Abstract
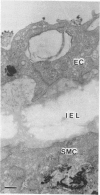
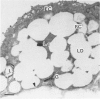
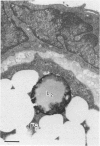
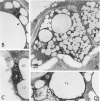
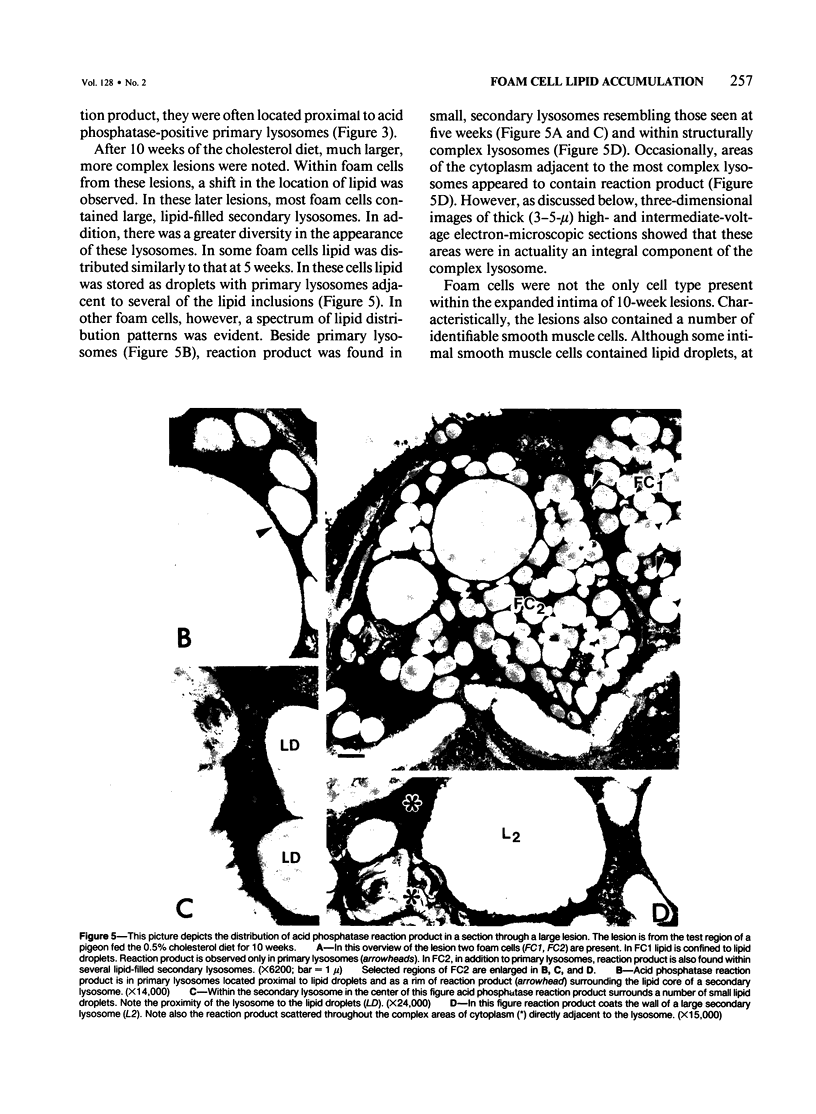
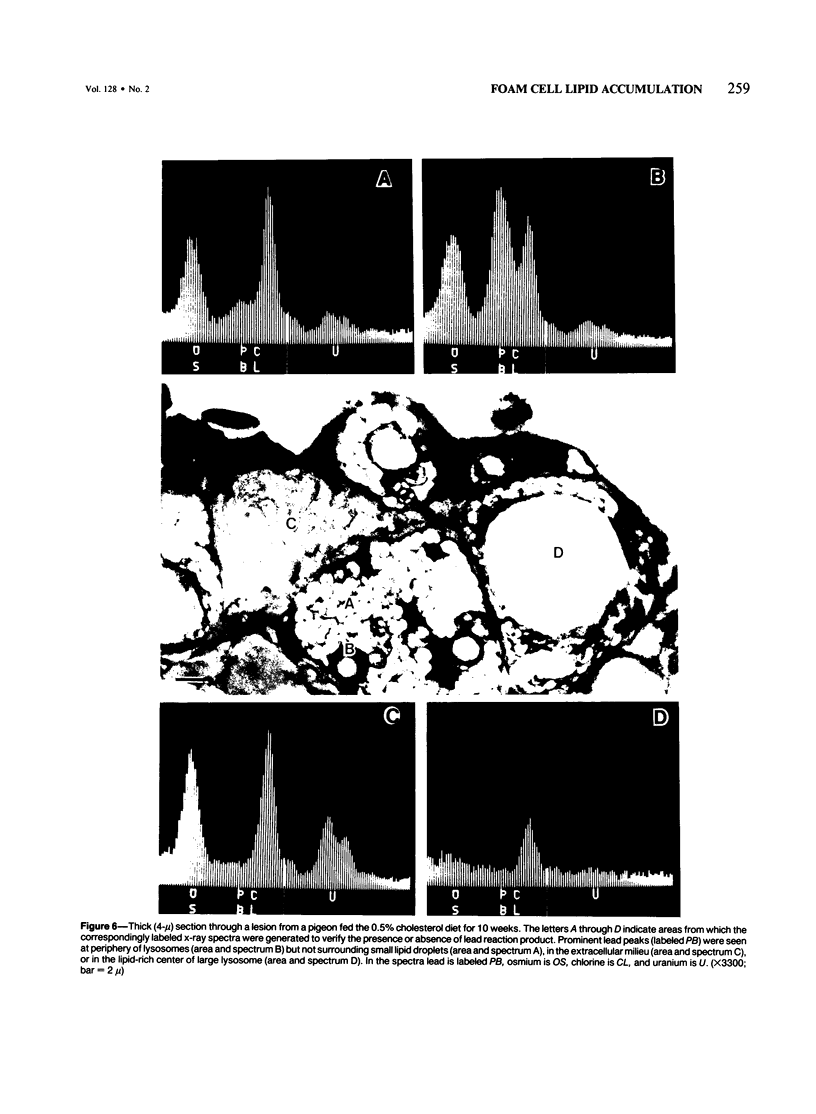
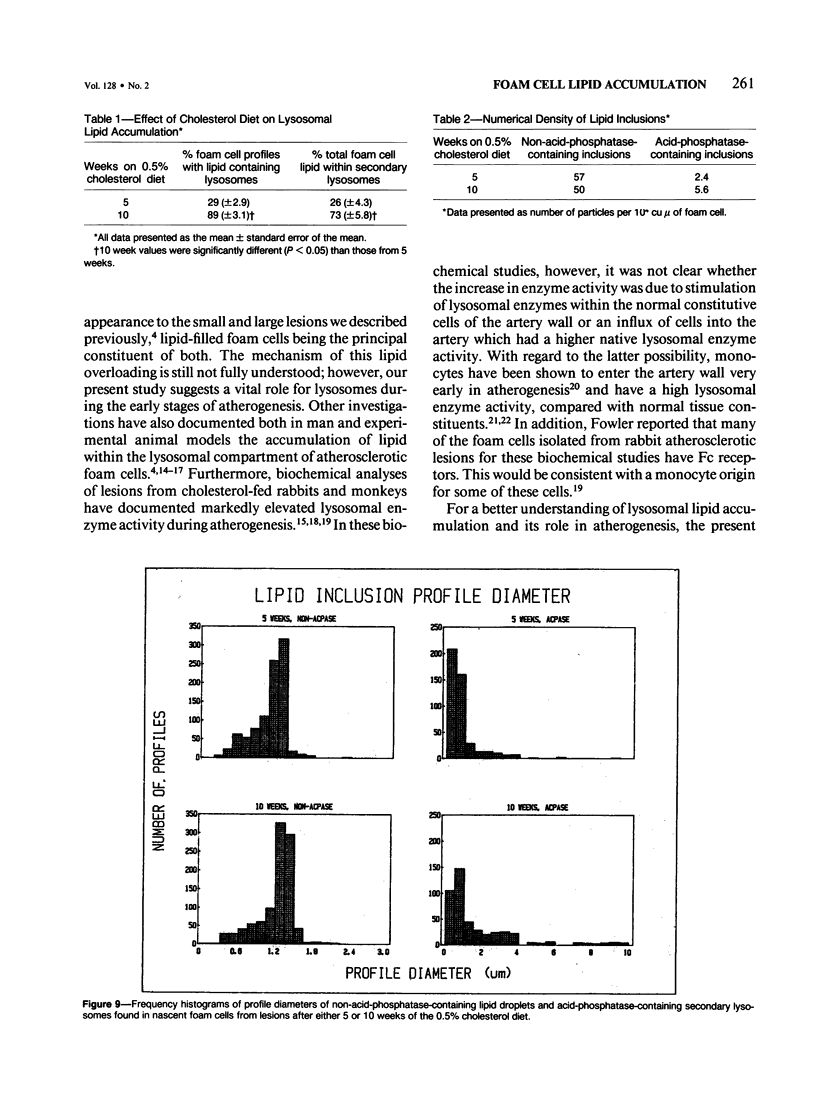
The role of lysosomes in aortic atherogenesis in White Carneau pigeons was examined by means of acid phosphatase cytochemistry. Foam cells were the major constituent of nascent atherosclerotic lesions in pigeons fed a 0.5% cholesterol diet for either 5 or 10 weeks. Seventy-four percent of foam cell lipid from animals at 5 weeks was in cytoplasmic droplets. The remaining lipid appeared in secondary lysosomes. After 10 weeks of cholesterol feeding, lysosomal lipid accounted for 73% of the lipid volume. The lipid accumulation correlated with increases in both size and number of lysosomes. An average of 2.4 lysosomes per 10(4) cu mu of cytoplasm was observed at 5 weeks. This value doubled by 10 weeks. The average lysosome diameter also increased between 5 and 10 weeks from 2.2 mu to 5.75 mu. Concomitantly, the complexity of lysosomes increased from simple, spherical organelles at 5 weeks to complex, multichambered organelles at 10 weeks. In contrast, lipid storage within cytoplasmic lipid droplets did not change either in size or in number. These observations suggest that by 5 weeks lipid storage within cytoplasmic droplets was maximized, and continued increases in lipid stores occurred predominantly through lysosomal loading.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Lewis J. C., Szejda P., Cowley L., McCall C. E. Activation of lysosomal acid phosphatase of eosinophil leukocytes. Lab Invest. 1981 May;44(5):403–409. [PubMed] [Google Scholar]
- Berberian P. A., Fowler S. The subcellular biochemistry of human arterial lesions. I. Biochemical constituents and marker enzymes in diseased and unaffected portions of human aortic specimens. Exp Mol Pathol. 1979 Feb;30(1):27–40. doi: 10.1016/0014-4800(79)90079-0. [DOI] [PubMed] [Google Scholar]
- Berberian P. A., Jenison M. W., Roddick V. Arterial prostaglandins and lysosomal function during atherogenesis. II. Isolated cells of diet-induced atherosclerotic aortas of rabbit. Exp Mol Pathol. 1985 Aug;43(1):36–55. doi: 10.1016/0014-4800(85)90053-x. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S., Berberian P. A., Shio H., Goldfischer S., Wolinsky H. Characterization of cell populations isolated from aortas of rhesus monkeys with experimental atherosclerosis. Circ Res. 1980 Apr;46(4):520–530. doi: 10.1161/01.res.46.4.520. [DOI] [PubMed] [Google Scholar]
- Fowler S. Characterization of foam cells in experimental atherosclerosis. Acta Med Scand Suppl. 1980;642:151–158. doi: 10.1111/j.0954-6820.1980.tb10947.x. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Schiller B., Wolinsky H. Lipid accumulation in smooth muscle cell lysosomes im primate atherosclerosis. Am J Pathol. 1975 Mar;78(3):497–504. [PMC free article] [PubMed] [Google Scholar]
- Haley N. J., Fowler S., de Duve C. Lysosomal acid cholesteryl esterase activity in normal and lipid-laden aortic cells. J Lipid Res. 1980 Nov;21(8):961–969. [PubMed] [Google Scholar]
- Haley N. J., Shio H., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. I. Resolution of aortic cell populations by metrizamide density gradient centrifugation. Lab Invest. 1977 Sep;37(3):287–296. [PubMed] [Google Scholar]
- Haust M. D. The morphogenesis and fate of potential and early atherosclerotic lesions in man. Hum Pathol. 1971 Mar;2(1):1–29. doi: 10.1016/s0046-8177(71)80019-9. [DOI] [PubMed] [Google Scholar]
- Hennig A., Elias H. A rapid method for the visual determination of size distribution of spheres from the size distribution of their sections. J Microsc. 1971 Apr;93(2):101–107. doi: 10.1111/j.1365-2818.1971.tb02271.x. [DOI] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C. Early atherogenesis in White Carneau pigeons. I. Leukocyte margination and endothelial alterations at the celiac bifurcation. Am J Pathol. 1984 Jul;116(1):56–68. [PMC free article] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C. Early atherogenesis in White Carneau pigeons. II. Ultrastructural and cytochemical observations. Am J Pathol. 1985 May;119(2):210–222. [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jerome W. G. Foam cell characteristics in coronary arteries and aortas of White Carneau pigeons with moderate hypercholesterolemia. Ann N Y Acad Sci. 1985;454:91–100. doi: 10.1111/j.1749-6632.1985.tb11847.x. [DOI] [PubMed] [Google Scholar]
- Lough J., Fawcett J., Wiegensberg B. Wolman's disease. An electron microscopic, histochemical, and biochemical study. Arch Pathol. 1970 Feb;89(2):103–110. [PubMed] [Google Scholar]
- Miller B. F., Kothari H. V. Increased activity of lysosomal enzymes in human atherosclerotic aortas. Exp Mol Pathol. 1969 Jun;10(3):288–294. doi: 10.1016/0014-4800(69)90058-6. [DOI] [PubMed] [Google Scholar]
- Musson R. A., Shafran H., Henson P. M. Intracellular levels and stimulated release of lysosomal enzymes from human peripheral blood monocytes and monocyte-derived macrophages. J Reticuloendothel Soc. 1980 Sep;28(3):249–264. [PubMed] [Google Scholar]
- Peters T. J., De Duve C. Lysosomes of the arterial wall. II. Subcellular fractionation of aortic cells from rabbits with experimantal atheroma. Exp Mol Pathol. 1974 Apr;20(2):228–256. doi: 10.1016/0014-4800(74)90057-4. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Müller M., De Duve C. Lysosomes of the arterial wall. I. Isolation and subcellular fractionation of cells from normal rabbit aorta. J Exp Med. 1972 Nov 1;136(5):1117–1139. doi: 10.1084/jem.136.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shio H., Farquhar M. G., de Duve C. Lysosomes of the arterial wall. IV. Cytochemical localization of acid phosphatase and catalase in smooth muscle cells and foam cells from rabbit atheromatous aorta. Am J Pathol. 1974 Jul;76(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Shio H., Haley N. J., Fowler S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. III. Intracellular localization of cholesterol and cholesteryl ester. Lab Invest. 1979 Aug;41(2):160–167. [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Fowler S. Participation of lysosomes in atherosclerosis. N Engl J Med. 1978 Nov 23;299(21):1173–1178. doi: 10.1056/NEJM197811232992107. [DOI] [PubMed] [Google Scholar]