Abstract
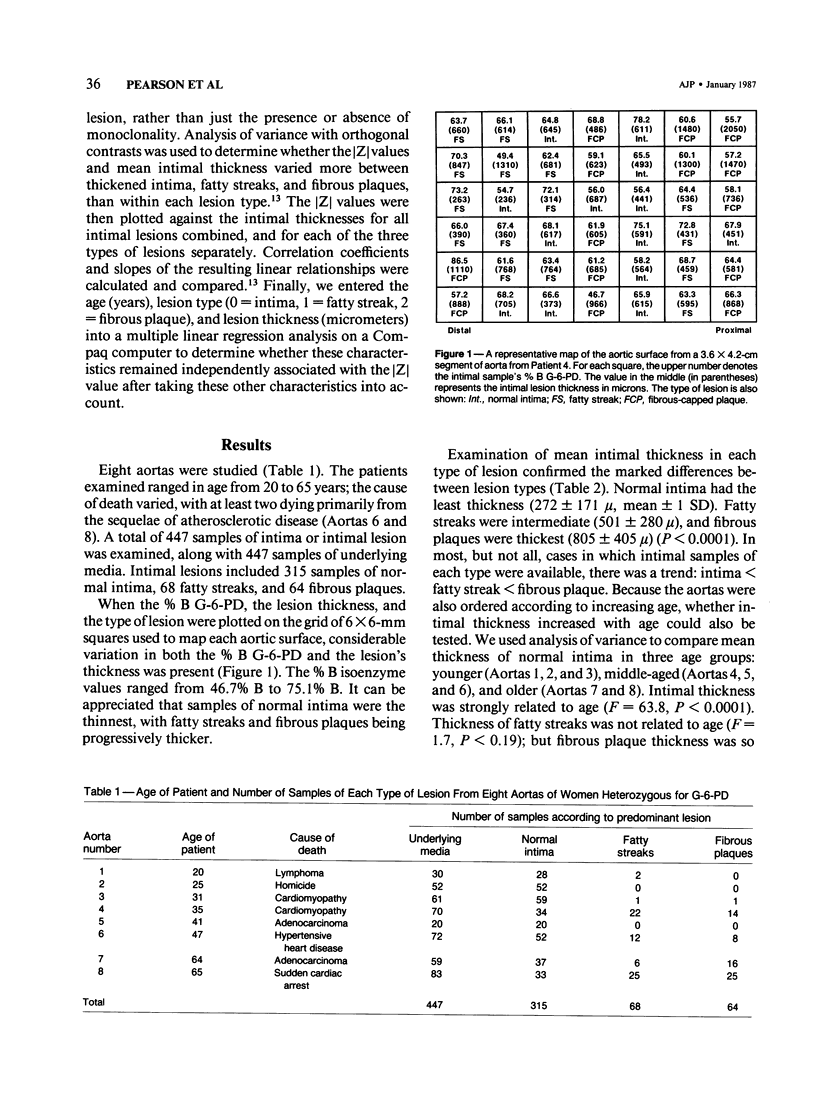
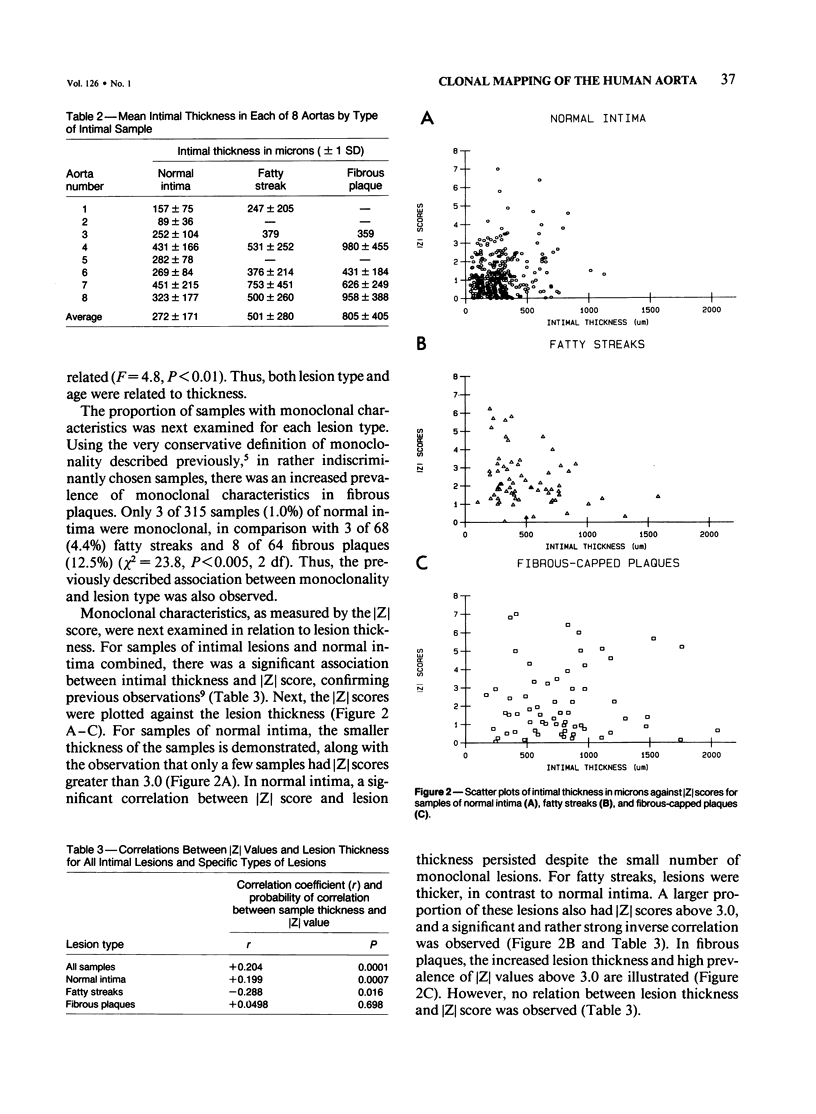
The surfaces of 8 aortas from women heterozygous for G-6-PD isoenzymes were mapped for an examination of the relationships of monoclonality, lesion type, lesion thickness, and age of the patient. The percent B isoenzyme value of samples of normal intima (n = 315), fatty steak (n = 68), or fibrous plaque (n = 64) was used to define monoclonality, expressed as the [Z] score, the number of standard deviations from the mean percent B isoenzyme of samples of underlying media. Intimal thickness increased significantly with type of lesion, such that intima less than fatty streak less than fibrous plaque, and with the age of the patient. The percentage of monoclonal portions also increased with lesion type, such that 1% of samples of normal intima were monoclonal, compared with 4.4% of fatty streaks and 12.5% of fibrous plaques (P less than 0.005). Monoclonality increased with intimal thickness when normal intima, fatty streaks, and fibrous plaques were combined (P = 0.0001). When examined separately, normal intima showed a direct correlation between monoclonality and intimal thickness. In contrast, the monoclonality of fatty streaks was inversely associated with thickness (P = 0.016) and the monoclonality of fibrous plaques not related to thickness. When entered into a multiple regression model, lesion type and age, but not lesion thickness, significantly predicted monoclonality. The lack of association of intimal thickness with monoclonality suggests that it is the type of lesion that determines monoclonality and not merely its thickness. This implies that mechanisms other than clonal selection are responsible for the monoclonal characteristics of human atherosclerotic lesions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P. Implications of the monoclonal character of human atherosclerotic plaques. Beitr Pathol. 1976 Sep;158(4):405–416. doi: 10.1016/s0005-8165(76)80137-0. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- Geer J. C., McGill H. C., Jr, Robertson W. B., Strong J. P. Histologic characteristics of coronary artery fatty streaks. Lab Invest. 1968 May;18(5):565–570. [PubMed] [Google Scholar]
- Linder D., Gartler S. M. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965 Oct 1;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- MOVAT H. Z., MORE R. H., HAUST M. D. The diffuse intimal thickening of the human aorta with aging. Am J Pathol. 1958 Nov-Dec;34(6):1023–1031. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Heptinstall R. H. The clonal characteristics of human aortic intima. Comparison with fatty streaks and normal media. Am J Pathol. 1983 Oct;113(1):33–40. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solex K., Heptinstall R. H. Clonal markers in the study of the origin and growth of human atherosclerotic lesions. Circ Res. 1978 Jul;43(1):10–18. doi: 10.1161/01.res.43.1.10. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solez K., Heptinstall R. H. Clonal characteristics of cutaneous scars and implications for atherogenesis. Am J Pathol. 1981 Jan;102(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solez K., Heptinstall R. H. Evidence for two populations of fatty streaks with different roles in the atherogenic process. Lancet. 1980 Sep 6;2(8193):496–498. doi: 10.1016/s0140-6736(80)91831-0. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Wang B. A., Solez K., Heptinstall R. H. Clonal characteristics of fibrous plaques and fatty streaks from human aortas. Am J Pathol. 1975 Nov;81(2):379–387. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Stary H. C. Proliferation of arterial cells in atherosclerosis. Adv Exp Med Biol. 1974;43(0):59–81. doi: 10.1007/978-1-4684-3243-5_4. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Reiner J. M., Janakidevi K., Florentin R. A., Lee K. T. Population dynamics of arterial cells during atherogenesis. X. Study of monotypism in atherosclerotic lesions of black women heterozygous for glucose-6-phosphate dehydrogenase (G-6-PD). Exp Mol Pathol. 1979 Dec;31(3):367–386. doi: 10.1016/0014-4800(79)90038-8. [DOI] [PubMed] [Google Scholar]
- WILENS S. L. The nature of diffuse intimal thickening of arteries. Am J Pathol. 1951 Sep-Oct;27(5):825–839. [PMC free article] [PubMed] [Google Scholar]


