Abstract
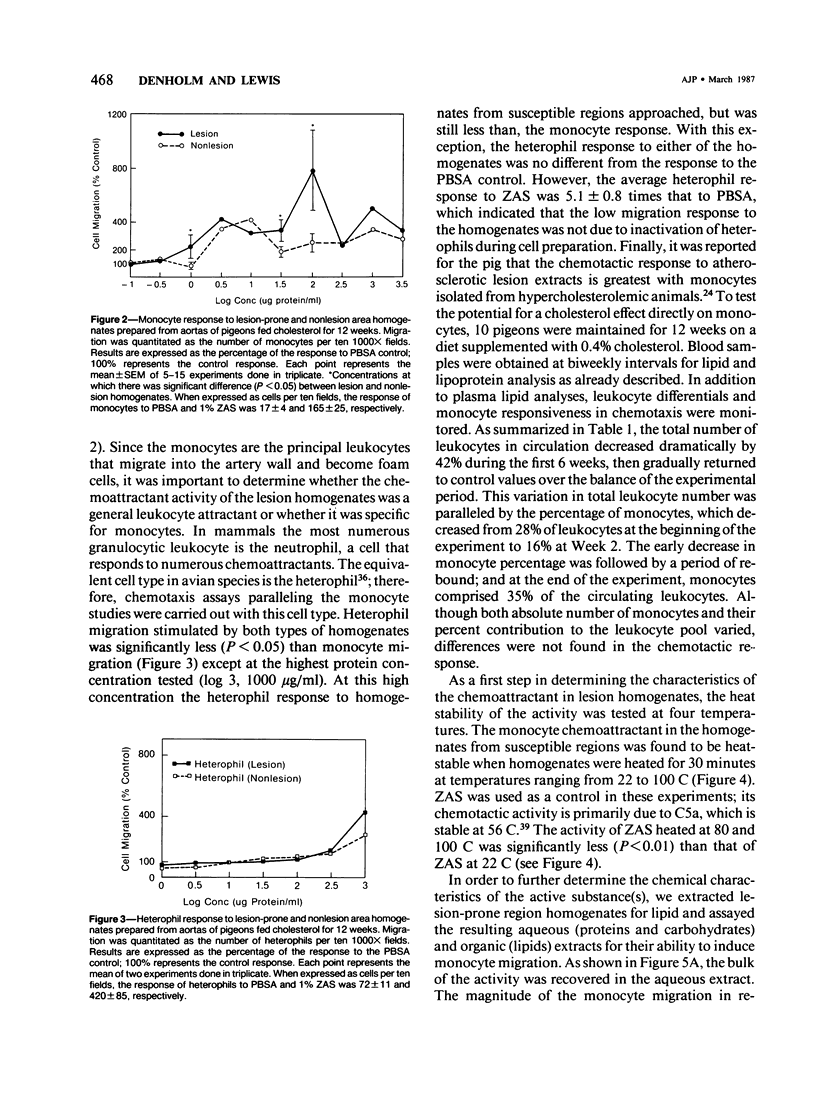
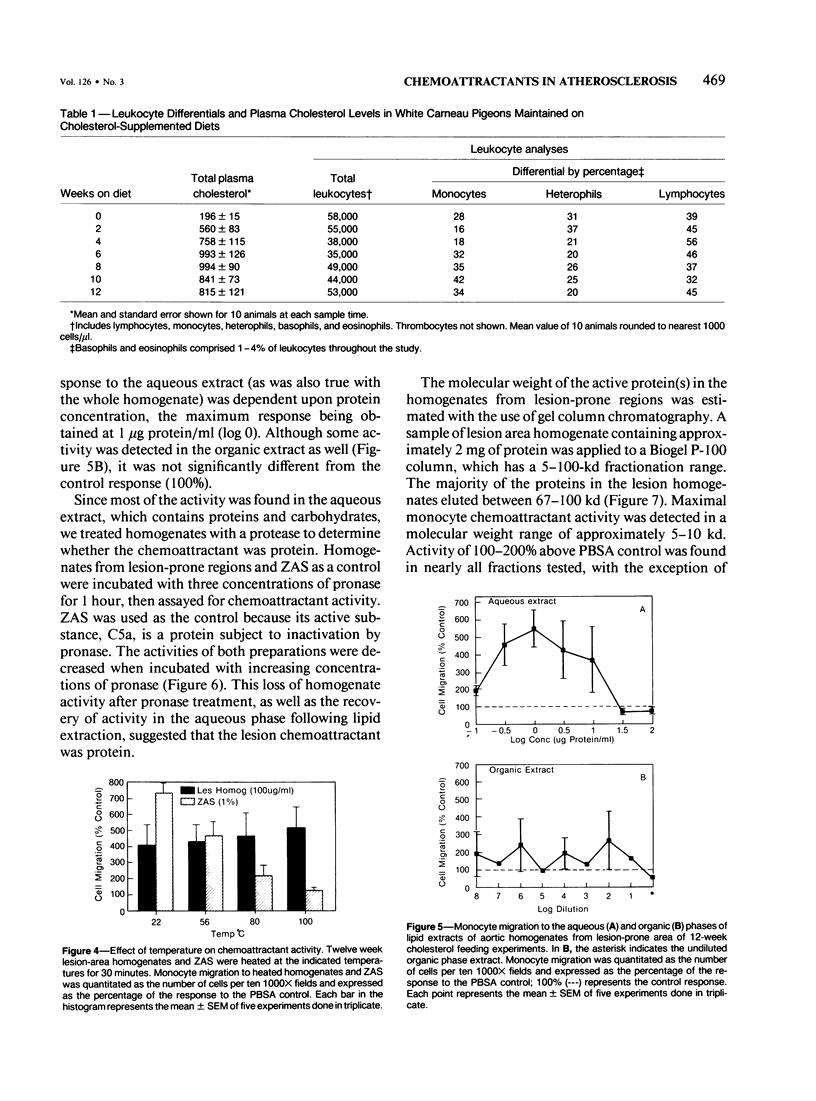
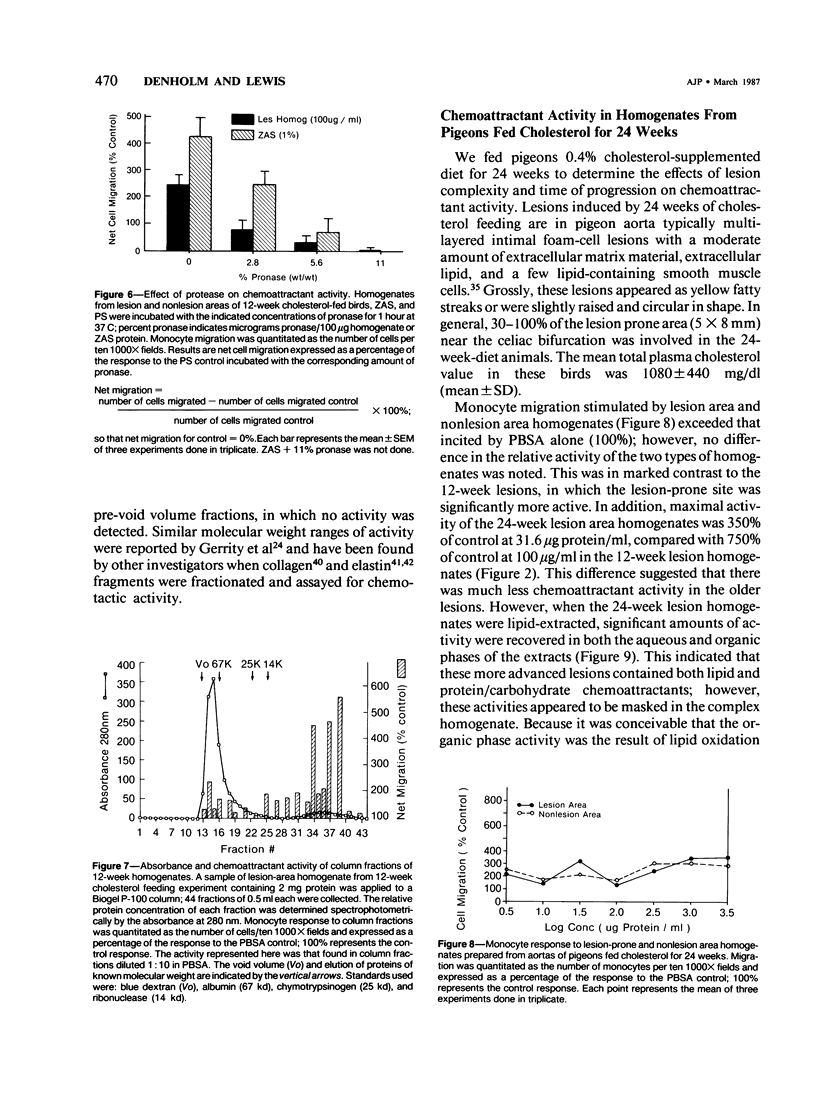
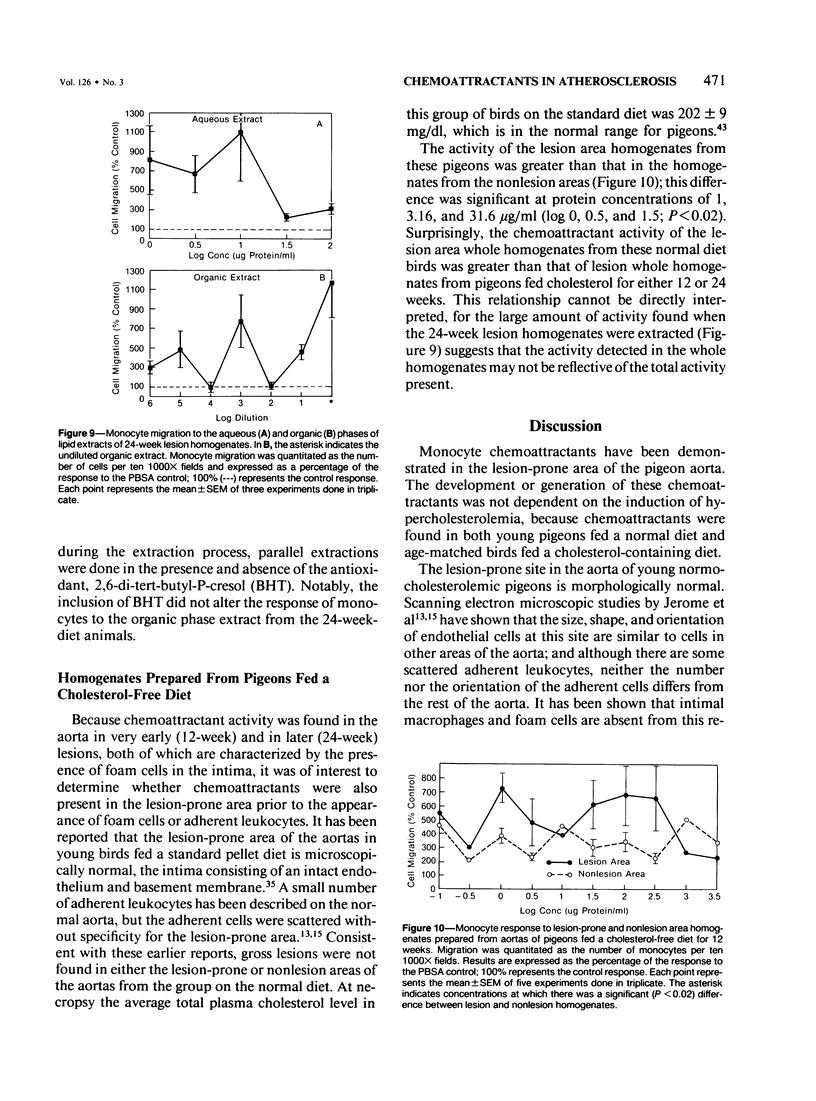
Atherosclerosis occurs in the aorta of White Carneau pigeons proximal to the celiac bifurcation, where monocyte adhesion and migration into lesions have been demonstrated. This study documents chemoattractants that might be responsible for monocyte adherence and migration. Ten-week-old pigeons were fed either a cholesterol-free (normal) diet or a 0.4% cholesterol diet for 12 or 24 weeks. Birds with a normal diet did not have lesions in the lesion-prone area of the aorta, whereas birds fed a cholesterol-containing diet had simple intimal foam-cell lesions (12 weeks) or foam-cell lesions complicated with extracellular lipid and fibrillar matrix material (24 weeks). Plasma cholesterol levels in birds on the cholesterol-containing diet were 780-1080 mg/dl versus 140-240 mg/dl in the normal diet control group(s) at necropsy. To assay for chemoattractants, tissue was collected from lesion-prone and nonsusceptible (nonlesion) areas of the aortas. Samples from the two types of regions were separately pooled, then homogenized and tested for chemoattractant activity for pigeon peripheral blood monocytes. Monocyte chemoattractants were demonstrated in lesion area homogenates from pigeons fed cholesterol for 12 or 24 weeks and also in analogous homogenates from pigeons fed a normal diet. Monocyte migration to lesion-prone homogenates was significantly greater than that to nonlesion area homogenates. The chemoattractants in homogenates were monocyte-specific. The chemoattractant activity in the birds fed cholesterol for 12 weeks was confined to the aqueous phase of lipid extracts. This activity was abolished by pronase but unaffected by heat (100 C, 30 minutes), which indicated that the chemoattractant(s) in these homogenates was heat-stable protein(s). Activity in lipid extracts of lesion area homogenates from birds fed a cholesterol-containing diet for 24 weeks was found in both the aqueous and organic phases, suggesting that these samples contained lipid as well as proteinaceous chemoattractants.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Bayliss O. B. Detection of macrophages in atherosclerotic lesions with cytochrome oxidase. Br J Exp Pathol. 1976 Feb;57(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Fenton J. W., 2nd, Wilner G. D. Chemotactic response of monocytes to thrombin. J Cell Biol. 1983 Jan;96(1):282–285. doi: 10.1083/jcb.96.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Walsh C. E., Thomas M. J., Wykle R. L., DeChatelet L. R., Waite B. M. Platelet activating factor. Stimulation of the lipoxygenase pathway in polymorphonuclear leukocytes by 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine. J Biol Chem. 1982 May 25;257(10):5402–5407. [PubMed] [Google Scholar]
- Cookson F. B. The origin of foam cells in atherosclerosis. Br J Exp Pathol. 1971 Feb;52(1):62–69. [PMC free article] [PubMed] [Google Scholar]
- Ernst J. D., Hartiala K. T., Goldstein I. M., Sande M. A. Complement (C5)-derived chemotactic activity accounts for accumulation of polymorphonuclear leukocytes in cerebrospinal fluid of rabbits with pneumococcal meningitis. Infect Immun. 1984 Oct;46(1):81–86. doi: 10.1128/iai.46.1.81-86.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Leonard E. J. Human monocyte chemotaxis: migrating cells are a subpopulation with multiple chemotaxin specificities on each cell. Infect Immun. 1980 Sep;29(3):953–959. doi: 10.1128/iai.29.3.953-959.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fowler S., Shio H., Haley N. J. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. IV. Investigation of macrophage-like properties of aortic cell populations. Lab Invest. 1979 Oct;41(4):372–378. [PubMed] [Google Scholar]
- Fritz K. E., Daoud A. S., Jarmolych J. Study of esterase-positive cells in swine atherosclerosis. Artery. 1980;8(3):220–224. [PubMed] [Google Scholar]
- Gaton E., Wolman M. The role of smooth muscle cells and hematogenous macrophages in atheroma. J Pathol. 1977 Oct;123(2):123–128. doi: 10.1002/path.1711230208. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Goss J. A., Soby L. Control of monocyte recruitment by chemotactic factor(s) in lesion-prone areas of swine aorta. Arteriosclerosis. 1985 Jan-Feb;5(1):55–66. doi: 10.1161/01.atv.5.1.55. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K. Ultrastructural identification of monocyte-derived foam cells in fatty streak lesions. Artery. 1980;8(3):208–214. [PubMed] [Google Scholar]
- Gerrity R. G., Richardson M., Somer J. B., Bell F. P., Schwartz C. J. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am J Pathol. 1977 Nov;89(2):313–334. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Klickstein L. B., Watt K. W., Wintroub B. U. The preferential human mononuclear leukocyte chemotactic activity of the substituent tetrapeptides of angiotensin II. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1097–1102. doi: 10.1016/0006-291x(80)91488-6. [DOI] [PubMed] [Google Scholar]
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Harvath L., Lazdins J. K., Alteri E., Leonard E. J. Differences in superoxide production by nonmigrating and migrating human monocyte subpopulations. Biochem Biophys Res Commun. 1982 Sep 16;108(1):392–398. doi: 10.1016/0006-291x(82)91879-4. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Davidson J. M., Rennard S., Szapiel S., Gadek J. E., Crystal R. G. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981 May 22;212(4497):925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- Janco R. L., English D. Regulation of monocyte oxidative metabolism: chemotactic factor enhancement of superoxide release, hydroxyl radical generation, and chemiluminescence. J Lab Clin Med. 1983 Dec;102(6):890–898. [PubMed] [Google Scholar]
- Jauchem J. R., Lopez M., Sprague E. A., Schwartz C. J. Mononuclear cell chemoattractant activity from cultured arterial smooth muscle cells. Exp Mol Pathol. 1982 Oct;37(2):166–174. doi: 10.1016/0014-4800(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C. Early atherogenesis in White Carneau pigeons. I. Leukocyte margination and endothelial alterations at the celiac bifurcation. Am J Pathol. 1984 Jul;116(1):56–68. [PMC free article] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C. Early atherogenesis in White Carneau pigeons. II. Ultrastructural and cytochemical observations. Am J Pathol. 1985 May;119(2):210–222. [PMC free article] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C., Taylor R. G., White M. S. Concurrent endothelial cell turnover and leukocyte margination in early atherosclerosis. Scan Electron Microsc. 1983;(Pt 3):1453–1459. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen M. P., Clarkson T. B., Prichard R. W. Animal models in atherosclerosis research. Exp Mol Pathol. 1985 Feb;42(1):1–28. doi: 10.1016/0014-4800(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Pepper D. S., McKenzie R. The identification of fibrinopeptide B as a chemotactic agent derived from human fibrinogen. Br J Haematol. 1974 Aug;27(4):669–677. doi: 10.1111/j.1365-2141.1974.tb06633.x. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Kaercher K., Plewa M., Fantone J. C., Ward P. A. Production of cyclooxygenase products and superoxide anion by macrophages in response to chemotactic factors. Prostaglandins. 1982 Dec;24(6):789–799. doi: 10.1016/0090-6980(82)90059-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis J. C., Kottke B. A. Endothelial damage and thrombocyte adhesion in pigeon atherosclerosis. Science. 1977 May 27;196(4293):1007–1009. doi: 10.1126/science.860128. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jerome W. G. Foam cell characteristics in coronary arteries and aortas of White Carneau pigeons with moderate hypercholesterolemia. Ann N Y Acad Sci. 1985;454:91–100. doi: 10.1111/j.1749-6632.1985.tb11847.x. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jones N. D., St Clair R. W., Cornhill J. F. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest. 1982 Feb;46(2):123–138. [PubMed] [Google Scholar]
- Mazzone T., Jensen M., Chait A. Human arterial wall cells secrete factors that are chemotactic for monocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5094–5097. doi: 10.1073/pnas.80.16.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey W., Snyderman R. Prostaglandins and inflammation: enhancement of monocyte chemotactic responsiveness by prostaglandin E2. Prostaglandins. 1976 Sep;12(3):415–426. doi: 10.1016/0090-6980(76)90022-8. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Newman H. A., Murad T. M., Geer J. C. Foam cells of rabbit atheromatous lesion. Identification and cholesterol uptake in isolated cells. Lab Invest. 1971 Dec;25(6):586–595. [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- PRICHARD R. W., CLARKSON T. B., GOODMAN H. O., LOFLAND H. B. AORTIC ATHEROSCLEROSIS IN PIGEONS AND ITS COMPLICATIONS. Arch Pathol. 1964 Mar;77:244–257. [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. J., Sprague E. A., Kelley J. L., Valente A. J., Suenram C. A. Aortic intimal monocyte recruitment in the normo and hypercholesterolemic baboon (Papio cynocephalus). An ultrastructural study: implications in atherogenesis. Virchows Arch A Pathol Anat Histopathol. 1985;405(2):175–191. doi: 10.1007/BF00704370. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J. K., Mergenhagen S. E. Biological activity of complement in vivo. Role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med. 1971 Nov 1;134(5):1131–1143. doi: 10.1084/jem.134.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbiah M. T. Prostaglandin E2 biosynthesis and effect in pigeon aorta. Possible role in atherogenesis. Atherosclerosis. 1978 Apr;29(4):487–495. doi: 10.1016/0021-9150(78)90177-6. [DOI] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Taylor K., Schaffner T., Wissler R. W., Glagov S. Immuno-morphologic identification and characterization of cells derived from experimental atherosclerotic lesions. Scan Electron Microsc. 1979;(3):815–822. [PubMed] [Google Scholar]
- Trillo A. A. The cell population of aortic fatty streaks in African green monkeys with special reference to granulocytic cells. An ultrastructural study. Atherosclerosis. 1982 Jun;43(2-3):259–275. doi: 10.1016/0021-9150(82)90027-2. [DOI] [PubMed] [Google Scholar]
- Turner S. R., Campbell J. A., Lynn W. S. Polymorphonulcear leukocyte chemotaxis toward oxidized lipid components of cell membranes. J Exp Med. 1975 Jun 1;141(6):1437–1441. doi: 10.1084/jem.141.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente A. J., Fowler S. R., Sprague E. A., Kelley J. L., Suenram C. A., Schwartz C. J. Initial characterization of a peripheral blood mononuclear cell chemoattractant derived from cultured arterial smooth muscle cells. Am J Pathol. 1984 Dec;117(3):409–417. [PMC free article] [PubMed] [Google Scholar]
- WEISS L. P., FAWCETT D. W. Cytochemical observations on chicken monocytes macrophages and giant cells in tissue culture. J Histochem Cytochem. 1953 Jan;1(1):47–65. doi: 10.1177/1.1.47. [DOI] [PubMed] [Google Scholar]
- Wagner W. D. Risk factors in pigeons genetically selected for increased atherosclerosis susceptibility. Atherosclerosis. 1978 Dec;31(4):453–463. doi: 10.1016/0021-9150(78)90141-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hirata M., Yoshikawa Y., Nagafuchi Y., Toyoshima H., Watanabe T. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985 Jul;53(1):80–90. [PubMed] [Google Scholar]
- Yonemasu K., Nakanishi A., Sasaki T., Kashiba S. Stimulation of locomotion of peripheral blood monocytes by human plasma fibronectin. Microbiol Immunol. 1983;27(3):283–290. doi: 10.1111/j.1348-0421.1983.tb03590.x. [DOI] [PubMed] [Google Scholar]



