Abstract
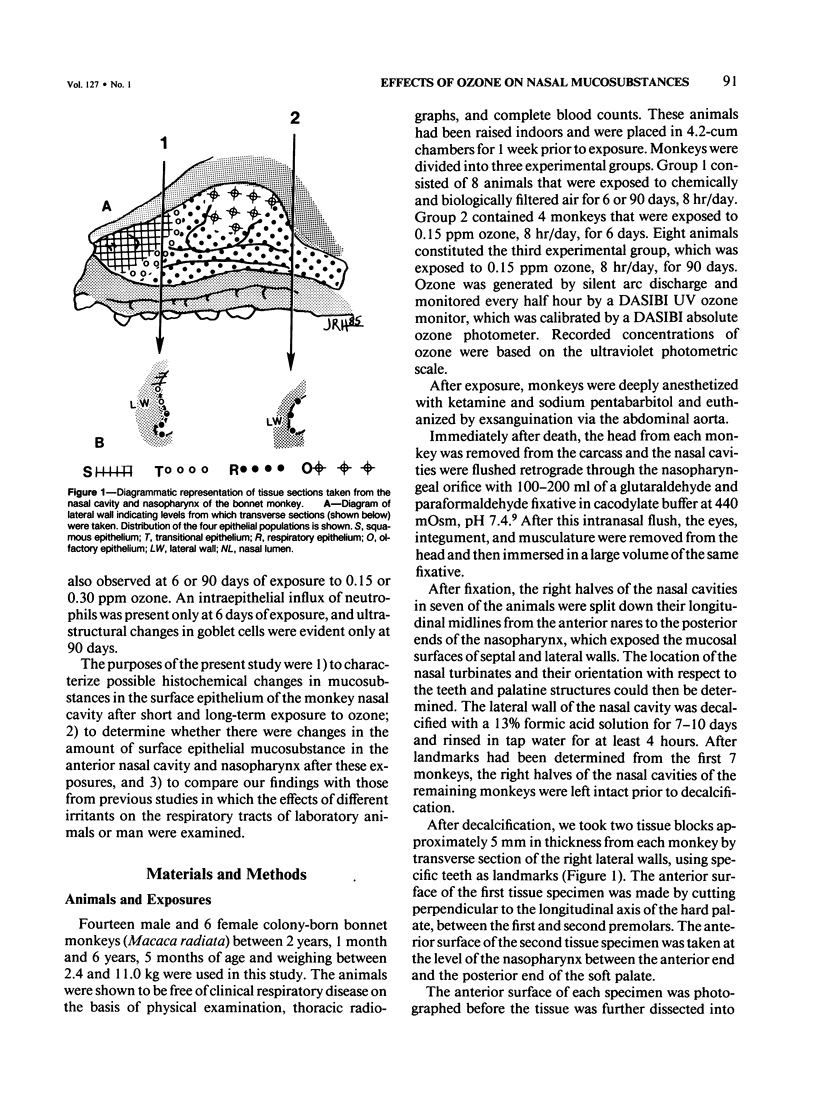
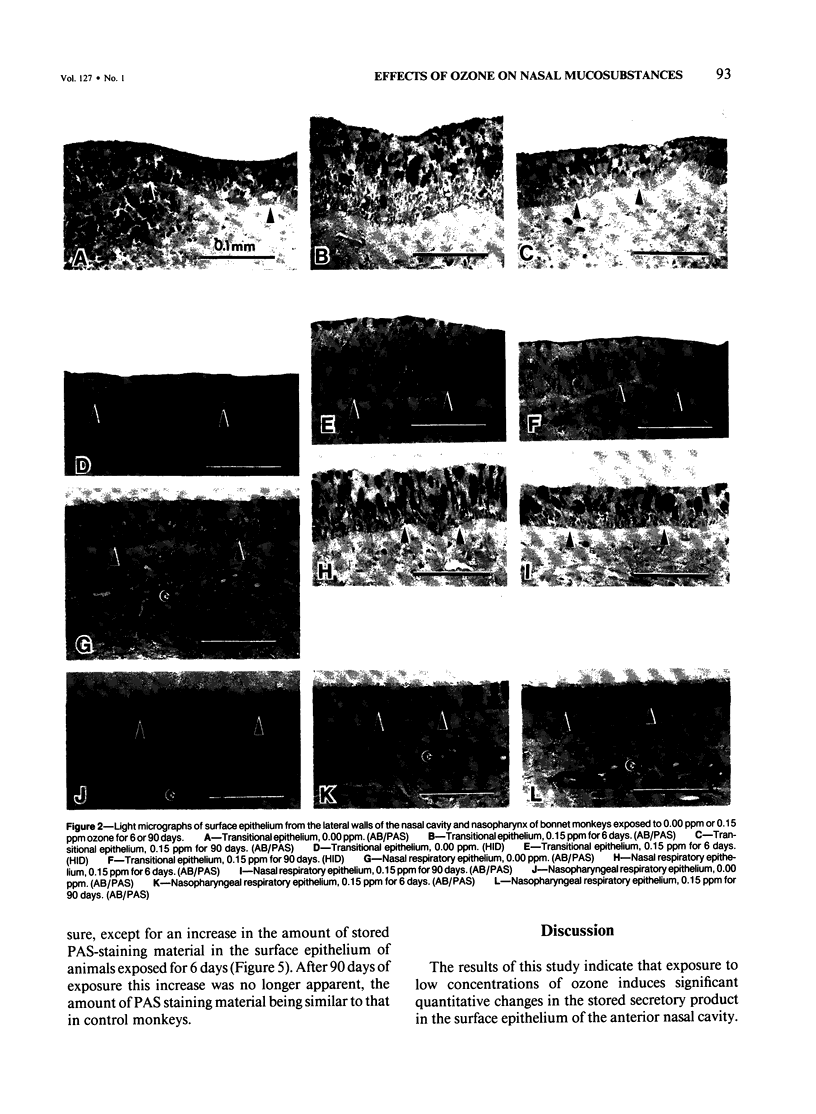
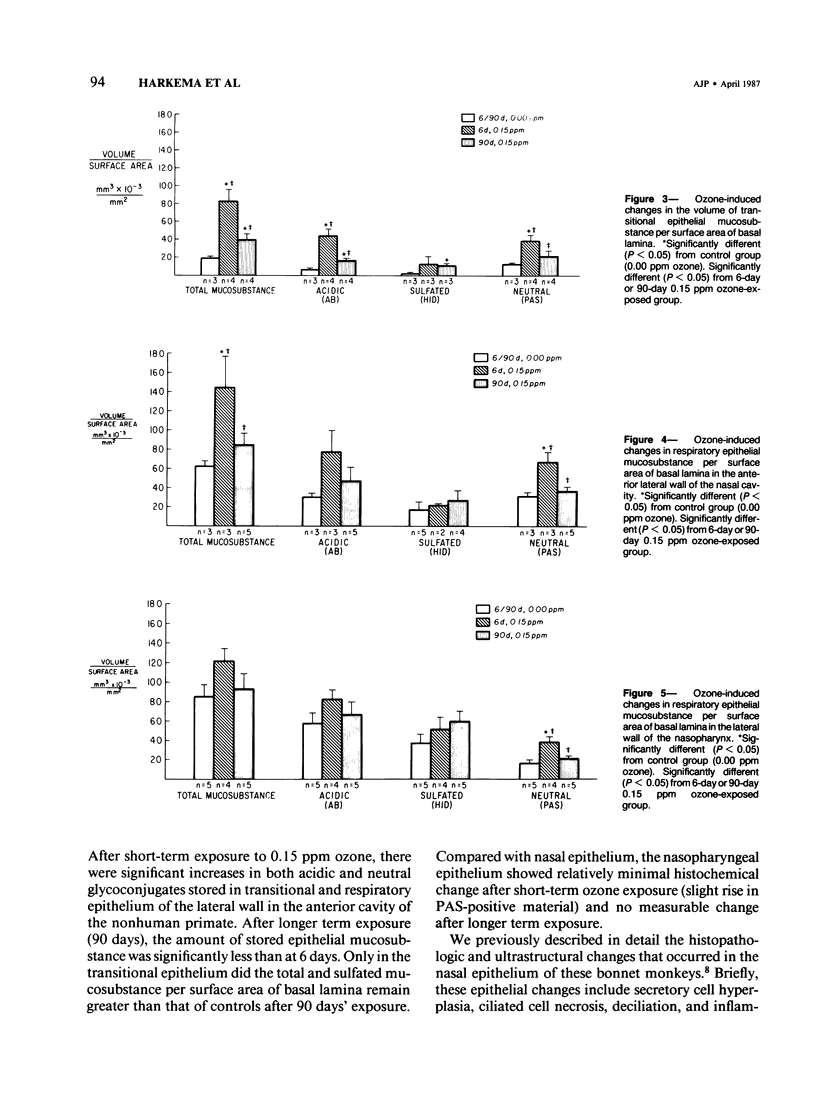
Despite the absorption of inhaled oxidant gases by the nasal cavity, little effort has been made to characterize the effects of these oxidants on the nasal mucosa. This study defines the effects of ambient concentrations of ozone on the character and amount of mucosubstances in epithelium of nasal mucosa. Bonnet monkeys were exposed to 0.00 or 0.15 ppm O3 (8 hr/day) for 6 or 90 days, anesthetized, and exsanguinated. Nasal cavities were fixed with Karnovsky's fixative, decalcified, and processed for light microscopy, and sections were stained with alcian blue (pH 2.5)/periodic acid-Schiff or high iron diamine. Volume densities of secretory material in nasal epithelium were determined with the use of a Quantimet 900 image analyzer. After 6 days' exposure there were significant increases in both acidic and neutral glycoconjugates stored in transitional and respiratory epithelium. After 90 days there was significantly less mucosubstance than at 6 days. Only in the transitional epithelium did the total and sulfated mucosubstance remain greater than that of controls. Nasopharyngeal epithelium was minimally affected after 6 days of O3 and unchanged after 90 days. It is concluded that exposures to ambient levels of O3 induce significant changes in the stored secretory product of nasal epithelium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castleman W. L., Dungworth D. L., Schwartz L. W., Tyler W. S. Acute respiratory bronchiolitis: an ultrastructural and autoradiographic study of epithelial cell injury and renewal in rhesus monkeys exposed to ozone. Am J Pathol. 1980 Mar;98(3):811–840. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Tyler W. S., Dungworth D. L. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol. 1977 Jun;26(3):384–400. doi: 10.1016/0014-4800(77)90041-7. [DOI] [PubMed] [Google Scholar]
- De Haller R., Reid L. Adult chronic bronchitis. Morphology, histochemistry and vascularisation of the bronchial mucous glands. Med Thorac. 1965;22(6):549–567. [PubMed] [Google Scholar]
- Eustis S. L., Schwartz L. W., Kosch P. C., Dungworth D. L. Chronic bronchiolitis in nonhuman primates after prolonged ozone exposure. Am J Pathol. 1981 Nov;105(2):121–137. [PMC free article] [PubMed] [Google Scholar]
- Frager N. B., Phalen R. F., Kenoyer J. L. Adaptations to ozone in reference to mucociliary clearance. Arch Environ Health. 1979 Jan-Feb;34(1):51–57. doi: 10.1080/00039896.1979.10667367. [DOI] [PubMed] [Google Scholar]
- Jones R., Reid L. Secretory cell hyperplasia and modification of intracellular glycoprotein in rat airways induced by short periods of exposure to tobacco smoke, and the effect of the antiinflammatory agent phenylmethyloxadiazole. Lab Invest. 1978 Jul;39(1):41–49. [PubMed] [Google Scholar]
- Jones R., Reid L. Secretory cells and their glycoproteins in health and disease. Br Med Bull. 1978 Jan;34(1):9–16. doi: 10.1093/oxfordjournals.bmb.a071466. [DOI] [PubMed] [Google Scholar]
- Kenoyer J. L., Phalen R. F., Davis J. R. Particle clearance from the respiratory tract as a test of toxicity: effect of ozone on short and long term clearance. Exp Lung Res. 1981 May;2(2):111–120. doi: 10.3109/01902148109052307. [DOI] [PubMed] [Google Scholar]
- LEV R., SPICER S. S. A HISTOCHEMICAL COMPARISON OF HUMAN EPITHELIAL MUCINS IN NORMAL AND IN HYPERSECRETORY STATES INCLUDING PANCREATIC CYSTIC FIBROSIS. Am J Pathol. 1965 Jan;46:23–47. [PMC free article] [PubMed] [Google Scholar]
- Lamb D., Reid L. Mitotic rates, goblet cell increase and histochemical changes in mucus in rat bronchial epithelium during exposure to sulphur dioxide. J Pathol Bacteriol. 1968 Jul;96(1):97–111. doi: 10.1002/path.1700960111. [DOI] [PubMed] [Google Scholar]
- Lamb D., Reid L. The tracheobronchial submucosal glands in cystic fibrosis: a qualitative and quantitative histochemical study. Br J Dis Chest. 1972 Oct;66(4):239–247. doi: 10.1016/0007-0971(72)90042-3. [DOI] [PubMed] [Google Scholar]
- Mellick P. W., Dungworth D. L., Schwartz L. W., Tyler W. S. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977 Jan;36(1):82–90. [PubMed] [Google Scholar]
- Proctor D. F., Adams G. K., Andersen I., Man S. F. Nasal mucociliary clearance in man. Ciba Found Symp. 1978;(54):219–234. doi: 10.1002/9780470720356.ch11. [DOI] [PubMed] [Google Scholar]
- SPICER S. S. DIAMINE METHODS FOR DIFFERENTIALING MUCOSUBSTANCES HISTOCHEMICALLY. J Histochem Cytochem. 1965 Mar;13:211–234. doi: 10.1177/13.3.211. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Chakrin L. W., Wardell J. R., Jr Effect of chronic sulfur dioxide inhalation on the carbohydrate histochemistry and histology of the canine respiratory tract. Am Rev Respir Dis. 1974 Jul;110(1):13–24. doi: 10.1164/arrd.1974.110.6P2.13. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Plopper C. G., Dungworth D. L. The response of the macaque tracheobronchial epithelium to acute ozone injury. A quantitative ultrastructural and autoradiographic study. Am J Pathol. 1984 Aug;116(2):193–206. [PMC free article] [PubMed] [Google Scholar]



