Abstract
Preantral granulosa cells (PAGCs) differentiate into cumulus cells following antrum formation. Cumulus cells, but not PAGCs, are competent to undergo expansion. Experiments reported here tested the respective roles of both oocytes and FSH in the transition of preantral granulosa cells to cumulus cells competent to undergo expansion. PAGC-oocyte complexes were cultured with or without a low dose of FSH (0.005 IU/ml) and isolated PAGCs were cultured with or without oocytes. At the end of culture, complexes or isolated PAGCs were tested for their ability to undergo cumulus expansion and upregulate expansion transcripts in response to EGF or FSH (0.5 IU/ml). The ability to undergo expansion in response to EGF required the presence of oocytes but not FSH during the culture period. Likewise, complexes isolated from the ovaries of hypogonadal mice, which lack circulating gonadotropins, underwent expansion in response to EGF, but not FSH. In contrast, the ability to activate MAPK3/1 and MAPK14 and undergo expansion in response to FSH required prior exposure to low doses of FSH. However, these low levels (0.005 or 0.025 IU FSH/ml) suppressed expression of Slc38a3 and Amh, two transcripts highly expressed in cumulus cells, suggesting opposing effects of FSH on cumulus cell differentiation. In conclusion, the ability to undergo expansion in response to FSH requires prior exposure to FSH during development, while oocyte-derived factors alone are sufficient to promote the ability to undergo expansion in response to EGF. These results highlight the crucial role of oocytes in driving the differentiation of PAGCs into cumulus cells during the preantral to antral follicle transition.
Keywords: Oocyte, preantral granulosa cells, cumulus cells, ovarian follicle
Introduction
The preantral to antral follicle transition is a striking and important step in ovarian follicle development. Antrum formation physically demarcates the former preantral granulosa cells (PAGC) into mural and cumulus granulosa cell populations (Fig. 1). Cumulus cells surround the oocyte and express certain transcripts, such as Slc38a3 (Eppig et al., 2005) and Amh (Salmon et al., 2004) at higher levels than mural cells and define, in part, the cumulus cell phenotype. Cumulus cells undergo a further transition after the preovulatory surge of LH. This surge initiates a cascade of events that leads to cumulus expansion, a defining feature of cumulus cells. Thus, cumulus cell differentiation is manifest as expression of cumulus marker transcripts before the LH surge and the ability to undergo cumulus expansion following the LH surge.
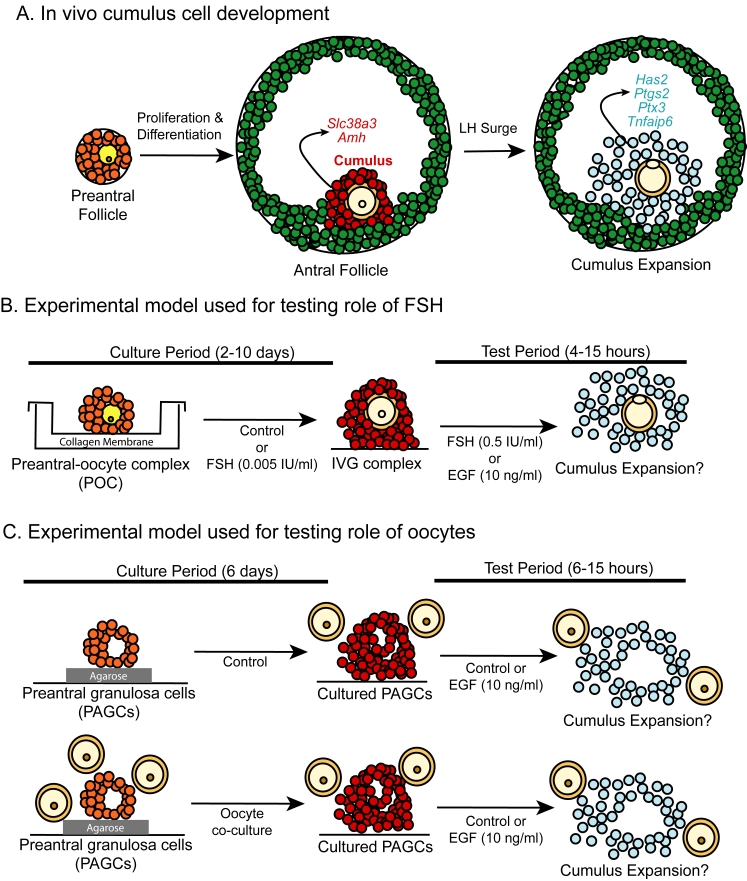
Figure 1.
Model of follicular development and experimental designs. (A) In-vivo follicular growth beginning with preantral follicles that differentiate into antral follicles with two populations of granulosa cells. The mural cells (green) localize to the follicular wall. Cumulus cells (red) surround the oocyte and express cumulus marker transcripts (Slc38a3 and Amh). Following the LH surge, the cumulus oophorus undergoes expansion with increases in expansion-related transcripts (Has2, Ptgs2, Ptx3 and Tnfaip6). Experimental models used to study acquisition of competence to undergo expansion in vitro. (B) PAGC-oocyte complexes were isolated from ovaries of 12 day old mice and cultured on collagen-coated membranes for 2 to 10 days in medium with or without FSH (0.005 IU/ml). Following culture complexes were removed from the membrane and treated with FSH (0.5 IU/ml) or EGF (10 ng/ml) to stimulate pathways leading to cumulus expansion. (C) To determine whether oocytes play a role in the preantral to antral follicle transition, PAGC-oocyte complexes were oocytectomized and cultured for 6 days on a thin layer of agarose either in medium only or co-cultured with fully-grown oocytes (FGO, 1 oocyte/μl). PAGCs co-cultured with oocytes received freshly isolated oocytes every 2 days. Following the culture period, PAGCs were removed from the agarose and treated with control medium or medium containing EGF (10 ng/ml) during the test period. Freshly isolated FGO (2 oocytes/μl) were also added to all treatment groups during the test period.
Expansion of the cumulus oophorous is induced by the preovulatory surge of LH in vivo, or by EGF or FSH treatment in vitro, and involves MAPK3/1-dependent upregulation of Has2, Ptgs2, Ptx3 and Tnfaip6 mRNAs and the production of a mucified matrix by the cumulus cells (Diaz et al., 2006; Su et al., 2003). Each of these transcripts is essential for cumulus expansion since null mutations of Ptgs2, Ptx3, or Tnfaip6 or pharmacological inhibition of HAS2 activity, all result in deficient or defective cumulus expansion (Chen et al., 1993; Fulop et al., 2003; Ochsner et al., 2003a; Ochsner et al., 2003b; Varani et al., 2002). Cumulus-expansion enabling factors (CEEFs) secreted by fully-grown oocytes (FGO) are also required for expansion to occur (Buccione et al., 1990; Diaz et al., 2006; Vanderhyden et al., 1990), underscoring the complex regulation of cumulus expansion.
Preantral granulosa cells are the precursor cells of both cumulus and mural cells (Fig. 1). However, preantral granulosa cells share more similarities with cumulus cells than with mural cells. For example, both PAGCs from large secondary follicles and cumulus cells express Slc38a3 (Eppig et al., 2005) and Amh (Salmon et al., 2004) mRNA, which are expressed at much lower levels in mural cells. Thus, although these can be used as markers to distinguish cumulus from mural cells, these transcripts begin to be expressed in large preantral follicles just before antrum formation. Another feature in common between preantral and cumulus cells is the strong immunostaining for the cell surface antigen OA-1 (Erickson et al., 1985). Since expansion does not occur in preantral or mural granulosa cells, competence to undergo expansion is a unique characteristic of cumulus cell differentiation (Diaz et al., 2006). The factors that promote acquisition of competence to undergo expansion during the preantral to antral follicle transition are unknown, but could involve oocyte-derived paracrine factors, FSH, and/or other factor(s). In an in vitro-model of cumulus-oocyte development, a low level of FSH (0.005 IU/ml) that does not stimulate cumulus expansion was required to promote the development of acquisition of competence to undergo expansion to a high dose of FSH (0.5 IU/ml) (Eppig et al., 1998). This observation suggests that FSH may have a role in promoting cumulus cell development during the preantral to antral follicle transition.
Oocyte-derived factors may also promote differentiation of the cumulus cell phenotype by promoting acquisition of the ability to undergo cumulus expansion and stimulating expression of cumulus marker transcripts. In mice lacking both copies of the Bmp15 gene and one copy of the Gdf9 gene, hereafter called double mutant (DM) mice, the cumulus cells are defective in their ability to undergo expansion even when cultured with wildtype oocytes (CEEFs) and EGF (Su et al., 2004). Thus, the cumulus cells from DM mice do not acquire full competence to undergo expansion due to deficiencies in oocyte-derived ligands (Gdf9 and Bmp15) during the preantral to antral follicle transition (Su et al., 2004; Yan et al., 2001). GDF9 and BMP15 signal through SMAD2/3 and SMAD1/5/9 pathways respectively (Moore et al., 2003; Roh et al., 2003). Recently oocyte factors signaling through the SMAD2/3 pathway were demonstrated to enable cumulus expansion (Dragovic et al., 2006). Both the SMAD2/3 and SMAD1/5/8 signaling pathways require the co-SMAD, SMAD4 for full activity. Deletion of Smad4 in granulosa cells, which would abrogate signaling through both BMP15 and GDF9 pathways, also leads to compromised cumulus cell differentiation, including lack of competence to undergo normal expansion (Pangas et al., 2006). In fact, the minimal expansion response in DM (Bmp15−/− Gdf9+/−) mice is remarkably similar to that observed in the cumulus oophorus of Smad4 conditional knockout mice, suggesting that BMP15 and/or GDF9 and SMAD4 are part of the same pathway promoting acquisition of competence to undergo expansion.
Preantral granulosa cells (PAGCs) and cumulus cells differ in their ability to undergo expansion (Diaz et al., 2006). PAGCs lack competence to undergo expansion and this deficiency was correlated with a failure to robustly upregulate expansion-related transcripts, particularly Tnfaip6, following stimulation with EGF and CEEFs. The lack of competence to undergo expansion was not due to deficiencies in MAPK3/1 or MAPK14 (also known as p38MAPK) activation; rather other unknown cumulus cell pathways are lacking in PAGCs that result in failure to undergo expansion. Having defined the differences between PAGCs and cumulus cells in terms of competence to undergo expansion (Diaz et al., 2006), the current study tests the hypothesis that FSH and/or oocyte-derived paracrine factors are required to promote the differentiation of PAGCs into cells having competence to undergo expansion in response to EGF or FSH. This study utilized a system in which PAGC-oocyte complexes were cultured with or without FSH and PAGCs where cultured with or without oocytes during the period coincident with the preantral to antral follicle transition in vivo (Fig. 1). At the end of culture, the ability to undergo cumulus expansion was tested. The results demonstrate that oocytes are indispensable, but that FSH also contributes to promoting the preantral granulosa cell to cumulus cell transition as indicated by acquisition of competence to undergo cumulus expansion.
Materials and Methods
Animals
Female B6SJLF1 mice were produced and raised in the research colony of the investigators at The Jackson Laboratory and were used for most experiments. Ovaries from 22 day old mice, 48 hours after I.P. injection of 5 IU eCG (National Hormone and Peptide Program, NIDDK) and untreated 12 day old mice were used for all experiments. Hypogonadal mice devoid of circulating gonadotropins caused by the hpg mutation in the Gnrh1 gene (Gnrh1hpg/Gnrh1hpg), hereafter referred to as hpg−/−mice, were used in some experiments (Halpin et al., 1986). Mice were maintained according to the Guide for the Care and Use of Laboratory Animals (Institute for Learning and Animal Research).
Isolation and culture of preantral-oocyte complexes
The experimental design is illustrated in Figure 1. PAGC-oocyte complexes were isolated from ovaries of 12 day old mice by collagenase digestion as described previously (Vanderhyden et al., 1990). The complexes were grown in vitro (IVG) as described previously (Eppig et al., 1998; Joyce et al., 1999; Vanderhyden et al., 1990) on collagen coated membranes for 2 to 10 days in bicarbonate-buffered MEM-α (Life Technologies, Inc., Grand Island, NY) with Earles salts, supplemented with 75 mg/L penicillin G, 50 mg/L streptomycin sulfate, 0.23 mM pyruvate, and 3 mg/ml crystallized lyophilized bovine serum albumen, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium). To some cultures, 0.005 IU) rhFSH (Organon, USA) was added to the culture medium during IVG (referred to as the “culture period” in Fig. 1). This concentration of FSH is 100 fold less than that needed to stimulate cumulus expansion (0.5 IU/ml). In some experiments, PAGCs were isolated from preantral-oocyte complexes (POC) by oocytectomy (Vanderhyden et al., 1990). PAGCs were cultured in control medium or co-cultured with oocytes for 6 days in 96 well plates coated with a thin layer of agarose and in MEM-α medium containing 10 μM milrinone to maintain the co-cultured fully grown oocytes (1 oocyte/μl) in the germinal vesicle (GV) stage. Oocytes used during co-culture with PAGCs were replaced every 2 days with freshly isolated oocytes
Isolation and culture of oocytes and cumulus-oocyte complexes
Cumulus-oocyte complexes (COCs) containing GV-stage oocytes were collected from antral follicles of 22 day old B6SJLF1 hybrid mice, hpg+/? mice and from the very small antral follicles of hpg−/− ovaries by gentle puncture with a syringe and needle. Fully-grown denuded oocytes were isolated by gentle pipetting COCs as described previously (Diaz et al., 2006).
Expansion assay used during the test period
At the end of the culture period, PAGC-oocyte complexes and PAGCs, and freshly isolated COCs were stimulated with EGF (10 ng/ml) or FSH (0.5 IU/ml) in medium (MEMα) containing 5% FBS and 10 μM milrinone, or cultured in control medium without either EGF or FSH, to determine if the cells had acquired the competence to undergo expansion, including the ability to activate MAPK14, MAPK3/1 and upregulate expansion-related transcripts (Test period. Fig. 1). For co-culture experiments, fully-grown denuded oocytes (FGOs) at a concentration of 2 oocyte/μl were used during the test period. All cultures were maintained at 37°C in a modular incubation chamber (Billups Rothenberg, Del Mar, CA) in an atmosphere of 5% O2, 5% CO2, and 90% N2. All samples were immediately frozen at the end of the culture period in liquid nitrogen and stored at −70°C until analyzed for protein or steady-state mRNA levels as described below. All reagents were purchased from Sigma Chemical Company (St. Louis, MO) unless otherwise noted.
Quantification of mRNA transcripts
For each sample, total RNA was isolated from 25 complexes and reverse transcribed into cDNA as described previously (Diaz et al., 2006). Quantification of expansion-specific transcripts was conducted using transcript-specific primers as described previously (Diaz et al., 2006). Primers used to analyze Amh (forward, CAGGCCCTGTTAGTGCTATACCCT; reverse, GAAGTCCACGGTTAGCACCAAA) and Slc38a3 (forward, TATCTTCGCCCCCAACATCTT; reverse, TGGGCATGATTCGGAAGTAGA) mRNA levels were designed using primer express software (Applied Biosystems, Foster City, CA). Only one product of the appropriate size was identified for each set of primers and all amplification products were sequenced to confirm specificity of the PCR reaction. Expression of Rpl19 mRNA was used to normalize specific expression of mural, cumulus and expansion-related transcripts according to the formula 2-(Ct gene of interest - Ct Rpl19), where Ct is the cycle number at which point the fluorescent intensity for each sample crosses a threshold level set above background. All experiments were repeated three or four times and values shown are the mean ± SEM.
Immunoblots
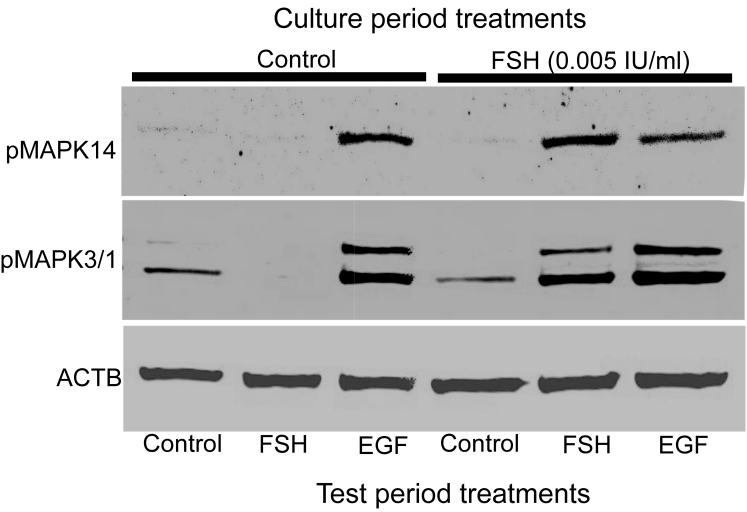
IVG complexes were cultured with or without FSH as described above. Complexes grown for 10 days in medium only (control) or medium with FSH (0.005 IU/ml) were removed from the collagen membrane and treated during the test period with medium only (control) or EGF (10 ng/ml) for 4 hours. Samples were simultaneously denatured by boiling in 1X loading buffer for 5 minutes followed by quenching on ice for 5 minutes. Proteins were separated on a 10% SDS PAGE gel and transferred to PVDF membrane. Membranes were blocked in 1X blocking buffer (Odyssey Blocking buffer, Licor Bioscience, Lincoln, Nebraska) for 1 hour with shaking at room temperature followed by incubation with specific anti-pMAPK3/1 antibody (1:2000, Sigma), anti-pMAPK14 antibody (1:1000, Cell Signaling Technology, Danvers, MA) or ß-actin (ACTB) antibody (1:6000, Sigma) diluted in blocking buffer with 0.1% Tween-20 for 2 to 12 hours at room temperature. Following incubation, blots were washed 3X for 10 minutes each with wash buffer (PBS, 0.1% Tween-20). Fluorescent-labeled secondary antibodies (IRDye™ 800 anti-mouse or anti-rabbit, Rockland Immunochemicals, Inc. Gilbertsville, PA) were diluted at 1:5000 and incubated with the blots for 1 hour at room temperature. Blots were washed as above with an additional final wash in PBS without Tween-20. Detection was accomplished with an infrared scanner (Licor Bioscience, Lincoln, Nebraska). Blots representative of three or four independent experiments are shown in Figure 8.
Figure 8.
Activation of MAPK14 and MAPK3/1 in complexes cultured with control medium or FSH (0.005 IU/ml) for 10 days followed by treatment with control medium, FSH (0.5 IU/ml), or EGF (10 ng/ml) for 4 hours and immunostaining for MAPK14, MAPK3/1 and ACTB on the same blot. A representative blot of three independent experiments is shown.
Statistical analyses
Results from mRNA expression experiments were analyzed by either two-way ANOVA within treatment group (control, FSH, EGF) followed by Tukeys HSD post-hoc test if a positive F-test was detected or by Student's t-test as indicated in the figure legends. Microsoft Excel was used for Student's t-test analysis and JMP 5.1 statistical analysis software was used for ANOVA analyses (SAS, Cary, NC).
Results
Acquisition of competence to undergo expansion in response to EGF
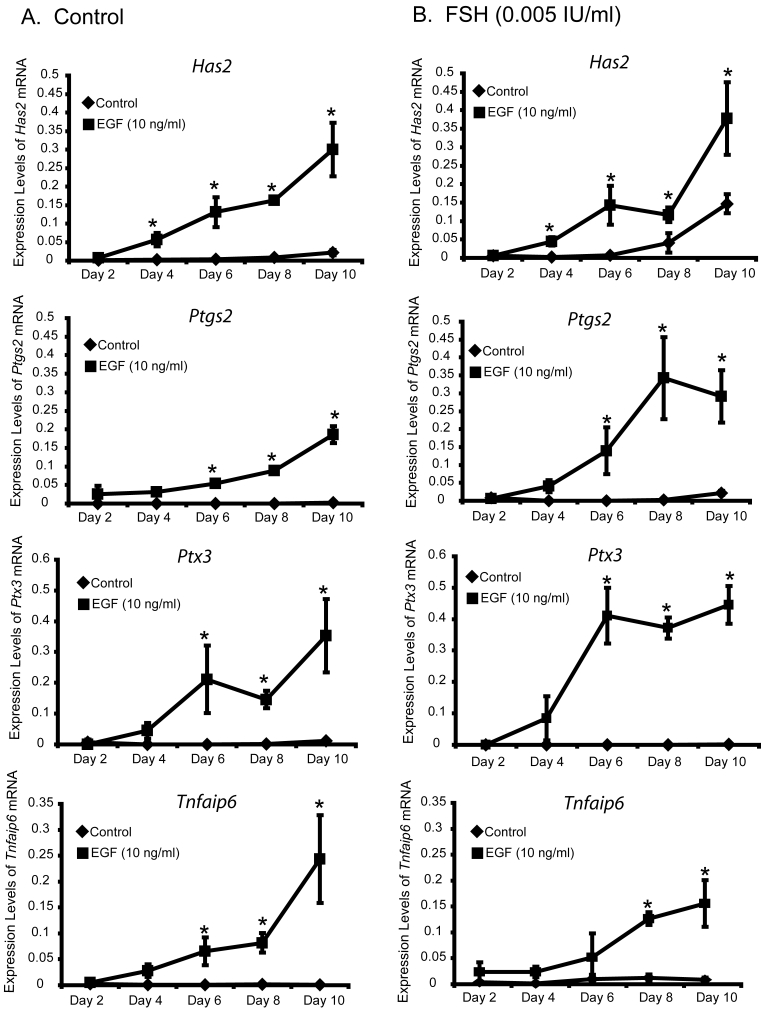
The competence of cumulus cells to undergo expansion in response to EGF is a characteristic of cumulus cells grown in vivo. The first issues addressed were (1) whether FSH is required for development of competence to undergo expansion, and (2) when do cultured PAGC-oocyte complexes acquire expansion competence during the culture period. PAGC-oocyte complexes were grown (IVG) in control medium or medium containing 0.005 IU/ml FSH for 2 to 10 days on a collagen membrane (Fig. 1). FSH was used at a concentration 0.005 IU/ml FSH during the grown period because this dose promotes optimal oocyte growth (Thomas et al., 2005) but does not stimulate expansion. Following the culture period, complexes were removed from the collagen membrane and treated during the test period with control medium or EGF (10 ng/ml) for 12 (mRNA assay) or 15 (expansion assay) hours. Complexes cultured in control medium or medium plus FSH acquired competence to undergo expansion in response to EGF by day 6 (Fig 2), however, in some cases, 1-3 complexes out of approximately 20-25/replicate, grown in the presence of FSH developed competence to undergo expansion by day 4. Development of the ability to undergo expansion correlated with the ability to upregulate expansion-related transcripts (Fig. 3).
Figure 2.
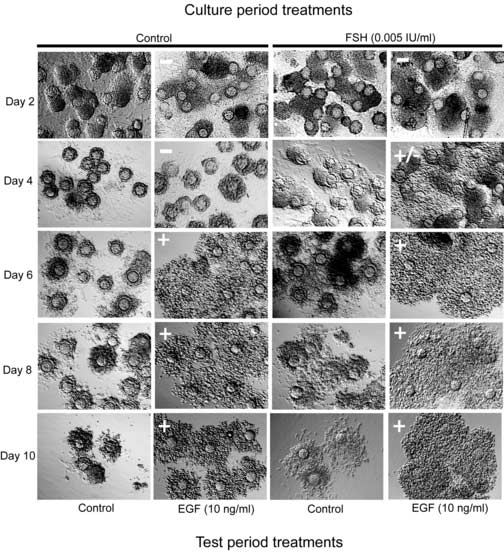
Timing of the acquisition of competence to undergo expansion in vitro. Complexes were cultured in control medium or medium supplemented with a low concentration of FSH (0.005 IU/ml) for 2, 4, 6, 8 and 10 days. Following the culture period, complexes were cultured in control medium or medium with EGF (10 ng/ml) for 15 hours during the test period to stimulate cumulus expansion. (+) indicate positive expansion, (−) negative expansion and (+/−) mixed response.
Figure 3.
Expression of Has2, Ptgs2, Ptx3 and Tnfaip6 mRNAs in complexes cultured with (A) control medium, or (B) FSH (0.005 IU/ml) for 2, 4, 6, 8 and 10 days followed by treatment with control medium or EGF (10 ng/ml) for 12 hours during the test period. *Denotes value different from control as determined by Student's t-test, P<0.05.
Oocyte-derived factors are required to promote development of competence to undergo expansion
Development of competence to undergo normal cumulus expansion is defective in mice lacking Bmp15, and this deficiency is exacerbated by heterozygosity for Gdf9 (Su et al., 2004; Yan et al., 2001). In mice, BMP15 and GDF9 are produced in the ovary only by oocytes (Dong et al., 1996; Elvin et al., 1999; Yan et al., 2001). Therefore, we directly tested the requirement for oocytes on development of competence to undergo expansion. PAGC-oocyte complexes from the secondary follicles of 12 day old mice were oocytectomized and the preantral granulosa cells were cultured individually on a thin layer of agarose to promote retention of their 3-dimensional architecture. PAGCs were cultured with or without FGO (1 oocyte/μl) for 6 days in control medium. On day 6, all PAGCs were co-cultured with fresh FGO (2 oocytes/μl), to provide CEEFs, and treated with either medium only (control) or EGF (10 ng/ml) for 6 hours (mRNA assays) or 15 hours (expansion assays). The experimental design is shown in Figure 1C. PAGCs grown in the absence of oocytes did not develop competence to undergo expansion even when stimulated with EGF and CEEFs secreted from FGO during the test period (Fig. 4A). In contrast, PAGCs cultured in the presence of FGO for 6 days responded to EGF by undergoing expansion (Fig. 4A). Oocyte-secreted factors also promoted the competence of PAGCs to upregulate expansion-related transcripts in response to EGF (Fig. 4B). Even though PAGCs cultured in the absence of FGO modestly upregulated expansion-related transcripts, these levels were much lower than in PAGCs cultured with FGO, and were not sufficient to produce an expansion response. Clearly, oocyte-derived factors are necessary and sufficient to promote competence to undergo expansion in response to EGF.
Figure 4.
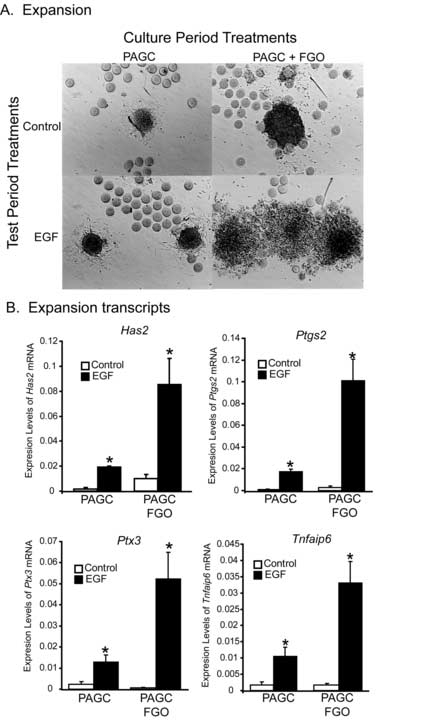
Effect of oocyte co-culture on development of competence to undergo expansion (A) and expression of expansion transcripts (B) in oocytectomized PAGCs cultured in control medium or co-cultured with FGO (1 oocytes/μl) for 6 days (culture period). At the end of the culture period, PAGCs were removed from the agarose and treated with control medium or EGF (10 ng/ml) for 6 hours (mRNA levels) or 15 hours (expansion assay). Freshly isolated oocytes were added to all PAGC treatment groups during the test period only (2 oocytes/μl). Test period treatments, including the oocytes added to provide CEEFs, are shown in the both 4A and 4B. *Denotes value different from control as determined by Student's t-test, P<0.05.
Competence to undergo expansion by complexes from hpg−/− mice
Results from experiments depicted in Figures 2-3 indicate that FSH is not required in vitro for development of the competence to undergo expansion in response to EGF. To determine how these results compare with complexes that develop in vivo in the absence of FSH, COCs were collected from mice with the hpg mutation in the Gnrh1 gene (Halpin et al., 1986). These mice have no detectable circulating FSH or LH, but some follicles develop to the small antral stage. Treatment with EGF stimulated expansion in both hpg−/− and control hpg+/? COCs (Fig. 5). In contrast, hpg−/− COCs did not undergo expansion in response to high dose of FSH (0.5 IU/ml) (Fig. 5). Thus, it appears that gonadotropin stimulation in vivo is required to develop expansion competence, as tested in vitro with FSH (0.5 IU/ml). However, development of competence to undergo expansion in response to EGF is intrinsic to the oocyte-granulosa cell complex.
Figure 5.
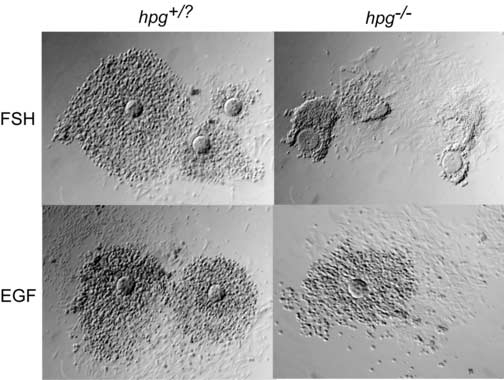
Competence to undergo expansion by COCs collected from hpg−/− mutant or hpg+/? littermate controls treated with FSH (0.5 IU/ml) or EGF (10ng/ml) for 15 hours.
Competence to undergo expansion in response to FSH requires prior gonadotropin stimulation
Lack of circulating gonadotropins in hpg−/− mice results in an inability of the cumulus oophorus to respond to a high dose of FSH (0.5 IU/ml) by undergoing expansion (Fig. 5), while expansion stimulated by EGF appears normal (Fig. 5). To further explore this difference between FSH- and EGF-induced expansion, we analyzed the competence to undergo expansion in PAGC-oocyte complexes cultured with or without a low level of FSH (0.005 IU/ml) for 10 days. At the end of the culture period, complexes were removed from the membrane and stimulated with EGF or a high dose of FSH (0.5 IU/ml) during the test period. EGF stimulated expansion regardless of whether the complexes had prior exposure to gonadotropins. In contrast, high levels of FSH (0.5 IU/ml) did not stimulate expansion unless the complexes were previously exposed to 0.005 IU/ml of FSH during the culture period (Fig. 6). Thus, FSH-induced expansion required prior exposure to gonadotropins, but EGF-induced expansion does not.
Figure 6.
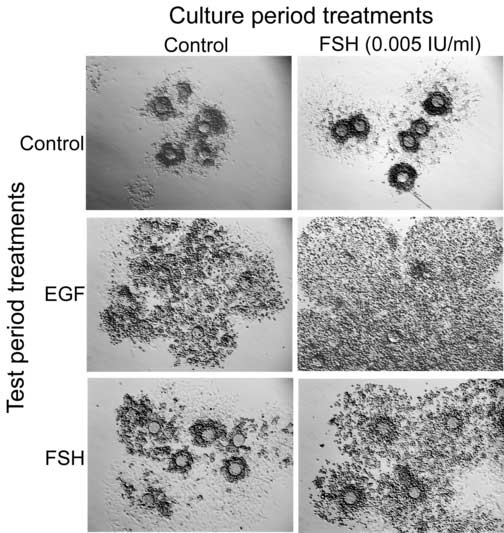
Competence to undergo expansion of complexes cultured in control medium or FSH (0.005 IU/ml) for 10 days followed by treatment with control medium (control), 10 ng/ml EGF (middle) or 0.5 IU/ml FSH (bottom) for 15 hours to stimulate expansion.
Activation of MAPK and upregulation of expansion-related transcripts
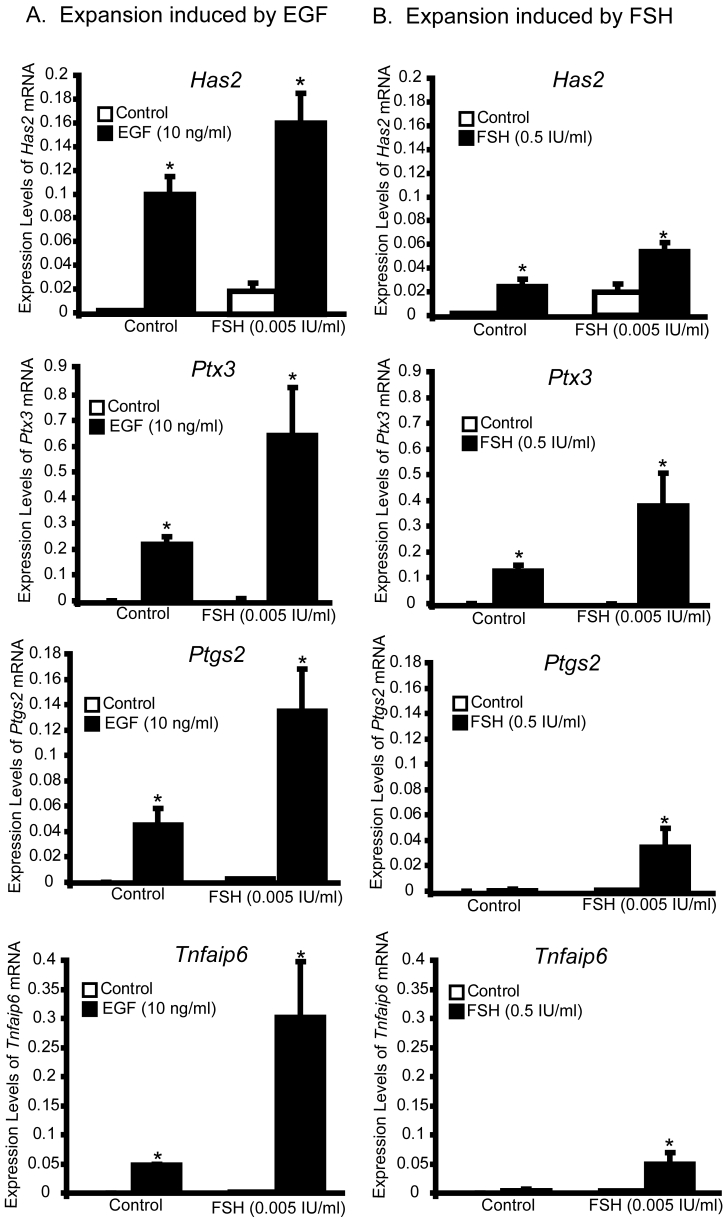
To examine which FSH-induced pathways are deficient in complexes grown in control medium (no FSH), we examined MAPK14 and MAPK3/1 activation and upregulation of expansion-related transcripts because these pathways are normally activated by FSH during cumulus expansion. EGF stimulated MAPK14 and MAPK3/1 activation (Fig. 8) and increased levels of expansion-related transcripts (Fig. 7A) during the test period, regardless of prior FSH (0.005 IU/ml) exposure during the culture period. In contrast, high dose FSH (0.5 IU/ml) during the test period did not stimulate activation of MAPKs or robustly stimulate increased in expansion-related transcripts unless complexes had been treated with FSH during the culture period (Fig. 7B and 8). Thus, prior exposure to FSH is necessary to promote the ability of cumulus cells to undergo expansion in response to a high dose of FSH, including the ability to activate MAPK3/1 and MAPK14 and to stimulate increased levels of expansion-related transcripts, particularly Ptgs2 and Tnfaip6.
Figure 7.
Steady-state levels of Has2, Ptgs2, Ptx3, and Tnfaip6 mRNA in complexes cultured with control medium or FSH (0.005 IU/ml) for 10 days followed by treatment with EGF (10 ng/ml) (A) or FSH (0.5 IU/ml) (B) for 6 hours. *Denotes value different from control as determined by Student's t-test, P<0.05.
Effect of FSH-treatment during the culture period on expression of cumulus marker transcripts
Before the LH surge, cumulus cells express different subsets of transcripts compared to mural cells. For example, levels of Slc38a3 and Amh mRNA are higher in cumulus cells compared to mural cells and their expression is dependent on oocyte-derived factors (Eppig et al., 2005; Salmon et al., 2004). Therefore, we assessed whether increasing levels of FSH during in-vitro development of preantral-oocyte complexes affects expression levels of cumulus marker transcripts compared to COC complexes that developed in vivo. Levels of Amh and Slc38a3 mRNA were suppressed by FSH-treatment during the culture period when compared with cumulus cells developed in vivo (Fig. 9). Thus, FSH-treatment during culture suppresses some phenotypic characteristics of the cumulus cell phenotype despite promoting competence to undergo expansion in response to high dose FSH during the test period, a characteristic of cumulus cells developed in vivo.
Figure 9.
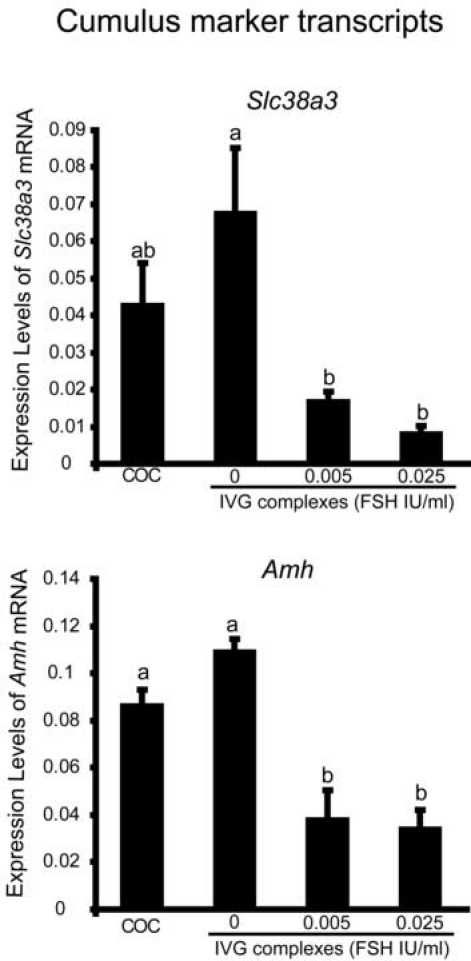
Expression of cumulus marker transcripts (Slc38a3 and Amh) in COCs grown in vivo and in vitro (from preantral granulosa cell-oocyte complexes for 10 days with 0, 0.005 or 0.025 IU/ml FSH). abcValues with different superscripts are significantly different, p<0.05.
Discussion
Preantral granulosa cells are the progenitors of cumulus cells, but lack many of the attributes of cumulus cells, particularly competence to undergo cumulus expansion (Diaz et al., 2006; Vanderhyden et al., 1990). The present study used an in-vitro model where PAGC-oocyte complexes or isolated PAGCs (without oocytes) were cultured during the period coincident with antrum formation and the PAGC to cumulus cell transition in vivo. The ability to undergo expansion and express cumulus marker transcripts were used to indicate that preantral granulosa cells had differentiated into cumulus-like cells. Competence to undergo expansion in response to EGF was dependent on prior exposure to oocyte-secreted factors, but did not require prior FSH exposure. In contrast, competence to undergo expansion in response to high levels of FSH (0.5 IU/ml) required prior exposure to low dose FSH (0.005 IU/ml). The relevance of this study conducted in vitro to the requirements for cumulus cell development in vivo was demonstrated using complexes isolated from the small antral follicles of hpg−/− mice, which lack circulating gonadotropins. Complexes of hpg−/− mice were competent to undergo expansion in response to EGF but not of FSH, confirming our observations of cultured PAGC-oocyte complexes.
Recent evidence strongly suggests that EGF-like factors, such as amphiregulin and epiregulin are the proximal signals that induce cumulus expansion in vivo (Park et al., 2004; Shimada et al., 2006). In vitro, EGF stimulates expansion (Diaz et al., 2006) and therefore can be used to test for the ability of cultured complexes to undergo cumulus expansion. Cumulus expansion also occurs following stimulation with a high dose of FSH in vitro (0.5 IU/ml) and this protocol was used to stimulate cumulus expansion for decades before the seminal paper by Park and colleagues (Park et al., 2004). Therefore, both EGF and FSH were used to stimulate cumulus expansion and, interestingly, some notable differences emerged with respect to acquisition to competence to respond to the two stimuli.
Cumulus cells that develop in vivo express FSH receptors. Since mice with null mutations in the Fshb gene (Kumar et al., 1997) or Fshr gene (Dierich et al., 1998) fail to form preovulatory follicles, we hypothesized that FSH may be important for cumulus cell differentiation as indicated by development of the ability to undergo expansion. Using complexes that developed in vivo (hpg−/−) or in vitro (Fig 2-3) in the absence of FSH, we show that the ability to expand in response to EGF does not depend on prior FSH treatment. Thus, FSH is not required during cumulus cell development for their ability to undergo expansion to EGF. This interesting finding demonstrates that the ability to undergo expansion to EGF develops intrinsically in cumulus-oocyte complexes.
The oocyte has a dominant role in directing follicle function. Oocytes from large secondary follicles direct follicular somatic cells from primordial follicles to increase their rate of development and to differentiate into cumulus-like cells at an earlier time then normal (Eppig et al., 2002). In DM mice (Bmp15 null; Gdf9 heterozygote), cumulus expansion even when co-cultured with wildtype oocytes (Su et al., 2004; Yan et al., 2001). GDF9 and BMP15 signal through SMAD2/3 and SMAD1/5/9 pathways respectively (Moore et al., 2003; Roh et al., 2003) and recently cumulus expansion enabling factors secreted by oocytes where shown to signal through the SMAD2/3-mediated pathways (Dragovic et al., 2006). However, left unanswered from these studies was whether oocyte-secreted products were required during the differentiation of PAGC to cumulus cells for acquisition of competence to undergo expansion, in addition to enabling the process of cumulus expansion itself. We tested the requirement for oocyte factors during differentiation of PAGCs to cumulus cells by culturing PAGCs with or without oocytes for 6 days (Fig. 1C). At the end of culture, PAGCs cultured with oocytes, but not those cultured alone had developed the ability to undergo expansion and robustly upregulate expansion transcripts when treated with EGF. These observations add to the growing list of effects that oocytes have on cumulus cells and support the concept that oocytes have a dominant role in specifying important aspects of the cumulus cell phenotype independently of FSH.
Although EGF is the proximal signal that stimulated expansion in vivo, cumulus cells that develop in vivo acquire the ability to undergo expansion to high dose FSH (Diaz et al., 2006). Thus the ability to undergo expansion in response to FSH can also be used as a marker of cumulus cell differentiation. Our previous report indicated that EGF stimulated MAPK3/1 activation in PAGCs, while FSH did not (Diaz et al., 2006). Thus, competence to undergo expansion in response to FSH requires development of the ability to activate MAPK3/1 in cumulus cells. Here we show that prior exposure to FSH (FSH priming) both in vivo (hpg−/−) and in vitro (Fig. 6) is required for development of the ability to undergo expansion to a high dose of FSH in vitro, but is not required for EGF-induced expansion. Why do complexes cultured in control medium lack the ability to undergo expansion to a high dose FSH? To answer this question, we analyzed the expression of expansion transcripts and MAPKs activation in complexes cultured with or without FSH (0.005 IU/ml) during the culture period. Not surprisingly, EGF-treatment led to activation of MAPK14 and MAPK3/1 and upregulation of expansion-related transcripts regardless of prior FSH exposure. In contrast, FSH (0.5 IU/ml) did not activate either MAPK14 or MAPK3/1 or robustly upregulate expansion-related transcripts, unless complexes had been primed with FSH (0.005 IU/ml) during the culture period. Thus, low dose FSH priming during the culture period causes changes in the granulosa cells that allow an expansion-inducing dose of FSH to stimulate MAPK14 and MAPK3/1 activation and upregulate expansion-related transcripts. The ability to activate expansion-related pathways in response to FSH is also a feature of COC that develop in vivo. Here we show that this ability is dependent on FSH priming during the period coincident with the preantral to antral transition. Thus, FSH priming promotes the ability of FSH to activate MAPK pathways, which results in cumulus expansion in vitro, but this observation is probably not physiologically relevant since expansion is likely stimulated by EGF-like peptides in vivo. Nevertheless, these observations indicate that the sensitivity to FSH changes as PAGCs differentiate into cumulus cells. In particular, cumulus cells acquire the ability to activate MAPK3/1 in response to FSH and this could have other important functions during cumulus cell differentiation. For example, the stimulatory effect of FSH on granulosa cell proliferation is dependent on MAPK activation (Yang and Roy, 2006), which we now show is dependent on low level FSH priming. Also, FSH in conjunction with oocyte-derived GDF9 promotes preantral follicle growth and development during a period similar to that of the present study (Orisaka et al., 2005). Thus even though FSH priming is not required for acquisition of competence to undergo expansion in response to EGF, FSH may still play a role during cumulus cell differentiation by promoting its own ability to activate MAPKs. These responses may be involved in mediating FSH-stimulated effects in granulosa cells not related to expansion per se, but to other processes downstream of MAPK activation.
The role of FSH in cumulus cell differentiation is more complicated than promoting activation of MAPKs and acquisition of competence to undergo expansion to a high dose of FSH. The expression of Scl38a3 and Amh mRNA begins in granulosa cells of large preantral follicles and is maintained in cumulus cells by oocyte-derived factors, but not in mural cells (Eppig et al., 2005; Salmon et al., 2004). These transcripts are important for defining the cumulus cell phenotype as distinct from the mural phenotype. To determine whether FSH during the culture period affects levels of these transcripts, their expression was measured in complexes cultured with or without 0.005 IU/ml or 0.025 IU/ml of FSH for 10 days. Levels of Slc38a3 and Amh mRNA were suppressed by FSH during culture of preantral complexes. These observations suggest that either the oocyte's ability to promote elevated levels of these transcripts is compromised or other factors present in the follicle milieu, such as steroids or other growth factors, are missing from the culture system used in this study.
The present study supports a direct role of oocytes in cumulus cell differentiation by promoting the ability to undergo expansion, but also provides evidence that FSH during the preantral to antral transition promotes some characteristics of cumulus cells, such as the ability to activate MAPK14 and MAPK3/1 and competence to undergo expansion to high FSH in vitro. At the same time, low levels of FSH suppress expression of cumulus marker transcripts Slc38a3 and Amh, suggesting that the balance between oocyte- and FSH-signaling is critical for optimal cumulus-oocyte complex development. How oocyte-derived factors and FSH interact to affect cumulus cell differentiation is unknown. However, these interactions between paracrine oocyte-secreted factors and endocrine-derived FSH provide an additional layer of complexity in the control of follicular development.
Footnotes
This work was supported by HD23839 from the NICHD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev. Biol. 1990;138:16–25. doi: 10.1016/0012-1606(90)90172-f. [DOI] [PubMed] [Google Scholar]
- Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol. Reprod. Dev. 1993;34:87–93. doi: 10.1002/mrd.1080340114. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: Development of competence to undergo expansion. Dev. Biol. 2006;299:91–104. doi: 10.1016/j.ydbio.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, SassoneCorsi P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol. Reprod. 2006 doi: 10.1095/biolreprod.106.057471. DOI:10.1095/biolreprod.106.057471. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol. Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc. Natl. Acad. Sci. USA. 2002;99:2890–2894. doi: 10.1073/pnas.052658699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol. Reprod. 2005;73:351–7. doi: 10.1095/biolreprod.105.041798. [DOI] [PubMed] [Google Scholar]
- Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–61. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- Halpin DMG, Jones A, Fink G, Charlton HM. Postnatal ovarian follicle development in hypogonadal (hpg) and normal mice and associated changes in the hypothalamic-pituitary ovarian axis. J. Reprod. Fert. 1986;77:287–296. doi: 10.1530/jrf.0.0770287. [DOI] [PubMed] [Google Scholar]
- Joyce IM, Pendola FL, Wigglesworth K, Eppig JJ. Oocyte regulation of kit ligand expression in mouse ovarian follicles. Dev. Biol. 1999;214:342–353. doi: 10.1006/dbio.1999.9437. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu NF, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S. Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J. Biol. Chem. 2003;278:304–10. doi: 10.1074/jbc.M207362200. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003a;144:4376–4384. doi: 10.1210/en.2003-0487. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Russell DL, Day AJ, Breyer RM, Richards JS. Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology. 2003b;144:1008–19. doi: 10.1210/en.2002-220435. [DOI] [PubMed] [Google Scholar]
- Orisaka M, Orisaka S, Jiang JY, Craig J, Wang Y, Kotsuji F, Tsang BK. Growth differentiation factor 9 Is antiapoptotic during follicular development from preantral to early antral stage. Mol. Endocrinol. 2005;20:2456–2468. doi: 10.1210/me.2005-0357. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific smad4 knockout mice. Mol. Endocrinol. 2006;20:1406–1422. doi: 10.1210/me.2005-0462. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Roh JS, Bondestam J, Mazerbourg S, Kaivo-Oja N, Groome N, Ritvos O, Hsueh AJW. Growth differentiation factor-9 stimulates inhibin production and activates Smad2 in cultured rat granulosa cells. Endocrinology. 2003;144:172–178. doi: 10.1210/en.2002-220618. [DOI] [PubMed] [Google Scholar]
- Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Mullerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev. Biol. 2004;266:201–208. doi: 10.1016/j.ydbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of EGF-like factors in cumulus oocyte complexes (COCs) and granulosa cells: key roles for prostanglandin synthase 2 (Ptgs2) and progesterone receptor (Pgr) Mol. Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus complex. Dev. Biol. 2003;263:126–138. doi: 10.1016/s0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JA, Matzuk MM, Eppig JJ. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev. Biol. 2004;276:64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Ethier JF, Shimasaki S, Vanderhyden BC. Follicle stimulating hormone regulates oocyte growth by modulation of expression of oocyte and granulosa cell factors. Endocrinology. 2005;146:941–949. doi: 10.1210/en.2004-0826. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus-expansion enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev. Biol. 1990;140:307–317. doi: 10.1016/0012-1606(90)90081-s. [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol. Endocrinol. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol. Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Yang PC, Roy SK. A Novel Mechanism of FSH Regulation of DNA Synthesis in the Granulosa Cells of Hamster Preantral Follicles: Involvement of a Protein Kinase C-Mediated MAP Kinase 3/1 Self-Activation Loop. Biol. Reprod. 2006;75:149–157. doi: 10.1095/biolreprod.105.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]