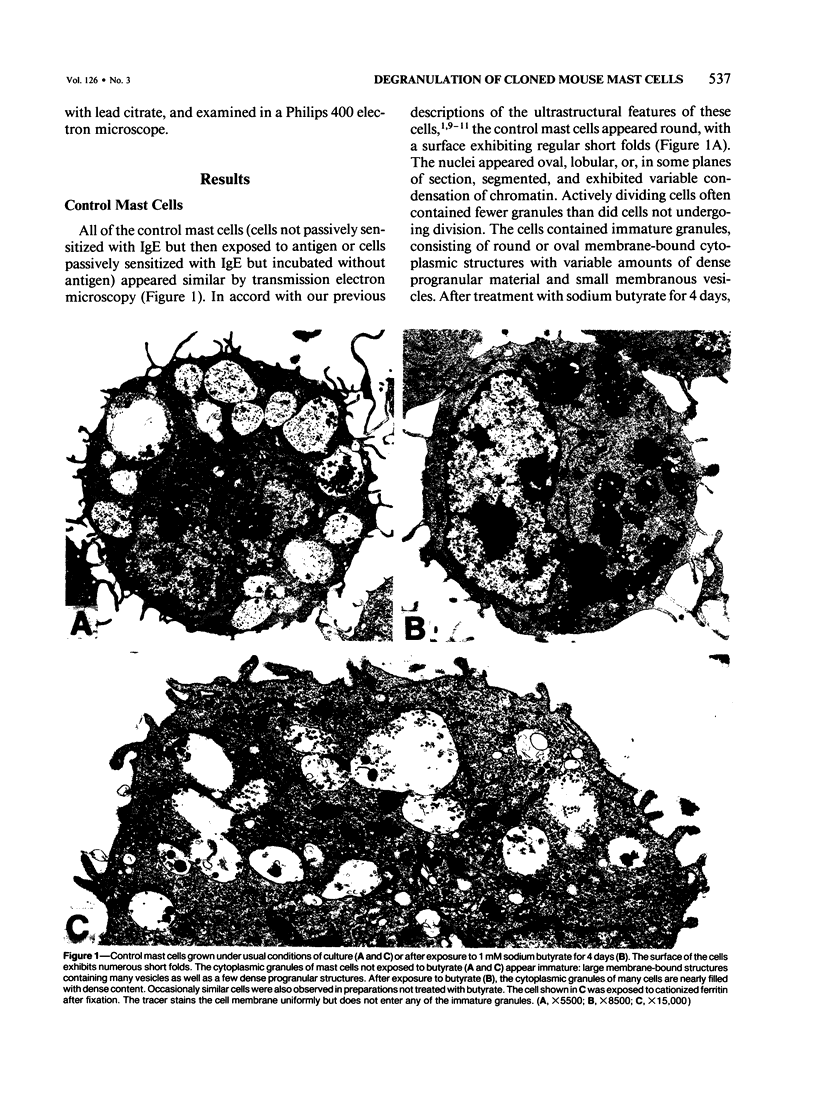
Abstract
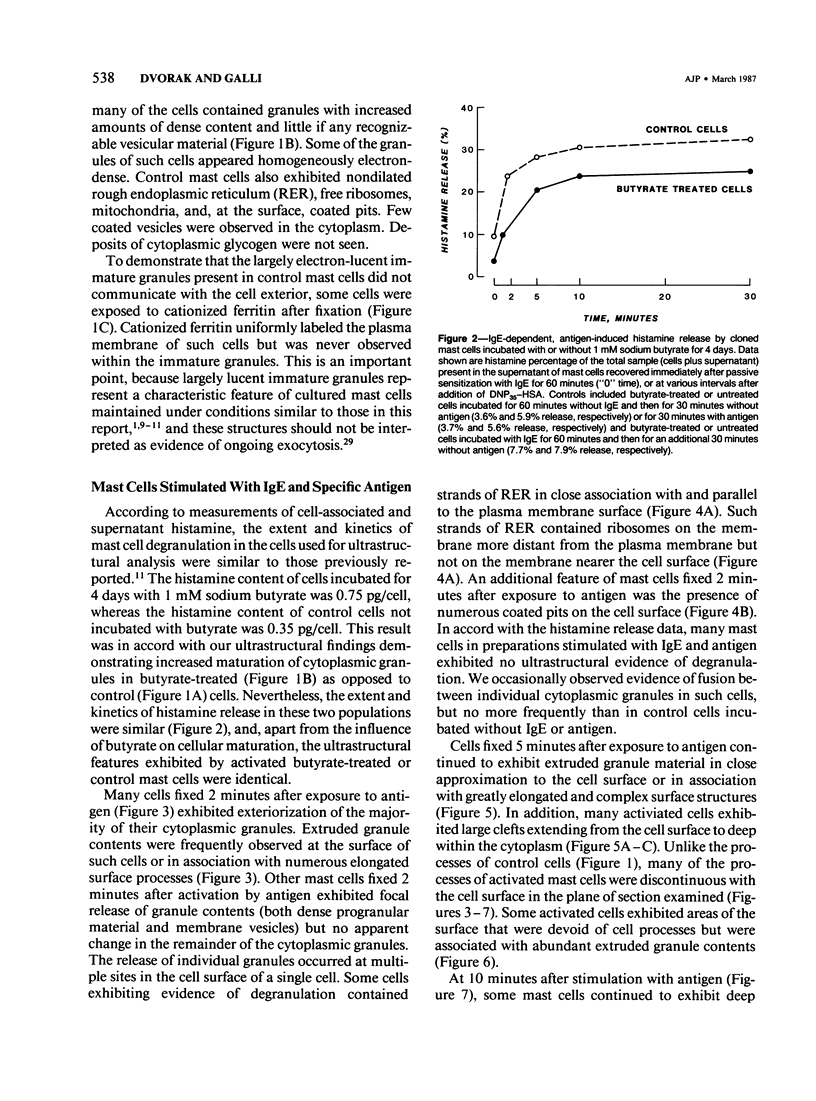
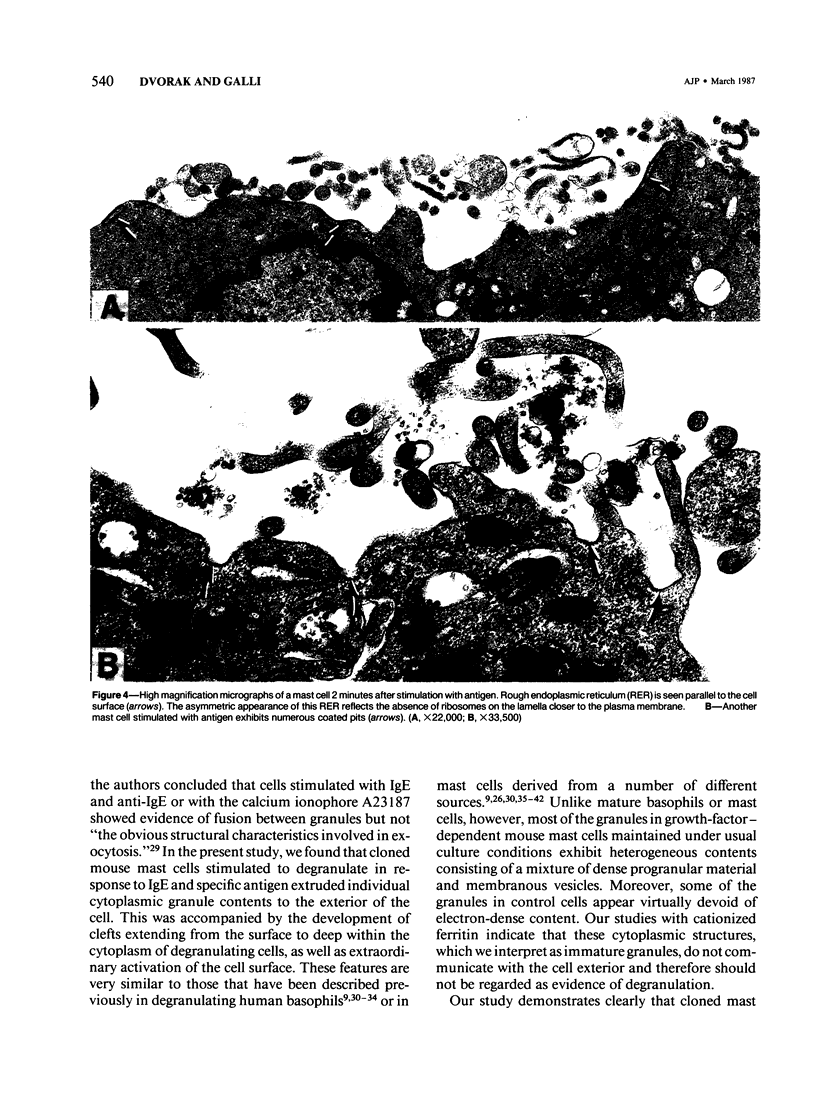
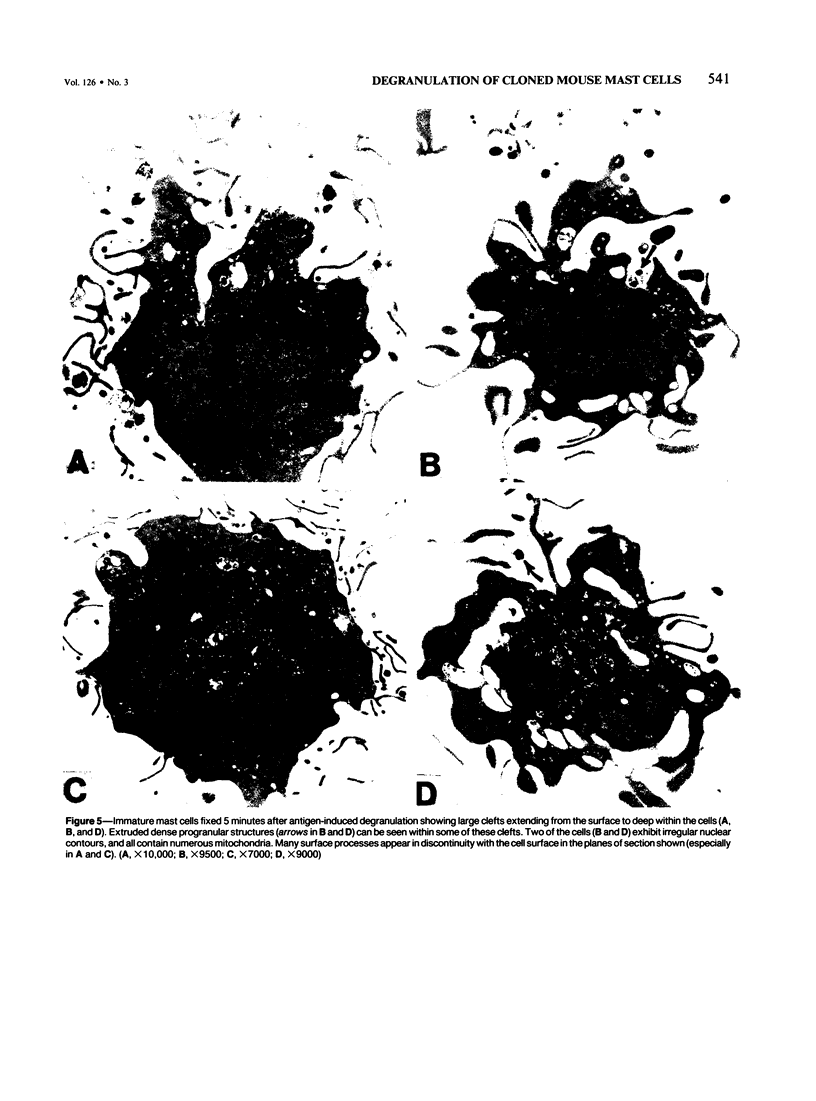
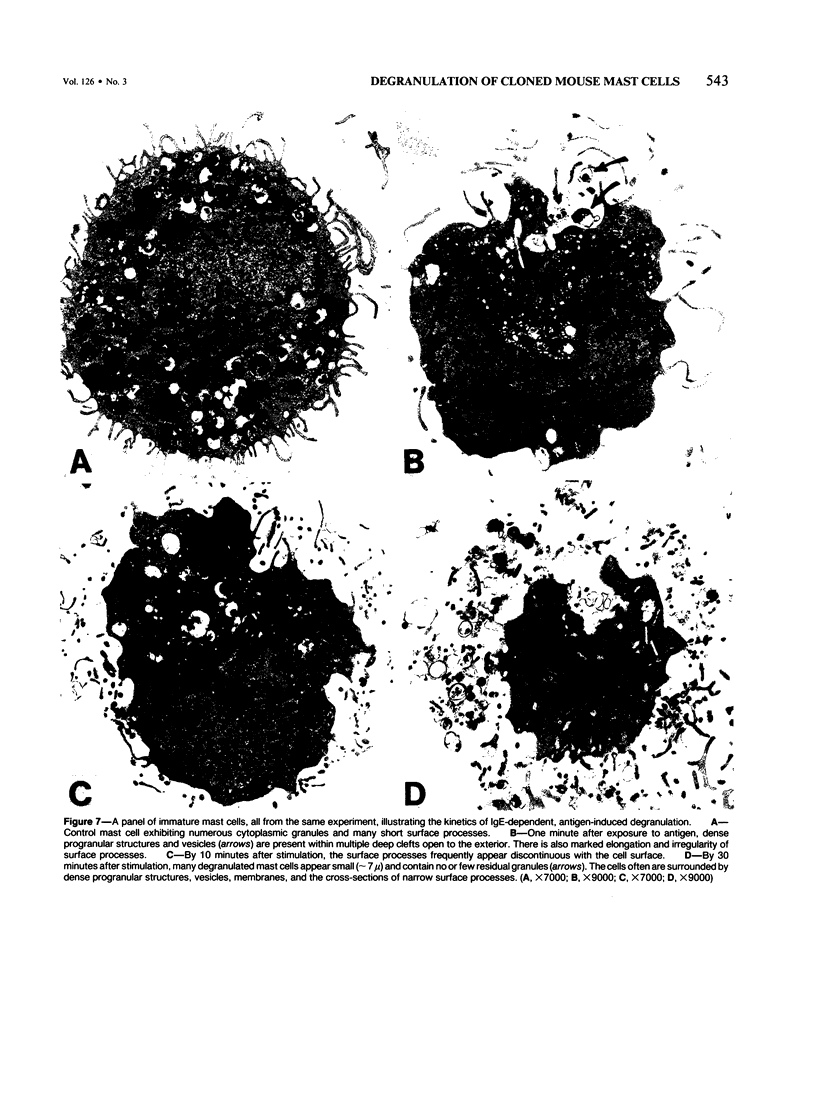
Cloned, immature mast cells derived from normal mice were passively sensitized with mouse monoclonal IgE antibodies with specificity for DNP, and then stimulated to degranulate with DNP35-HSA. Cells were fixed for transmission electron microscopy or recovered for quantitation of histamine release at various intervals up to 30 minutes after antigen challenge. The cloned mast cells rapidly extruded the contents of their immature granules (dense progranular material and membrane-bound vesicles) to the exterior via multiple openings in the plasma membrane. Degranulation was associated with striking activation of the cell surface, characterized initially by elongation of surface processes, as well as by close approximation of strands of rough endoplasmic reticulum to the cell surface and by the development of coated pits. At later times after stimulation, degranulated mast cells had released nearly all of their granules and exhibited angular surfaces lacking elongated processes. These findings demonstrate for the first time that cloned, immature mast cells, like their mature counterparts, can undergo classic morphologic release reactions involving exocytosis of granules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. C. Calcification processes. Pathol Annu. 1980;15(Pt 2):45–75. [PubMed] [Google Scholar]
- Black P. H. Shedding from the cell surface of normal and cancer cells. Adv Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- Burwen S. J., Satir B. H. Plasma membrane folds on the mast cell surface and their relationship to secretory activity. J Cell Biol. 1977 Sep;74(3):690–697. doi: 10.1083/jcb.74.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Come S. E., Shohet S. B., Robinson S. H. Surface remodeling vs. whole-cell hemolysis of reticulocytes produced with erythroid stimulation or iron deficiency anemia. Blood. 1974 Dec;44(6):817–830. [PubMed] [Google Scholar]
- Cooper E. H., Bedford A. J., Kenny T. E. Cell death in normal and malignant tissues. Adv Cancer Res. 1975;21:59–120. doi: 10.1016/s0065-230x(08)60971-9. [DOI] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Dvorak H. F., Galli S. J. Surface membrane traffic in guinea pig basophils exposed to cationic ferritin. Int Arch Allergy Appl Immunol. 1985;77(1-2):267–273. doi: 10.1159/000233807. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Galli S. J., Schulman E. S., Lichtenstein L. M., Dvorak H. F. Basophil and mast cell degranulation: ultrastructural analysis of mechanisms of mediator release. Fed Proc. 1983 May 15;42(8):2510–2515. [PubMed] [Google Scholar]
- Dvorak A. M., Hammel I., Schulman E. S., Peters S. P., MacGlashan D. W., Jr, Schleimer R. P., Newball H. H., Pyne K., Dvorak H. F., Lichtenstein L. M. Differences in the behavior of cytoplasmic granules and lipid bodies during human lung mast cell degranulation. J Cell Biol. 1984 Nov;99(5):1678–1687. doi: 10.1083/jcb.99.5.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Hammond M. E., Dvorak H. F., Karnovsky M. J. Loss of cell surface material from peritoneal exudate cells associated with lymphocyte-mediated inhibition of macrophage migration from capillary tubes. Lab Invest. 1972 Dec;27(6):561–574. [PubMed] [Google Scholar]
- Dvorak A. M., Lett-Brown M. A., Thueson D. O., Pyne K., Raghuprasad P. K., Galli S. J., Grant J. A. Histamine-releasing activity (HRA). III. HRA induces human basophil histamine release by provoking noncytotoxic granule exocytosis. Clin Immunol Immunopathol. 1984 Aug;32(2):142–150. doi: 10.1016/0090-1229(84)90116-8. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Lett-Brown M., Thueson D., Grant J. A. Complement-induced degranulation of human basophils. J Immunol. 1981 Feb;126(2):523–528. [PubMed] [Google Scholar]
- Dvorak A. M., Newball H. H., Dvorak H. F., Lichtenstein L. M. Antigen-induced IgE-mediated degranulation of human basophils. Lab Invest. 1980 Aug;43(2):126–139. [PubMed] [Google Scholar]
- Dvorak A. M., Schulman E. S., Peters S. P., MacGlashan D. W., Jr, Newball H. H., Schleimer R. P., Lichtenstein L. M. Immunoglobulin E-mediated degranulation of isolated human lung mast cells. Lab Invest. 1985 Jul;53(1):45–56. [PubMed] [Google Scholar]
- Dvorak H. F., Quay S. C., Orenstein N. S., Dvorak A. M., Hahn P., Bitzer A. M., Carvalho A. C. Tumor shedding and coagulation. Science. 1981 May 22;212(4497):923–924. doi: 10.1126/science.7195067. [DOI] [PubMed] [Google Scholar]
- Findlay S. R., Dvorak A. M., Kagey-Sobotka A., Lichtenstein L. M. Hyperosmolar triggering of histamine release from human basophils. J Clin Invest. 1981 Jun;67(6):1604–1613. doi: 10.1172/JCI110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Dvorak H. F. Basophils and mast cells: morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982 Nov;95(2 Pt 1):435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Nabel G., Goldin J. M., Rosenberg R. D., Cantor H., Dvorak H. F. Mouse mast cell clones: modulation of functional maturity in vitro. Monogr Allergy. 1983;18:166–170. [PubMed] [Google Scholar]
- HOGBERG B., UVNAS B. The mechanism of the disruption of mast cells produced by compound 48/80. Acta Physiol Scand. 1957 Dec 31;41(4):345–369. doi: 10.1111/j.1748-1716.1957.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Hasthorpe S. A hemopoietic cell line dependent upon a factor in pokeweed mitogen-stimulated spleen cell conditioning medium. J Cell Physiol. 1980 Nov;105(2):379–384. doi: 10.1002/jcp.1041050221. [DOI] [PubMed] [Google Scholar]
- Kinsolving C. R., Johnson A. R., Moran N. C. The uptake of a substituted acridone by rat mast cells in relationship to histamine release: a possible indicator of exocytosis-induced expansion of the plasma membrane. J Pharmacol Exp Ther. 1975 Mar;192(3):654–669. [PubMed] [Google Scholar]
- Lagunoff D. Contributions of electron microscopy to the study of mast cells. J Invest Dermatol. 1972 May;58(5):296–311. doi: 10.1111/1523-1747.ep12540314. [DOI] [PubMed] [Google Scholar]
- Lagunoff D. Membrane fusion during mast cell secretion. J Cell Biol. 1973 Apr;57(1):252–259. doi: 10.1083/jcb.57.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Razin E., Ringel E. W., Corey E. J., Hoover D., Austen K. F., Lewis R. A. Immunologic and ionophore-induced generation of leukotriene B4 from mouse bone marrow-derived mast cells. J Immunol. 1983 Apr;130(4):1885–1890. [PubMed] [Google Scholar]
- Nabel G., Galli S. J., Dvorak A. M., Dvorak H. F., Cantor H. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981 May 28;291(5813):332–334. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- Nagao K., Yokoro K., Aaronson S. A. Continuous lines of basophil/mast cells derived from normal mouse bone marrow. Science. 1981 Apr 17;212(4492):333–335. doi: 10.1126/science.7209531. [DOI] [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983 Jul;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Radley J. M., Scurfield G. The mechanism of platelet release. Blood. 1980 Dec;56(6):996–999. [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo A., Minick C. R., Good R. A. Studies on the exocytosis of cultured mast cells derived from mouse bone marrow. Exp Hematol. 1982 Jul;10(6):524–532. [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo C., Good R. A. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Mencia-Huerta J. M., Lewis R. A., Corey E. J., Austen K. F. Generation of leukotriene C4 from a subclass of mast cells differentiated in vitro from mouse bone marrow. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4665–4667. doi: 10.1073/pnas.79.15.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Rennick D. M., Lee F. D., Yokota T., Arai K. I., Cantor H., Nabel G. J. A cloned MCGF cDNA encodes a multilineage hematopoietic growth factor: multiple activities of interleukin 3. J Immunol. 1985 Feb;134(2):910–914. [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs D. H., Kiszkiss P., Kim K. J. Release of Ia antigens by a cultured B cell line. J Immunol. 1980 May;124(5):2130–2136. [PubMed] [Google Scholar]
- Schrader J. W. Bone marrow differentiation in vitro. Crit Rev Immunol. 1983;4(3):197–277. [PubMed] [Google Scholar]
- Schrader J. W. In in vitro production and cloning of the P cell, a bone marrow-derived null cell that expresses H-2 and Ia-antigens, has mast cell-like granules, and is regulated by a factor released by activated T cells. J Immunol. 1981 Feb;126(2):452–458. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraganian R. P. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974 Feb;57(2):383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Sredni B., Friedman M. M., Bland C. E., Metcalfe D. D. Ultrastructural, biochemical, and functional characteristics of histamine-containing cells cloned from mouse bone marrow: tentative identification as mucosal mast cells. J Immunol. 1983 Aug;131(2):915–922. [PubMed] [Google Scholar]
- Tachiwaki O., Wollman S. H. Shedding of dense cell fragments into the follicular lumen early in involution of the hyperplastic thyroid gland. Lab Invest. 1982 Jul;47(1):91–98. [PubMed] [Google Scholar]
- Tertian G., Yung Y. P., Guy-Grand D., Moore M. A. Long-term in vitro culture of murine mast cells. I. Description of a growth factor-dependent culture technique. J Immunol. 1981 Aug;127(2):788–794. [PubMed] [Google Scholar]
- Weitzman G., Galli S. J., Dvorak A. M., Hammel I. Cloned mouse mast cells and normal mouse peritoneal mast cells. Determination of serotonin content and ability to synthesize serotonin in vitro. Int Arch Allergy Appl Immunol. 1985;77(1-2):189–191. doi: 10.1159/000233782. [DOI] [PubMed] [Google Scholar]
- Williams D. S. Photoreceptor membrane shedding and assembly can be initiated locally within an insect retina. Science. 1982 Nov 26;218(4575):898–900. doi: 10.1126/science.7134980. [DOI] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung Y. P., Moore M. A. Mast cell growth factor. Lymphokine Res. 1983;2(4):127–131. [PubMed] [Google Scholar]
- van Blitterswijk W. J., Emmelot P., Hilkmann H. A., Oomenmeulemans E. P., Inbar M. Differences in lipid fluidity among isolated plasma membranes of normal and leukemic lympocytes and membranes exfoliated from their cell surface. Biochim Biophys Acta. 1977 Jun 16;467(3):309–320. doi: 10.1016/0005-2736(77)90308-x. [DOI] [PubMed] [Google Scholar]