Abstract
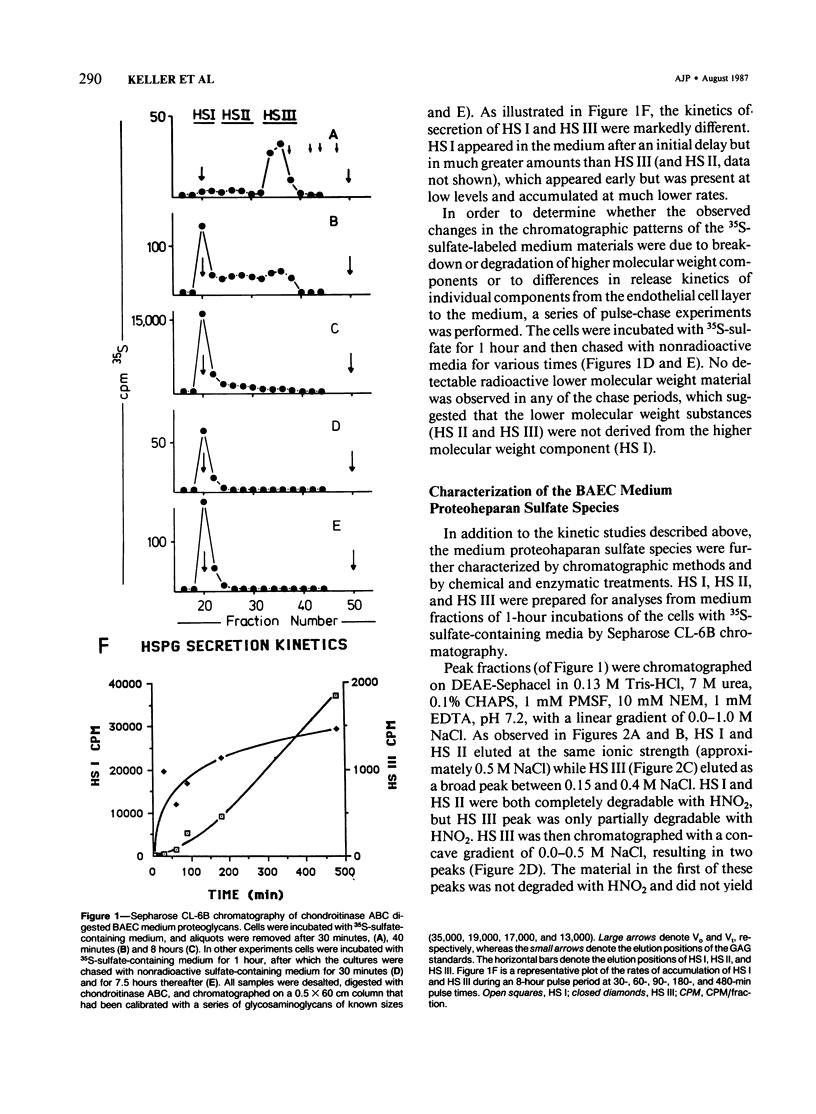
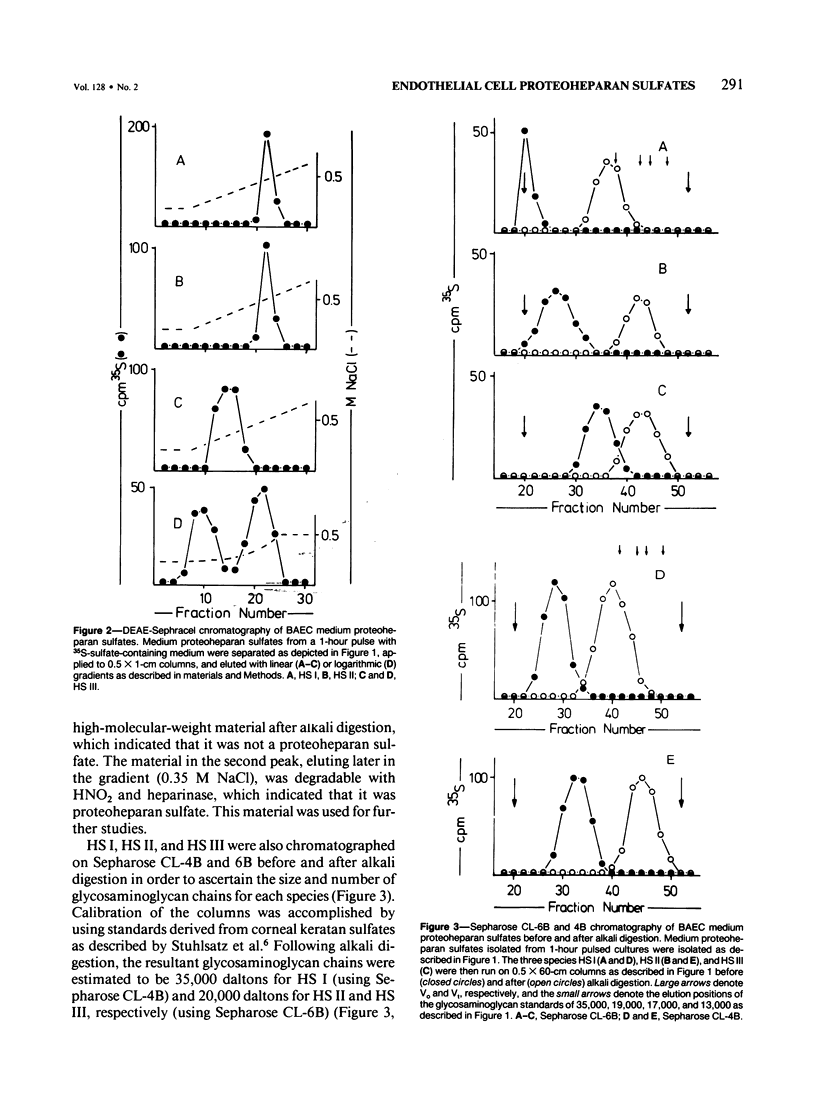
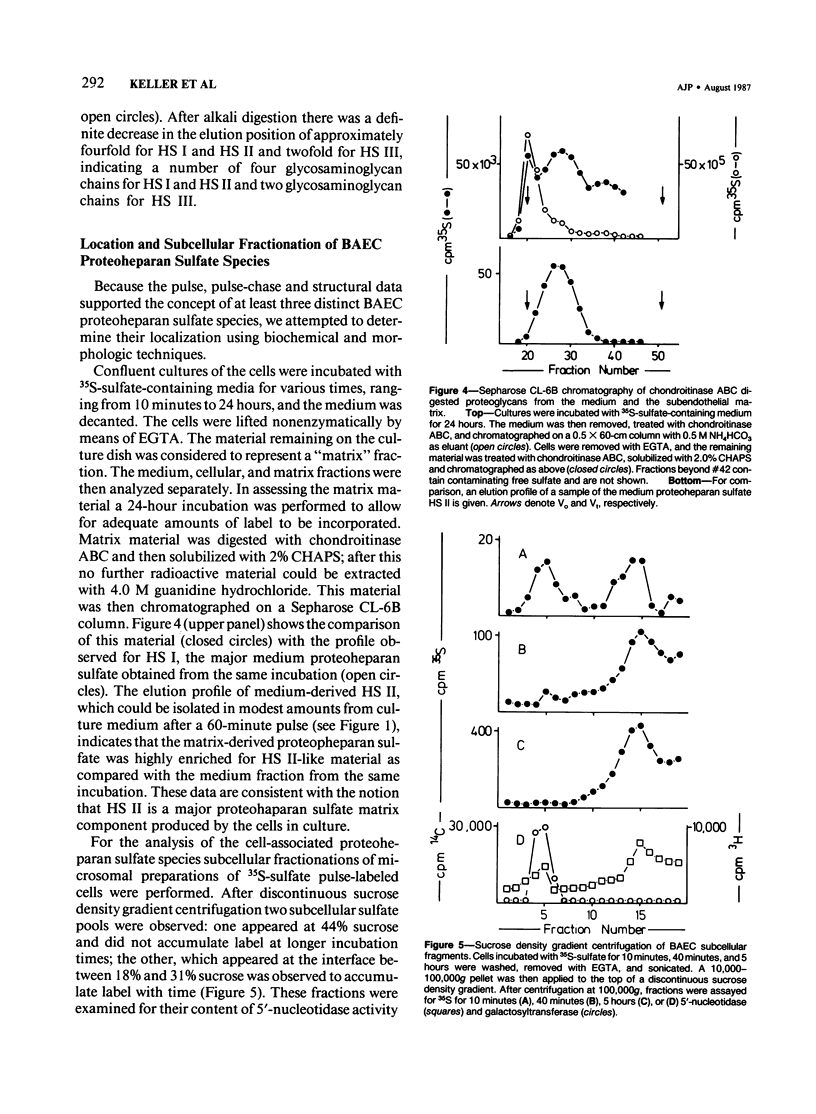
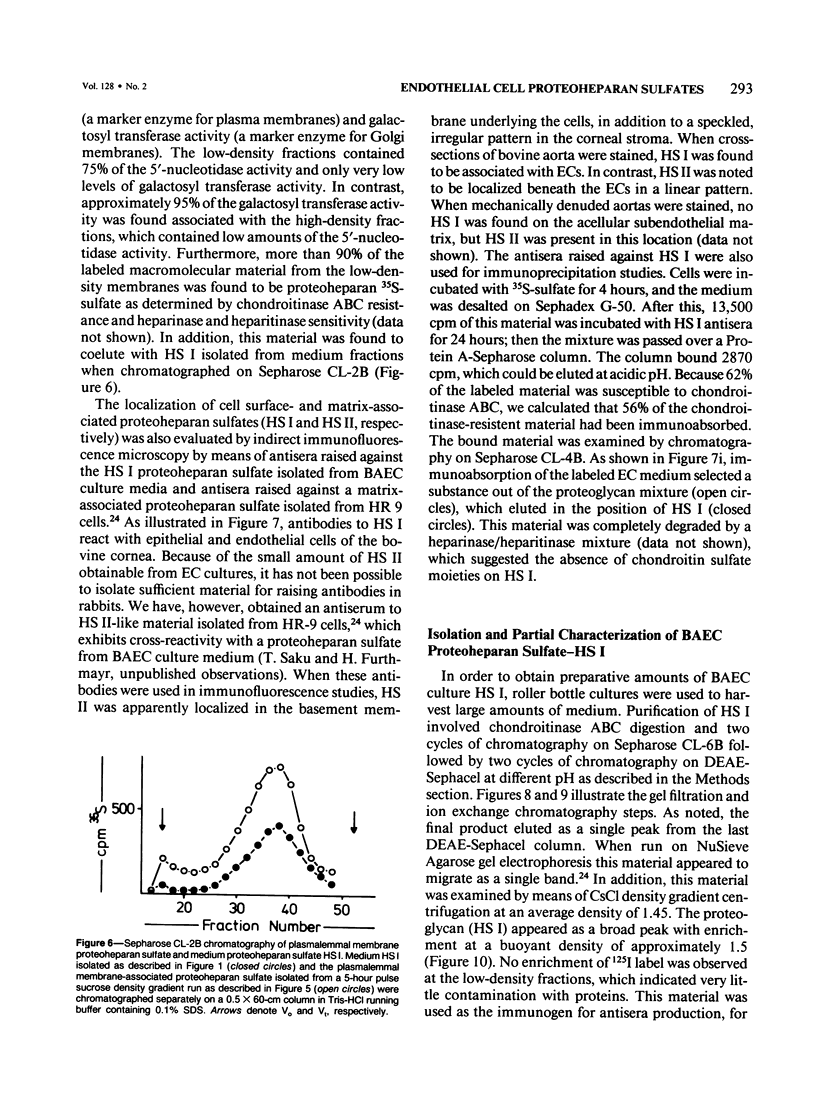
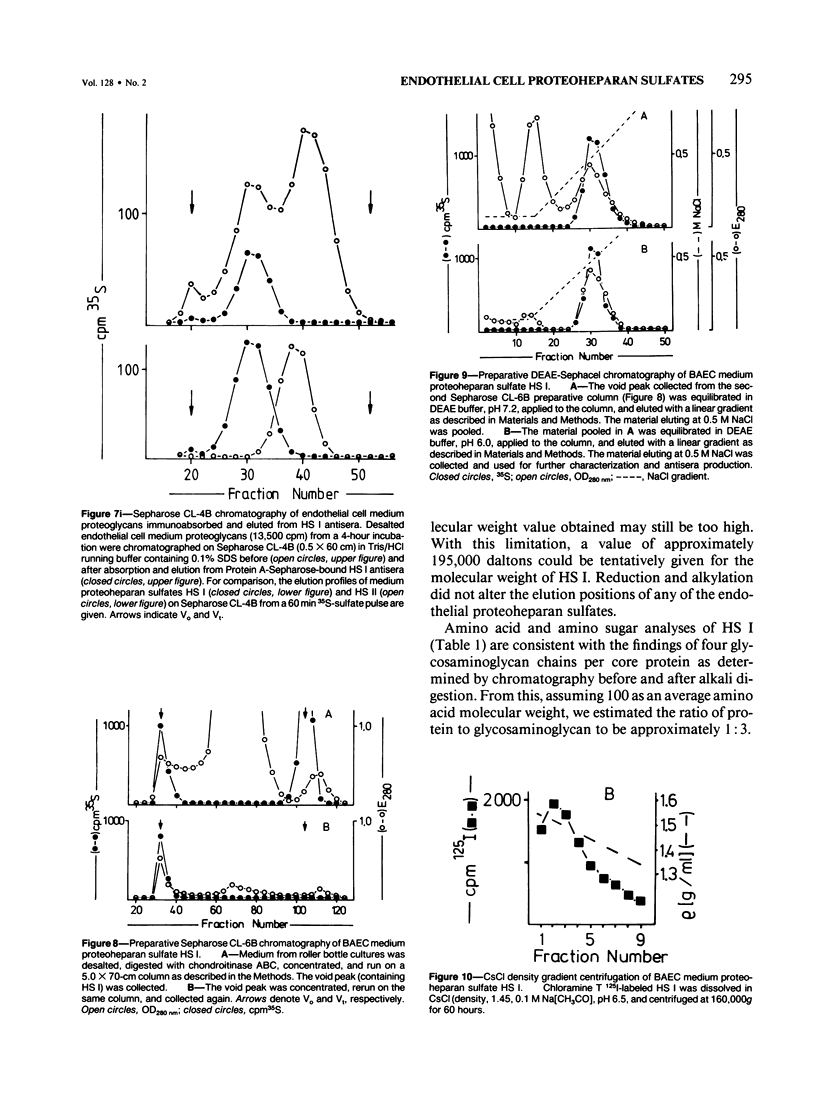
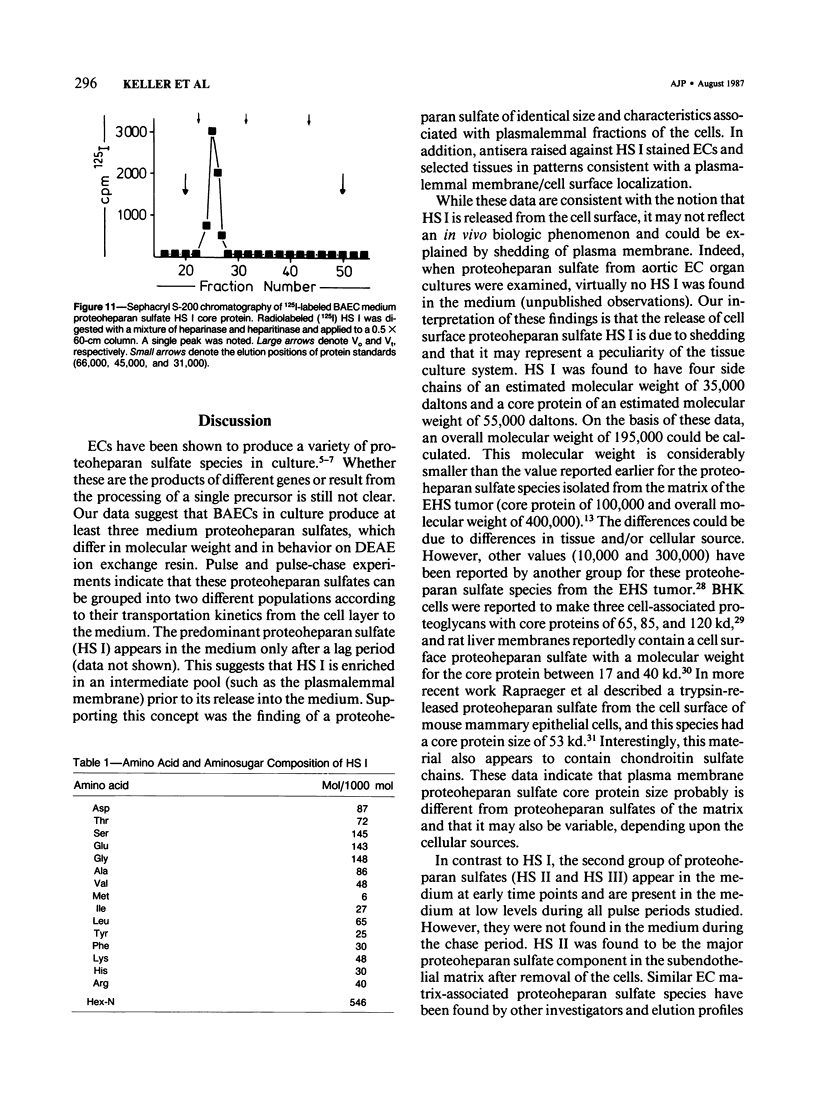
Proteoheparan sulfate biosynthesis was studied in cultured bovine aortic endothelial cells by means of pulse and pulse-chase experiments and subcellular fractionations. Three proteoheparan sulfate species were found in the medium. The major species, which the authors have called HS I, appeared in the medium only after an initial lag period and was also found associated with the plasma membrane. The other two (HS II and HS III) appeared in small amounts in the medium at early time points. At later times these were not readily observed because the large amounts of HS I present in the medium. The major medium species, HS I, appeared to be composed of approximately four heparan sulfate chains of approximately 35,000 daltons and a core protein of approximately 55,000 daltons apparent molecular weight. HS I appeared to be homogeneous by gel filtration on Sepharose CL 2B and 6B and elution from DEAE Sephacel, electrophoresis on Nu-Sieve agarose, and CsCl density centrifugation. After digestion with heparinase the core protein appeared to be homogeneous by S-200 Sephacel chromatography. HS I was also found associated with plasma membrane fractions of the cultured bovine aortic endothelial cells, and antisera raised against it stained epithelial and endothelial cells in patterns consistent with a cell surface localization. Of the other two species found in the medium, one (HS II) also appeared to be a component of the cell layer. This species appeared to contain approximately four heparan sulfate chains of approximately 20,000 daltons apparent molecular weight. Antisera raised against a similar molecule produced by HR 9 cell cultures stained basement membranes intensely, supporting the subcellular matrix localization of this molecule. The third species (HS III) was detected in culture medium only and apparently contained two heparan sulfate chains of approximately 20,000 daltons apparent molecular weight. These results support the concept of multiple endothelial cell proteoheparan sulfate species which exhibit differences in structure and localization and possibly diverse specialized functions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- Bretscher M. S. Heparan sulphate proteoglycans and their polypeptide chains from BHK cells. EMBO J. 1985 Aug;4(8):1941–1944. doi: 10.1002/j.1460-2075.1985.tb03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonassisi V., Colburn P. Antibodies to the heparan sulfate proteoglycans synthesized by endothelial cell cultures. Biochim Biophys Acta. 1983 Oct 4;760(1):1–12. doi: 10.1016/0304-4165(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Conrad G. W., Hamilton C., Haynes E. Differences in glycosaminoglycans synthesized by fibroblast-like cells from chick cornea, heart, and skin. J Biol Chem. 1977 Oct 10;252(19):6861–6870. [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Wiedemann H., Timpl R., Lustig A., Engel J. Structure and interactions of heparan sulfate proteoglycans from a mouse tumor basement membrane. Eur J Biochem. 1984 Aug 15;143(1):145–157. doi: 10.1111/j.1432-1033.1984.tb08353.x. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Leyshon W. C., Ledbetter S. R., Tyree B., Suzuki S., Kato M., Kimata K., Kleinman H. K. Isolation of two forms of basement membrane proteoglycans. J Biol Chem. 1985 Jul 5;260(13):8098–8105. [PubMed] [Google Scholar]
- Karnovsky M. J. Endothelial--vascular smooth muscle cell interactions. Rous--Whipple Award Lecture. Am J Pathol. 1981 Dec;105(3):200–206. [PMC free article] [PubMed] [Google Scholar]
- Keller R., Furthmayr H. Isolation and characterization of basement membrane and cell proteoheparan sulphates from HR9 cells. Eur J Biochem. 1986 Dec 15;161(3):707–714. doi: 10.1111/j.1432-1033.1986.tb10497.x. [DOI] [PubMed] [Google Scholar]
- Kinsella M. G., Wight T. N. Modulation of sulfated proteoglycan synthesis by bovine aortic endothelial cells during migration. J Cell Biol. 1986 Mar;102(3):679–687. doi: 10.1083/jcb.102.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I. Basement membrane-like matrix of teratocarcinoma-derived endodermal cells: presence of laminin and heparan sulfate in the matrix at points of attachment to cells. J Histochem Cytochem. 1983 Jan;31(1):35–45. doi: 10.1177/31.1.6187802. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Barwick K. W. Use of avidin-biotin complex in an ELISA system: a quantitative comparison with two other immunoperoxidase detection systems using keratin antisera. Lab Invest. 1983 Jan;48(1):98–107. [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Madri J. A., Foellmer H. G., Furthmayr H. Type V collagens of the human placenta: trimer alpha-chain composition, ultrastructural morphology and peptide analysis. Coll Relat Res. 1982 Jan;2(1):19–29. doi: 10.1016/s0174-173x(82)80038-1. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Anticoagulantly active heparin-like molecules from vascular tissue. Biochemistry. 1984 Apr 10;23(8):1730–1737. doi: 10.1021/bi00303a023. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Kjellén L., Hök M. Cell-surface heparan sulfate. Isolation and characterization of a proteoglycan from rat liver membranes. J Biol Chem. 1979 Sep 10;254(17):8505–8510. [PubMed] [Google Scholar]
- Oohira A., Wight T. N., Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983 Feb 10;258(3):2014–2021. [PubMed] [Google Scholar]
- Pratt B. M., Harris A. S., Morrow J. S., Madri J. A. Mechanisms of cytoskeletal regulation. Modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol. 1984 Dec;117(3):349–354. [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Endo E., Koda J., Bernfield M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. J Biol Chem. 1985 Sep 15;260(20):11046–11052. [PubMed] [Google Scholar]
- Rollins B. J., Culp L. A. Preliminary characterization of the proteoglycans in the substrate adhesion sites of normal and virus-transformed murine cells. Biochemistry. 1979 Dec 11;18(25):5621–5629. doi: 10.1021/bi00592a016. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D. Role of heparin and heparinlike molecules in thrombosis and atherosclerosis. Fed Proc. 1985 Feb;44(2):404–409. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Stuhlsatz H. W., Hirtzel F., Keller R., Cosma S., Greiling H. Studies on the polydispersity and heterogeneity of proteokeratan sulfate from calf and porcine cornea. Hoppe Seylers Z Physiol Chem. 1981 Jul;362(7):841–852. doi: 10.1515/bchm2.1981.362.2.841. [DOI] [PubMed] [Google Scholar]
- Tyree B., Horigan E. A., Klippenstein D. L., Hassell J. R. Heterogeneity of heparan sulfate proteoglycans synthesized by PYS-2 cells. Arch Biochem Biophys. 1984 Jun;231(2):328–335. doi: 10.1016/0003-9861(84)90395-3. [DOI] [PubMed] [Google Scholar]



