Abstract
Self-filling blind loops were created experimentally in jejunal segments of specific pathogen-free male Wistar rats, and the loop contents and mucosa were examined over an 8-week period for evaluation of the interaction between mucus and luminal bacteria. Corresponding jejunal segments from rats that did not undergo surgery were used as controls. Proliferation of anaerobic bacteria developed in the test animals by the first week after surgery. Despite anaerobic bacterial proliferation, no adherence by bacteria to the intestinal microvillus surface was observed by scanning or transmission electron microscopy. Rather, bacteria were present within the mucus layer overlying the intestinal mucosal surface. Immunoassay of goblet cell mucin demonstrated an increase in the proportion of mucin present in the intestinal lumen and a decrease in mucin levels in the jejunal mucosa. These results suggest that the interaction of bacteria with mucus is an important mechanism of protection of the mucosal surface in experimental small bowel bacterial overgrowth.
Full text
PDF


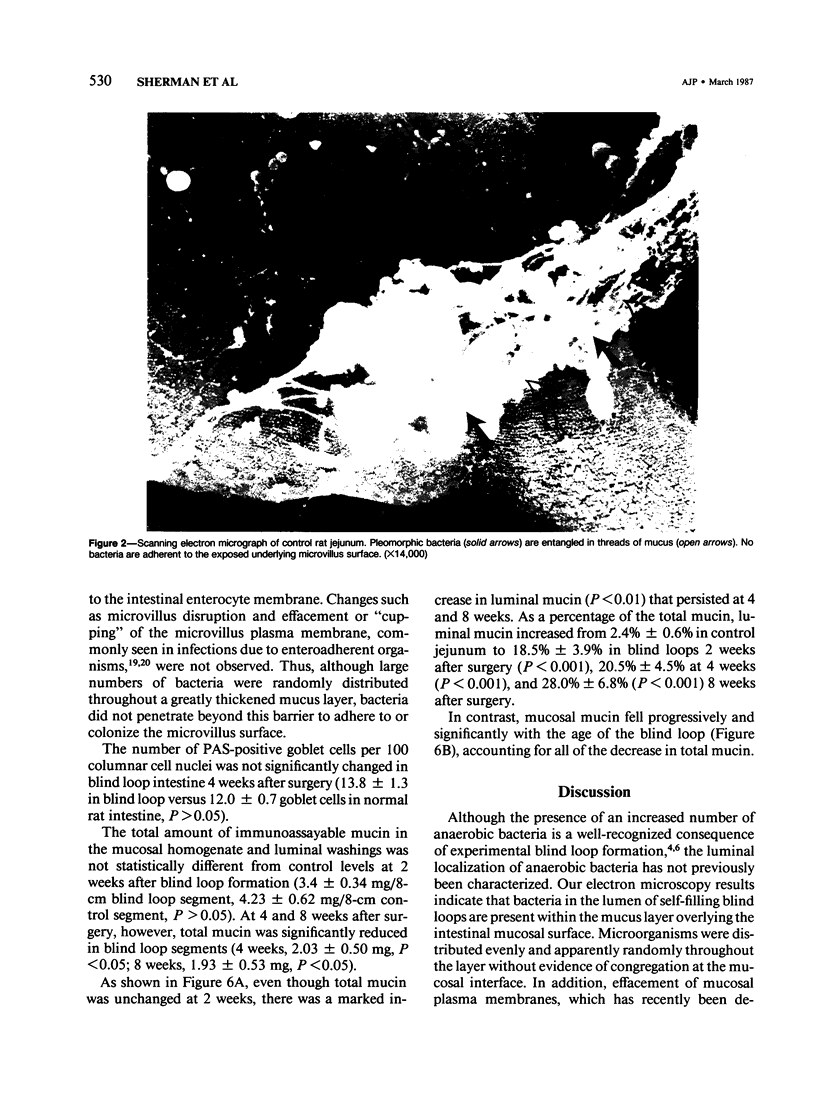
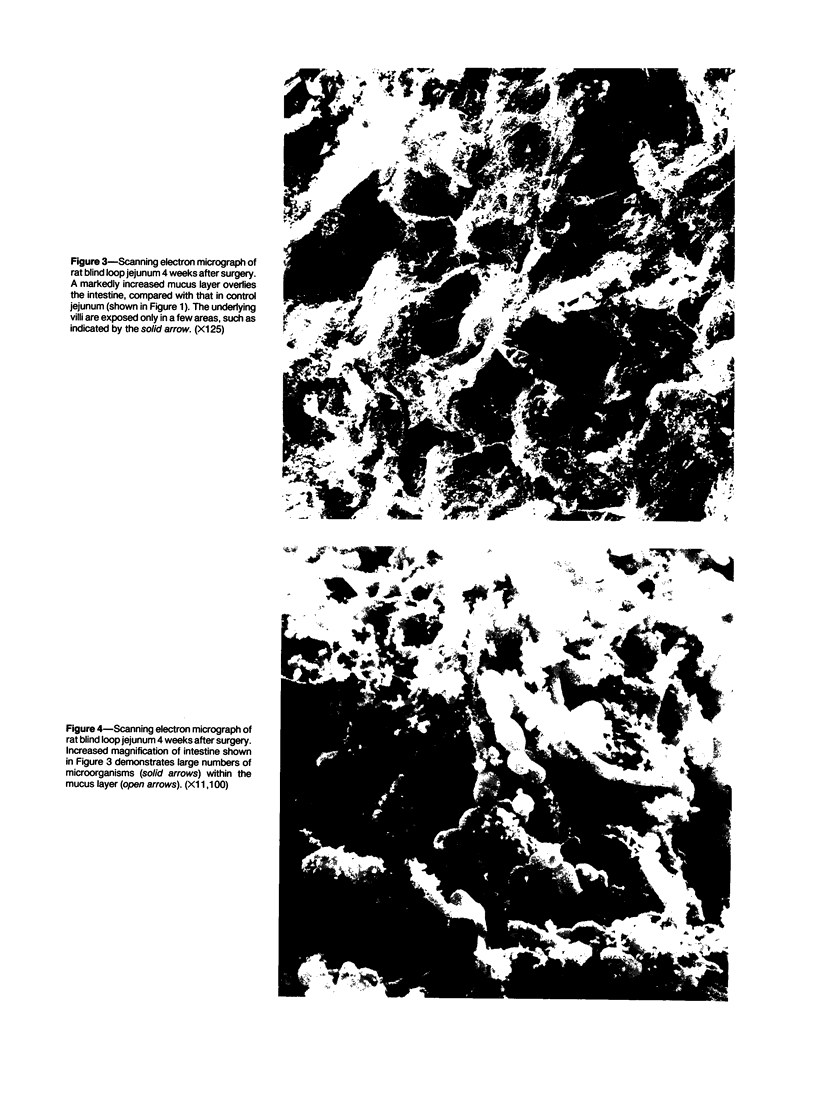
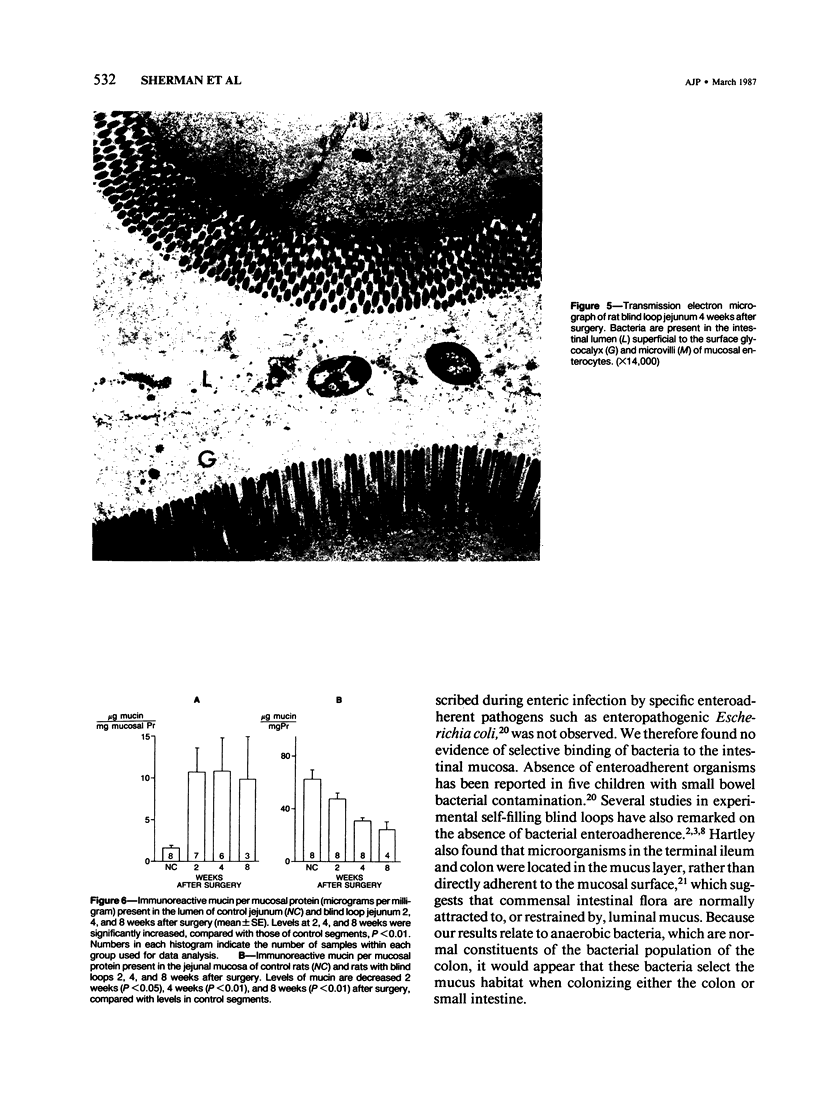


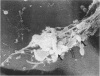
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ament M. E., Shimoda S. S., Saunders D. R., Rubin C. E. Pathogenesis of steatorrhea in three cases of small intestinal stasis syndrome. Gastroenterology. 1972 Nov;63(5):728–747. [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F. Intestinal mucins in health and disease. Digestion. 1978;17(3):234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Toskes P. P. Jejunal brush border injury and impaired sugar and amino acid uptake in the blind loop syndrome. Gastroenterology. 1974 Nov;67(5):965–974. [PubMed] [Google Scholar]
- Gracey M., Burke V., Oshin A., Barker J., Glasgow E. F. Bacteria, bile salts, and intestinal monosaccharide malabsorption. Gut. 1971 Sep;12(9):683–692. doi: 10.1136/gut.12.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey M., Papadimitriou J., Bower G. Ultrastructural changes in the small intestines of rats with self-filling blind loops. Gastroenterology. 1974 Oct;67(4):646–651. [PubMed] [Google Scholar]
- Hampton J. C., Rosario B. The attachment of microorganisms to epithelial cells in the distal ileum of the mouse. Lab Invest. 1965 Aug;14(8):1464–1481. [PubMed] [Google Scholar]
- Hartley C. L., Neumann C. S., Richmond M. H. Adhesion of commensal bacteria to the large intestine wall in humans. Infect Immun. 1979 Jan;23(1):128–132. doi: 10.1128/iai.23.1.128-132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E. D. Progesterone level and response to oxytocin at term. Lancet. 1968 Sep 7;2(7567):570–570. doi: 10.1016/s0140-6736(68)92430-6. [DOI] [PubMed] [Google Scholar]
- Jonas A., Flanagan P. R., Forstner G. G. Pathogenesis of mucosal injury in the blind loop syndrome. Brush border enzyme activity and glycoprotein degradation. J Clin Invest. 1977 Dec;60(6):1321–1330. doi: 10.1172/JCI108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas A., Forstner G. The effect of biliary diversion on mucosal enzyme activity and brush border glycoprotein degradation in rats with self-filling blind loops. Eur J Clin Invest. 1979 Apr;9(2 Pt 1):167–173. doi: 10.1111/j.1365-2362.1979.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Jonas A., Krishnan C., Forstner G. Pathogenesis of mucosal injury in the blind loop syndrome. Gastroenterology. 1978 Nov;75(5):791–795. [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Short H. B. Enterotoxigenicity of colonising coliform bacteria in tropical sprue and blind-loop syndrome. Lancet. 1978 Aug 12;2(8085):342–344. doi: 10.1016/s0140-6736(78)92942-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Prizont R., Konigsberg N. Identification of bacterial glycosidases in rat cecal contents. Dig Dis Sci. 1981 Sep;26(9):773–777. doi: 10.1007/BF01309607. [DOI] [PubMed] [Google Scholar]
- Riepe S. P., Goldstein J., Alpers D. H. Effect of secreted Bacteroides proteases on human intestinal brush border hydrolases. J Clin Invest. 1980 Aug;66(2):314–322. doi: 10.1172/JCI109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Stanley R. A. In vitro utilization of mucin by Bacteroides fragilis. Appl Environ Microbiol. 1982 Feb;43(2):325–330. doi: 10.1128/aem.43.2.325-330.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomi N., Laburthe M., Fleming N., Crowther R., Forstner J. Cholera-induced mucin secretion from rat intestine: lack of effect of cAMP, cycloheximide, VIP, and colchicine. Am J Physiol. 1984 Aug;247(2 Pt 1):G140–G148. doi: 10.1152/ajpgi.1984.247.2.G140. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J. E. Isolation and identification of anaerobic bacteria. Hum Pathol. 1976 Mar;7(2):177–186. doi: 10.1016/s0046-8177(76)80021-4. [DOI] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Sherman P., Wesley A., Forstner G. Sequential disaccharidase loss in rat intestinal blind loops: impact of malnutrition. Am J Physiol. 1985 Jun;248(6 Pt 1):G626–G632. doi: 10.1152/ajpgi.1985.248.6.G626. [DOI] [PubMed] [Google Scholar]
- Specian R. D., Neutra M. R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol. 1980 Jun;85(3):626–640. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaqchali S. The pathophysiological role of small intestinal bacterial flora. Scand J Gastroenterol Suppl. 1970;6:139–163. [PubMed] [Google Scholar]
- Toskes P. P., Giannella R. A., Jervis H. R., Rout W. R., Takeuchi A. Small intestinal mucosal injury in the experimental blind loop syndrome. Light- and electron-microscopic and histochemical studies. Gastroenterology. 1975 May;68(5 Pt 1):1193–1203. [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]