Abstract
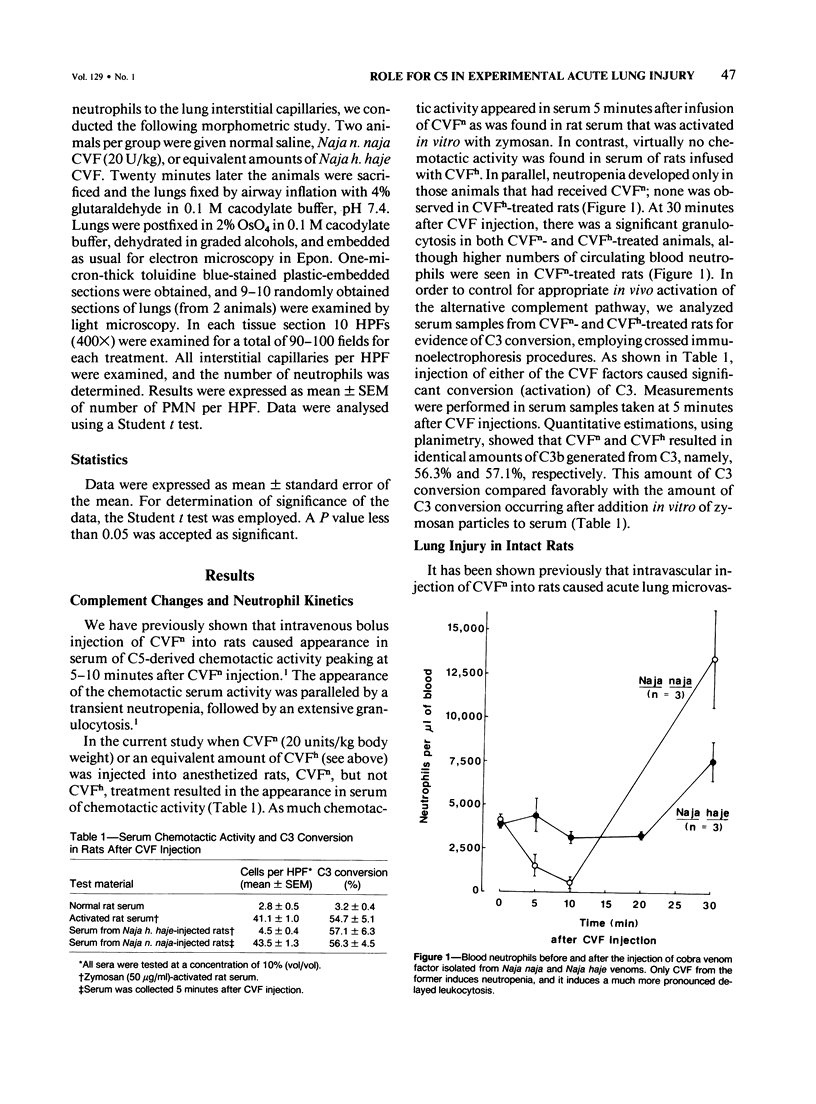
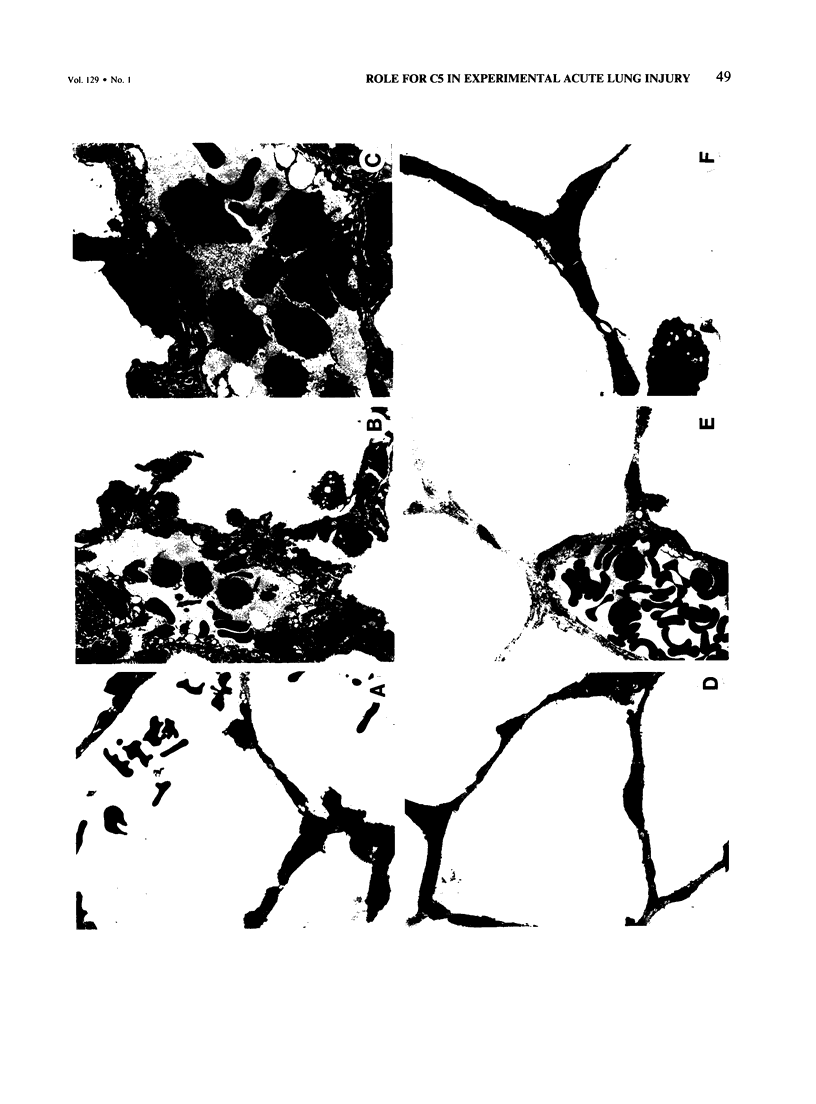
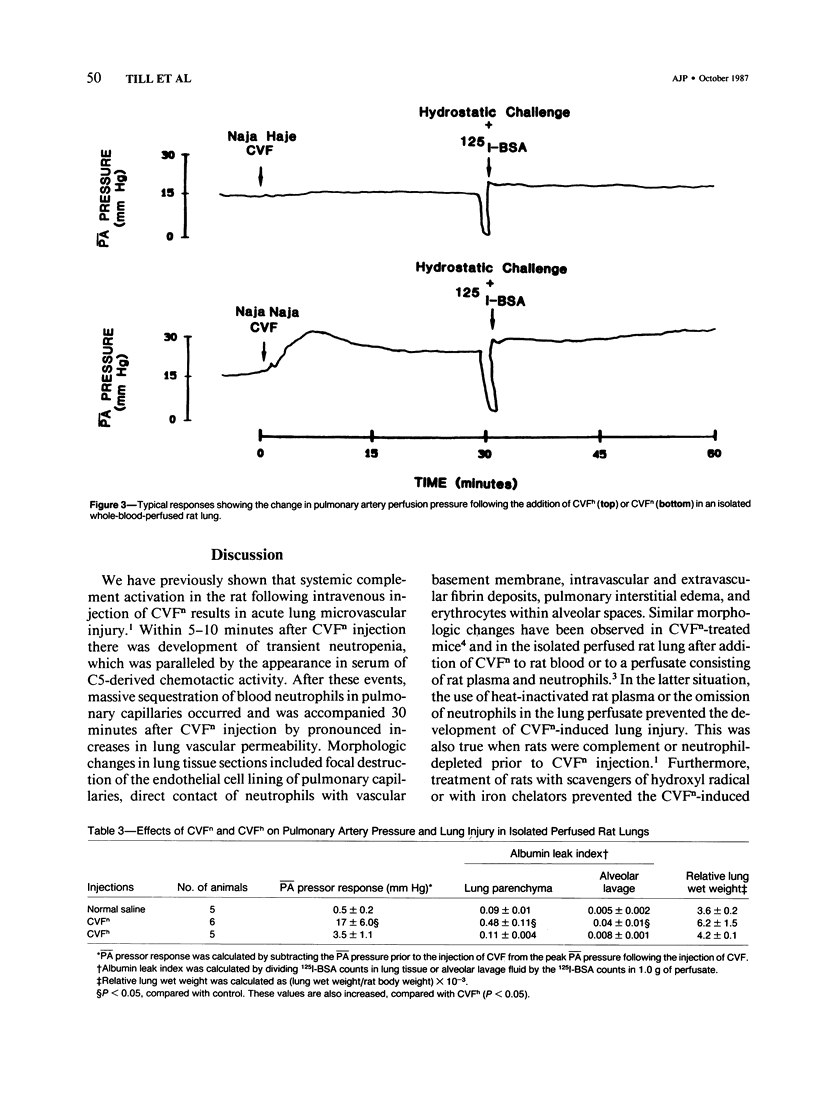
Cobra venom factor (CVF)-induced systemic activation of the complement system in the rat has been shown to result in the development of acute lung microvascular injury and appearance in lungs and plasma of lipid peroxidation products. The pathogenesis of these events is dependent on complement and neutrophils and is sensitive to pretreatment of experimental animals with iron chelators or scavengers of hydroxyl radical. In order to further analyze the role of complement in the pathogenesis of acute lung injury in rats after systemic complement activation, two different CVFs have been employed in the present study. One was the previously used CVFn isolated from Naja n. naja venom, whereas the other factor, CVFh, was isolated from Naja h. haje venom. Both factors have been shown to activate the alternative complement pathway by forming a potent C3 convertase but differ with respect to their ability to bind and activate C5. CVFn but not CVFh activates C5 and distant complement components. When equal doses of C3-activating activity of CVFn or CVFh were injected intravenously into rats, CVFh-treated rats failed to develop acute lung injury, whereas CVFn-treated animals showed pronounced increases in lung vascular permeability. Similarly, in isolated blood perfused rat lungs neither the lung injury nor pulmonary hypertension caused by CVFn were found after injection of CVFh. In addition, CVFh-treated animals failed to show transient neutropenia or appearance in plasma of C5-derived chemotactic activity, although the extent of C3 conversion in vivo was identical to that seen in CVFn-treated rats. Morphologic examination of the lungs of the experimental animals revealed no signs of injury in CVFh-treated rats, whereas the lungs from CVFn-treated animals revealed interstitial and alveolar edema, as well as plugging of pulmonary capillaries with neutrophils, blebbing and/or destruction of vascular endothelial cells, fibrin deposition, and hemorrhage. These studies provide evidence that activation of the complement system involving C3 but not extending further in the complement sequence is not sufficient to bring about acute injury of the lung microvasculature and that the activation sequence must at least also involve C5.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Campbell A. K., Morgan B. P. Monoclonal antibodies demonstrate protection of polymorphonuclear leukocytes against complement attack. Nature. 1985 Sep 12;317(6033):164–166. doi: 10.1038/317164a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. D., McDonald J. W., Ali M., Menkes E., Masterson J., Klement P. Prostaglandin production associated with the pulmonary vascular response to complement activation. Surgery. 1980 Aug;88(2):215–221. [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick M. R., Horn J. K., Hoeffel J. M., Goldstein I. M. Reduction of total hemolytic complement activity with Naja haje cobra venom factor does not prevent endotoxin-induced lung injury in sheep. Am Rev Respir Dis. 1986 Jan;133(1):62–67. doi: 10.1164/arrd.1986.133.1.62. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B., Müller-Eberhard H. J. C3e: an acidic fragment of human C3 with leukocytosis-inducing activity. J Immunol. 1979 Aug;123(2):616–621. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H. P. Leukotriene C4 (LTC4) elicits a respiratory burst in peritoneal macrophages. Eur J Pharmacol. 1983 Jul 15;91(1):159–160. doi: 10.1016/0014-2999(83)90384-9. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Parnham M. J., Winkelmann J., Englberger W., Hadding U. Platelet activating factor (PAF) induces the oxidative burst in macrophages. Int J Immunopharmacol. 1983;5(2):115–121. doi: 10.1016/0192-0561(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Larsen G. L., Webster R. O., Mitchell B. C., Goins A. J., Henson J. E. Pulmonary microvascular alterations and injury induced by complement fragments: synergistic effect of complement activation, neutrophil sequestration, and prostaglandins. Ann N Y Acad Sci. 1982;384:287–300. doi: 10.1111/j.1749-6632.1982.tb21379.x. [DOI] [PubMed] [Google Scholar]
- Huey R., Bloor C. M., Kawahara M. S., Hugli T. E. Potentiation of the anaphylatoxins in vivo using an inhibitor of serum carboxypeptidase N (SCPN). I. Lethality and pathologic effects on pulmonary tissue. Am J Pathol. 1983 Jul;112(1):48–60. [PMC free article] [PubMed] [Google Scholar]
- Johnson A., Cooper J. A., Malik A. B. Effect of complement activation with cobra venom factor on pulmonary vascular permeability. J Appl Physiol (1985) 1986 Dec;61(6):2202–2209. doi: 10.1152/jappl.1986.61.6.2202. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Wilson B. S., Till G. O., Ward P. A. Acute lung injury in rat caused by immunoglobulin A immune complexes. J Clin Invest. 1984 Aug;74(2):358–369. doi: 10.1172/JCI111431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- José P. J., Forrest M. J., Williams T. J. Human C5a des Arg increases vascular permeability. J Immunol. 1981 Dec;127(6):2376–2380. [PubMed] [Google Scholar]
- McCall C. E., De Chatelet L. R., Brown D., Lachmann P. New biological activity following intravascular activation of the complement cascade. Nature. 1974 Jun 28;249(460):841–843. doi: 10.1038/249841a0. [DOI] [PubMed] [Google Scholar]
- Morganroth M. L., Reeves J. T., Murphy R. C., Voelkel N. F. Leukotriene synthesis and receptor blockers block hypoxic pulmonary vasoconstriction. J Appl Physiol Respir Environ Exerc Physiol. 1984 May;56(5):1340–1346. doi: 10.1152/jappl.1984.56.5.1340. [DOI] [PubMed] [Google Scholar]
- Morganroth M. L., Till G. O., Kunkel R. G., Ward P. A. Complement and neutrophil-mediated injury of perfused rat lungs. Lab Invest. 1986 May;54(5):507–514. [PubMed] [Google Scholar]
- Nusbacher J., Rosenfeld S. I., MacPherson J. L., Thiem P. A., Leddy J. P. Nylon fiber leukapheresis: associated complement component changes and cranulocytopenia. Blood. 1978 Feb;51(2):359–365. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A. Neutropenia induced by systemic infusion of chemotactic factors. J Immunol. 1977 May;118(5):1586–1589. [PubMed] [Google Scholar]
- Perkowski S. Z., Havill A. M., Flynn J. T., Gee M. H. Role of intrapulmonary release of eicosanoids and superoxide anion as mediators of pulmonary dysfunction and endothelial injury in sheep with intermittent complement activation. Circ Res. 1983 Nov;53(5):574–583. doi: 10.1161/01.res.53.5.574. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Rother K. Leucocyte mobilizing factor: a new biological activity derived from the third component of complement. Eur J Immunol. 1972 Dec;2(6):550–558. doi: 10.1002/eji.1830020615. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Radin A., Smolen J. E., Korchak H., Samuelsson B., Weissmann G. Leukotriene B4 is a complete secretagogue in human neutrophils: a kinetic analysis. Biochem Biophys Res Commun. 1982 Aug;107(3):1006–1012. doi: 10.1016/0006-291x(82)90622-2. [DOI] [PubMed] [Google Scholar]
- Stevens J. H., O'Hanley P., Shapiro J. M., Mihm F. G., Satoh P. S., Collins J. A., Raffin T. A. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J Clin Invest. 1986 Jun;77(6):1812–1816. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimler N. P., Hugli T. E., Bloor C. M. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am J Pathol. 1980 Aug;100(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvedten H. W., Till G. O., Ward P. A. Mediators of lung injury in mice following systemic activation of complement. Am J Pathol. 1985 Apr;119(1):92–100. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zabern I., Hinsch B., Przyklenk H., Schmidt G., Vogt W. Comparison of Naja n. naja and Naja h. haje cobra-venom factors: correlation between binding affinity for the fifth component of complement and mediation of its cleavage. Immunobiology. 1980 Dec;157(4-5):499–514. doi: 10.1016/s0171-2985(80)80018-0. [DOI] [PubMed] [Google Scholar]



