of individual patients (compared with batch processing), offers a savings in cost and time. For diagnostic and monitoring purposes, the ability to test patients one at a time is clinically important. Currently, conventional testing using inexpensive acrylamide gel separation consumes about $10 in reagent costs per sample and about $125 in labor, whether one or multiple samples are analyzed. Although it is premature to make precise estimates, chip testing consumes about $10 in reagents plus labor, and product detection takes seconds rather than hours. Ultimately, for low-cost diagnostics and monitoring, it is likely that the chips used would be manufactured by a low-cost injection molding (or similar) method, with even greater cost savings.
The work reported here shows that at least five different primer sets [three different primer sets detecting t(4;14) breakpoints and two different primer sets detecting clonotypic VDJ transcripts] are readily interchangeable on the same chip using exactly the same protocols, confirming the versatility of chip and the system. The potential exists for more quantitative assessment of tumor burden using microfluidic chips, as recently demonstrated with comparable chips for detecting shed virus in the urine of organ transplant recipients.18 Although estimation of cell equivalents is not possible with an RT-PCR assay because of heterogeneity in transcript expression levels, real-time quantitative PCR is currently being implemented on-chip, holding promise for more precise evaluation of tumor burden. We are currently developing strategies to perform analysis directly from whole blood, thereby integrating sample processing with automated RT-PCR-CE on a single microchip, to realize the full benefit of miniaturization and automation. Cancer is highly heterogeneous, but single-cell analysis using conventional approaches is problematic for clinical laboratories. Although we have shown here and elsewhere39 that single-cell analysis is feasible on-chip, this would be time-consuming and laborious for large numbers of cells. We are currently developing capabilities for simultaneous analysis of thousands of individual cells. The ability to quickly and inexpensively monitor the response of patients to therapy as evaluated by monitoring the malignant clone, in real time at every clinic visit, would facilitate an optimally informed choice of the most effective therapy for patients who respond poorly to frequently used conventional treatments and would confer the ability to modify rapidly the treatment strategies when the genetic characteristics of the tumor change (as shown for patient 2). Use of microfluidic technology to monitor tumor burden is likely to change the way clinical trials are performed and the kind of response parameters that can affordably be collected by enabling frequent testing of, for example, both blood and BM in longitudinal monitoring of the malignant clone. Real-time on-chip detection of complex genetic abnormalities will allow sensitive detection of emerging aggressive variants as disease progresses and clinical assessment of individuals at risk of transforming to overt malignancy. For the effective use of microfluidic technology to screen cancer biomarkers, many of the functional components demonstrated here could be useful individually or as integrated systems. Ultimately, using on-chip sample processing and accelerated thermal cycling (in preparation) on highly integrated platforms with readily available and frequent screening for therapeutically important biomarkers will enable custom-tailored therapies to target the genetic vulnerabilities of the cancer cells in each individual patient, using point-of-care monitoring tools.
Acknowledgments
We thank Erin Strachan, who provided valuable technical assistance. The Nanofab at the University of Alberta and Paul Dumais assisted in the microfluidic chip development.
Footnotes
Supported by the Canadian Institutes of Health Research, the Natural Science and Engineering Research Council, Western Economic Diversification, and the Canada Research Chair Program. L.M.P. is Canada Research Chair in Biomedical Nanotechnology. J.V. was supported by Natural Science and Engineering Research Council and Alberta Heritage Foundation for Medical Research. G.K. was funded by the Canadian Institutes of Health Research Translational Research Training Program in the Department of Oncology and the National Institute of Nanotechnology. S.A. was funded by Alberta Heritage Foundation for Medical Research and National Institute of Nanotechnology.
J.V. and G.V.K. contributed equally to this work.
References
- Keats JJ, Maxwell CA, Taylor BJ, Hendzel MJ, Chesi M, Bergsagel PL, Larratt LM, Mant MJ, Belch AR, Pilarski LM. Overexpression of transcripts originating from the MMSET Locus characterizes all t(4;14)(p16;q32) positive multiple myeloma patients. Blood. 2005;105:4060–4069. doi: 10.1182/blood-2004-09-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsagel PL, Chesi M, Nardini E, Brents LA, Kirby SL, Kuehl WM. Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc Natl Acad Sci USA. 1996;93:13931–13936. doi: 10.1073/pnas.93.24.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelda R, Ronchetti D, Baldini L, Cro L, Viggiano L, Marzella R, Rocchi M, Otsuki T, Lombardi L, Maiolo AT. A novel chromosomal translocation t(4;14)(p16.3;q32) in multiple myeloma involves the fibroblast growth factor receptor gene. Blood. 1997;90:4062–4070. [PubMed] [Google Scholar]
- Chesi M, Nardini E, Lim RS, Smith KD, Kuehl M, Bergsagel PL. The t(4;14) translocation in myeloma dysregulates both FGFR3 and a novel gene, MMSET, resulting in IgH/MMSET hybrid transcripts. Blood. 1998;92:3025–3034. [PubMed] [Google Scholar]
- Keats JJ, Reiman T, Maxwell CA, Taylor BJ, Larratt LM, Mant MJ, Belch AR, Pilarski LM. In multiple myeloma, t(4;14)(p16;q32) is an adverse prognostic factor irrespective of FGFR3 expression. Blood. 2003;101:1520–1529. doi: 10.1182/blood-2002-06-1675. [DOI] [PubMed] [Google Scholar]
- Winkler JM, Greipp P, Fonseca R. t(4;14)(p16.3;q32) is strongly associated with a shorter survival in myeloma patients. Br J Haematol. 2003;120:170–171. doi: 10.1046/j.1365-2141.2003.03983_5.x. [DOI] [PubMed] [Google Scholar]
- Moreau P, Facon T, Leleu X, Morineau N, Huyghe P, Harousseau JL, Bataille R, Avet-Loiseau H. Recurrent 14q32 translocations determine the prognosis of multiple myeloma, especially in patients receiving intensive chemotherapy. Blood. 2002;100:1579–1583. doi: 10.1182/blood-2002-03-0749. [DOI] [PubMed] [Google Scholar]
- Jaksic W, Trudel S, Chang H, Trieu Y, Qi X, Mikhael J, Reece D, Chen C, Stewart AK. Clinical outcomes in t(4;14) multiple myeloma: a chemotherapy-sensitive disease characterized by rapid relapse and alkylating agent resistance. J Clin Oncol. 2005;23:7069–7073. doi: 10.1200/JCO.2005.17.129. [DOI] [PubMed] [Google Scholar]
- Finelli P, Fabris S, Zagano S, Baldini L, Intini D, Nobili L, Lombardi L, Maiolo AT, Neri A. Detection of t(4;14)(p16.4;q32) chromosomal translocations in multiple myeloma by double color fluorescent in situ hybridization. Blood. 1999;94:724–732. [PubMed] [Google Scholar]
- Keats JJ, Strachan E, Belch AR, Pilarski LM. The classical illegitimate switch translocation model Is unable to account for at least half of the translocation breakpoints from t(4;14)(p16;q32) multiple myeloma (Abstract). Blood. 2005;106:446A. [Google Scholar]
- Malgeri U, Baldini L, Perfetti V, Fabris S, Vignarelli MC, Colombo G, Lotti V, Compasso S, Bogni S, Lombardi L, Maiolo AT, Neri A. Detection of t(4;14)(p16.3;q32) chromosomal translocation in multiple myeloma by reverse transcription-polymerase chain reaction analysis of IGH-MMSET fusion transcripts. Cancer Res. 2000;60:4058–4061. [PubMed] [Google Scholar]
- Ferrance JP, Wu Q, Giordano B, Hernandez C, Kwok Y, Snow K, Thibodeau S, Landers JP. Developments towards a complete micro-total analysis system for Duchenne muscular dystrophy diagnosis. Anal Chim Acta. 2003;500:223–236. [Google Scholar]
- Verpoorte E. Microfluidic chips for clinical and forensic analysis. Electrophoresis. 2002;23:677–712. doi: 10.1002/1522-2683(200203)23:5<677::AID-ELPS677>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Lin YC, Huang MY, Young KC, Chang TT, Wu CY. A rapid micro-polymerase chain reaction system for hepatitis C virus amplification. Sens Actuators B Chem. 2000;71:2–8. [Google Scholar]
- Cho Y, Kim J, Lee Y, Kim Y, Namkoong K, Lim H, Oh K, Kim S, Han J, Park C, Pak YE, Ki C, Choi JR, Myeong H, Ko C. Clinical evaluation of micro-scale chip-based PCR system for rapid detection of hepatitis B virus. Biosens Bioelectron. 2006;21:2161–2169. doi: 10.1016/j.bios.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Liao C, Lee G, Liu H, Hsieh T, Luo C. Miniature RT-PCR system for diagnosis of RNA-based viruses. Nucleic Acids Res. 2005;33:e156. doi: 10.1093/nar/gni157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Yang M, Lin R, Johnson BN, Srivastava N, Razzacki SZ, Chomistek KJ, Heldsinger DC, Haque RM, Ugaz VM, Thwar PK, Chen Z, Alfrano K, Yim MB, Krishnana M, Fuller AO, Larson RG, Burke DT, Burns MA. An integrated microfluidic device for influenza and other genetic analyses. Lab Chip. 2005;5:1024–1032. doi: 10.1039/b505994a. [DOI] [PubMed] [Google Scholar]
- Kaigala GV, Huskins RJ, Preiksaitis J, Pang XL, Pilarski LM, Backhouse CJ. Automated surveillance using microfluidic chip-based PCR thermocycling and product detection to assess risk of polyoma BK virus associated neuropathy in renal transplant recipients. Electrophoresis. 2006;27:3753–3763. doi: 10.1002/elps.200600061. [DOI] [PubMed] [Google Scholar]
- Gulliksen A, Solli LA, Drese KS, Sorensen O, Karlsen F, Rogne H, Hovig E, Sirevag R. Parallel nanoliter detection of cancer markers using polymer microchips. Lab Chip. 2005;5:416–420. doi: 10.1039/b415525d. [DOI] [PubMed] [Google Scholar]
- Waters LC, Jacobson SC, Kroutchinina N, Khandurina J, Foote RS, Ramsey JM. Multiple sample PCR amplification and electrophoretic analysis on a microchip. Anal Chem. 1998;70:5172–5176. doi: 10.1021/ac980447e. [DOI] [PubMed] [Google Scholar]
- Liu J, Enzelberger M, Quake S. A nanoliter rotary device for polymerase chain reaction. Electrophoresis. 2002;23:1531–1536. doi: 10.1002/1522-2683(200205)23:10<1531::AID-ELPS1531>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Liu J, Hansen C, Quake SR. Solving the “world-to-chip” interface problem with a microfluidic matrix. Anal Chem. 2003;75:4718–4723. doi: 10.1021/ac0346407. [DOI] [PubMed] [Google Scholar]
- Hong JW, Fujii T, Seki M, Yamamoto T, Endo I. Integration of gene amplification and capillary gel electrophoresis on a polydimethylsiloxane-glass hybrid microchip. Electrophoresis. 2001;22:328–333. doi: 10.1002/1522-2683(200101)22:2<328::AID-ELPS328>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Roper MG, Easley CJ, Landers JP. Advances in polymerase chain reaction on microfludic chips. Anal Chem. 2005;77:3887–3894. doi: 10.1021/ac050756m. [DOI] [PubMed] [Google Scholar]
- Ibrahim MS, Lofts RS, Jahrling PB. Real-time microchip PCR for detecting single-base differences in viral and human DNA. Anal Chem. 1998;70:2013–2017. doi: 10.1021/ac971091u. [DOI] [PubMed] [Google Scholar]
- Burns MA, Johnson BN, Brahmasandra SN, Handique K, Webster JR, Kroishnan M, Sammarco TS, Man PM, Jones D, Heldsinger DC, Mastrangelo CH, Burke DT. An integrated nanoliter DNA analysis device. Science. 1998;282:484–487. doi: 10.1126/science.282.5388.484. [DOI] [PubMed] [Google Scholar]
- Gascoyne P, Satayavivad J, Ruchirawad M. Microfluidic approaches to malaria detection. Acta Tropica. 2004;89:357–369. doi: 10.1016/j.actatropica.2003.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JS, Anderson WF, Quake SR. Parallel picoliter RT-PCR assays using microfluidics. Anal Chem. 2006;78:956–958. doi: 10.1021/ac0513865. [DOI] [PubMed] [Google Scholar]
- Khandurina J, McKnight TE, Jacobson SC, Waters LC, Foote RS, Ramsey JM. Integrated system for rapid PCR-based DNA analysis in microfluidic devices. Anal Chem. 2000;72:2995–3000. doi: 10.1021/ac991471a. [DOI] [PubMed] [Google Scholar]
- Waters LC, Jacobson SC, Kroutchinina N, Khandurina J, Foote RS, Ramsey JM. Microchip device for cell lysis, multiplex PCR amplification, and electrophoretic sizing. Anal Chem. 1998;70:158–162. doi: 10.1021/ac970642d. [DOI] [PubMed] [Google Scholar]
- Woolley AT, Hadley D, Landre P, deMello AJ, Mathies RA, Northrup MA. Functional integration of PCR amplification and capillary electrophoresis in a microfabricated DNA analysis device. Anal Chem. 1996;68:4081–4086. doi: 10.1021/ac960718q. [DOI] [PubMed] [Google Scholar]
- Lagally ET, Scherer JR, Blazej RG, Toriello NM, Diep BA, Ramchandani M, Sensabaugh GF, Riley LW, Mathies RA. Integrated portable genetic analysis microsystem for pathogen/infectious disease detection. Anal Chem. 2004;76:3162–3170. doi: 10.1021/ac035310p. [DOI] [PubMed] [Google Scholar]
- Easley CJ, Karlinsey JM, Landers JP. On-chip pressure injection for integration of infrared-mediated DNA amplification with electrophoretic separation. Lab Chip. 2006;6:601–610. doi: 10.1039/b600039h. [DOI] [PubMed] [Google Scholar]
- Koh CG, Tan W, Zhao MQ, Ricco AJ, Fan ZH. Integrating polymerase chain reaction, valving, and electrophoresis in a plastic device for bacterial detection. Anal Chem. 2003;75:4591–4598. doi: 10.1021/ac0343836. [DOI] [PubMed] [Google Scholar]
- Obeid PJ, Christopoulos TK, Crabtree HJ, Backhouse CJ. Microfabricated device for DNA and RNA amplification by continuous-flow polymerase chain reaction and reverse transcription-polymerase chain reaction with cycle number selection. Anal Chem. 2003;75:288–295. doi: 10.1021/ac0260239. [DOI] [PubMed] [Google Scholar]
- Szczepek AJ, Seeberger K, Wizniak J, Mant MJ, Belch AR, Pilarski LM. A high frequency of circulating B cells share clonotypic Ig heavy-chain VDJ rearrangements with autologous bone marrow plasma cells in multiple myeloma, as measured by single-cell and in situ reverse transcriptase-polymerase chain reaction. Blood. 1998;92:2844–2855. [PubMed] [Google Scholar]
- Anonymous Going single, but not alone (Editorial). Nat Methods. 2006;3:581. doi: 10.1038/nmeth0806-581. [DOI] [PubMed] [Google Scholar]
- Pilarski PM, Adamia S, Backhouse CJ. An adaptable microvalving system for on-chip polymerase chain reactions. J Immunol Methods. 2005;305:48–58. doi: 10.1016/j.jim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Pilarski LM, Lauzon J, Strachan E, Adamia S, Atrazhev A, Belch AR, Backhouse CJ. Sensitive detection using microfluidics technology of single cell PCR products from high and low abundance IgH VDJ templates in multiple myeloma. J Immunol Methods. 2005;305:94–105. doi: 10.1016/j.jim.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Pilarski LM, Kaigala G, Adamia S, Chowdhury J, Huskins R, Lauzon J, Preiksaitas J, Belch AR, Backhouse CJ. Microfluidic devices for cancer, infectious disease and pharmacogenetics (Abstract). Transfus Med. 2005;15:337–367. [Google Scholar]
- Footz T, Somerville MJ, Tomaszewski R, Elyas B, Backhouse CJ. Integration of combined heteroduplex/restriction fragment length polymorphism analysis on an electrophoresis microchip for the detection of hereditary haemochromatosis. Analyst. 2004;129:25–31. doi: 10.1039/b309931h. [DOI] [PubMed] [Google Scholar]
- Manage DP, Zheng Y, Somerville MJ, Backhouse CJ. On-chip HA/SSCP for the detection of hereditary haemochromatosis. Micro Nanofluidics. 2005;1:364–372. [Google Scholar]
- Vahedi G, Kaler K, Backhouse CJ. An integrated method for mutation detection using on-chip sample preparation, single stranded conformation polymorphism, and heteroduplex analysis. Electrophoresis. 2004;25:2346–2356. doi: 10.1002/elps.200405957. [DOI] [PubMed] [Google Scholar]
- Ma R, Crabtree HJ, Backhouse CJ. A rejuvenation method for poly(N,N-dimethlacrylamide)-coated glass microfluidic chips. Electrophoresis. 2005;26:2692–2700. doi: 10.1002/elps.200410418. [DOI] [PubMed] [Google Scholar]
- Ma R, Kaler K, Backhouse CJ. A rapid performance assessment method for microfluidic chips. 2004 International Conference on MEMS, Nano, and Smart Systems (ICMENS 2004), 25–27 Aug 2004, Banff, Alberta, Canada. IEEE Computer Society. 2004;1:680–686. [Google Scholar]
- Pilarski LM, Seeberger K, Keats JJ, Taylor BJ, Coupland RW, Belch AR. Leukemic B cells clonally identical to myeloma plasma cells are myelomagenic in NOD SCID mice. Exp Hematol. 2002;30:221–228. doi: 10.1016/s0301-472x(01)00788-3. [DOI] [PubMed] [Google Scholar]
- Adamia S, Reiman T, Crainie M, Belch AR, Pilarski LM. Aberrant splicing of hyaluronan synthase 1 (HAS1) gene in multiple myeloma (MM): HAS1 and novel HAS1 splice variants in MM B cells have an adverse impact on patient survival. Blood. 2003;102:371B. [Google Scholar]
- Backhouse CJ, Caamano M, Oaks F, Nordman E, Carrilo A, Johnson B, Bay S. DNA sequencing in a monolitic microchannel device. Electrophoresis. 2000;21:150–156. doi: 10.1002/(SICI)1522-2683(20000101)21:1<150::AID-ELPS150>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee JN, Park C, Whitesides GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem. 2003;75:6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]