Abstract
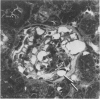
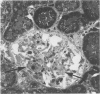
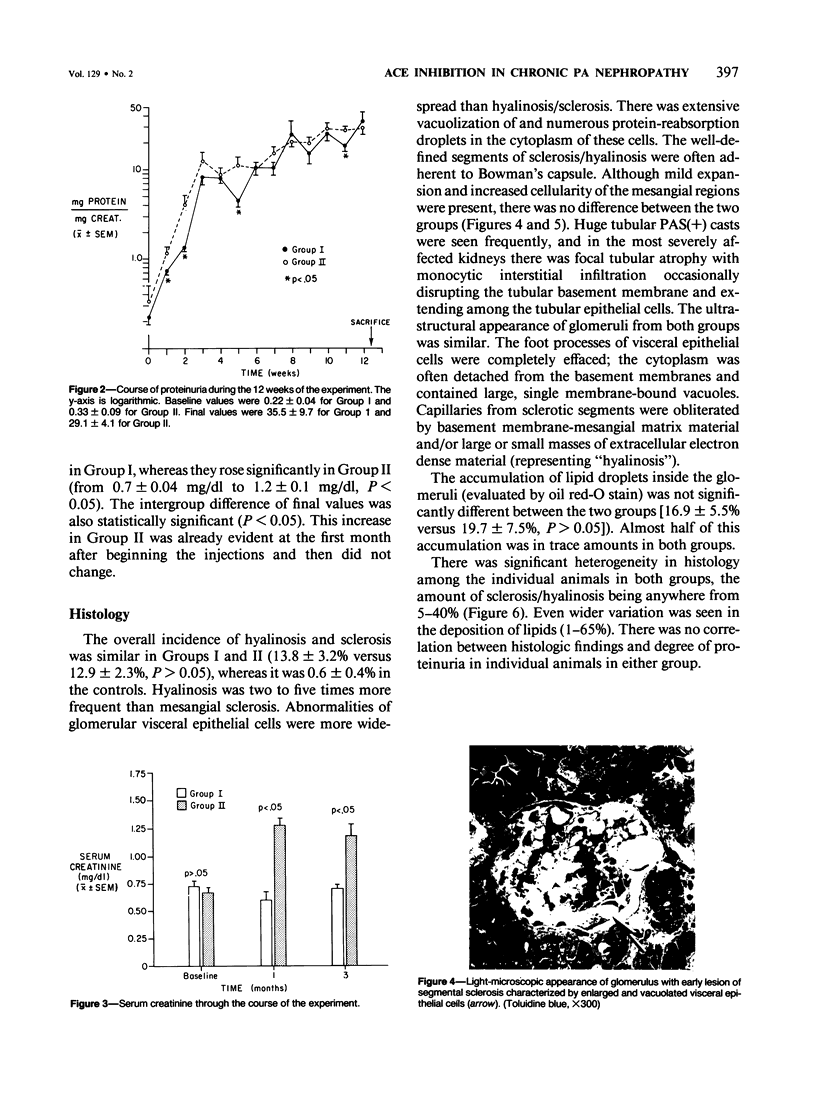
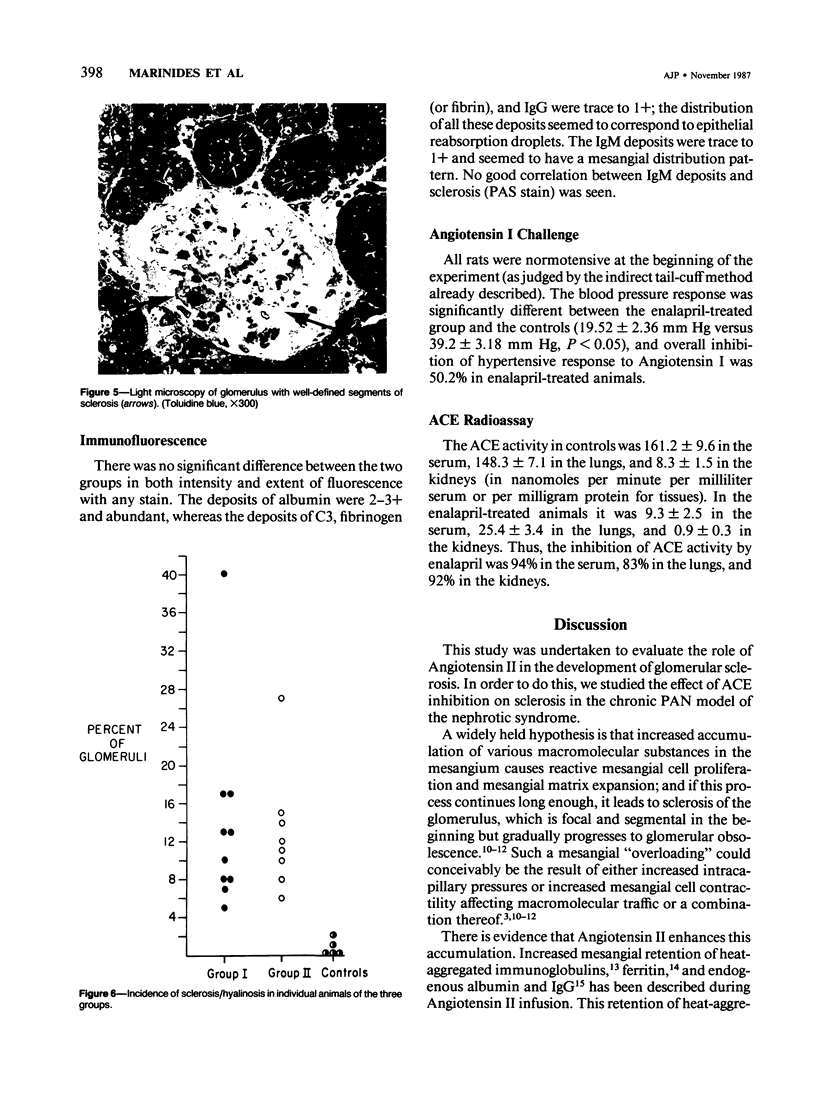
The effects of the angiotensin converting enzyme (ACE) inhibitor enalapril on the proteinuria and degree of focal glomerular sclerosis hyalinosis (FSH) in chronic puromycin aminonucleoside nephropathy (PAN) were examined. Chronic PAN was induced in male Sprague-Dawley rats by seven subcutaneous injections of puromycin aminonucleoside (20 mg/kg) over 10 weeks (Groups I and II). Group II rats also received enalapril 10 mg/kg/day in the drinking water throughout the study (12 weeks). Group III rats served as age-matched controls. Proteinuria was similar in Groups I and II (35.5 +/- 9.7 versus 29.1 +/- 4.1 mg protein/mg creatinine, mean +/- SEM, P greater than 0.05). Serum creatinine remained unchanged in Group I, but rose from 0.7 +/- 0.04 to 1.2 +/- 0.1 mg/dl (mean +/- SEM, P less than 0.05) in Group II. FSH was 13.8% in Group I, 12.9% in Group II (P greater than 0.05), and 0.6% in Group III. There was no significant difference in glomerular lipid content and in immunofluorescence for rat albumin, fibrinogen, IgM, IgG, and C3 between Groups I and II. ACE activity was inhibited by 94% in serum, 83% in lungs, and 92% in kidneys; and blood pressure response to. Angiotensin I challenge was decreased by 50% in rats similarly treated with enalapril versus controls. In summary, proteinuria and glomerular sclerosis in this model are not affected by ACE inhibition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausiello D. A., Kreisberg J. I., Roy C., Karnovsky M. J. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980 Mar;65(3):754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani T., Poggi A., Pozzoni R., Delaini F., Sacchi G., Thoua Y., Mecca G., Remuzzi G., Donati M. B. Adriamycin-induced nephrotic syndrome in rats: sequence of pathologic events. Lab Invest. 1982 Jan;46(1):16–23. [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Robertson C. R., Brenner B. M., Troy J. L., Willis W. T. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J Clin Invest. 1977 Jul;60(1):152–161. doi: 10.1172/JCI108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. H., Mampaso F., Zamboni L. Glomerular podocyte degeneration in human renal disease: an ultrastructural study. Lab Invest. 1977 Jul;37(1):30–42. [PubMed] [Google Scholar]
- Cohen M. L., Kurz K. D. Angiotensin converting enzyme inhibition in tissues from spontaneously hypertensive rats after treatment with captopril or MK-421. J Pharmacol Exp Ther. 1982 Jan;220(1):63–69. [PubMed] [Google Scholar]
- Couser W. G., Stilmant M. M. Mesangial lesions and focal glomerular sclerosis in the aging rat. Lab Invest. 1975 Nov;33(5):491–501. [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis following a single intravenous dose of puromycin aminonucleoside. Am J Pathol. 1986 Mar;122(3):481–487. [PMC free article] [PubMed] [Google Scholar]
- Dorer F. E., Kahn J. R., Lentz K. E., Levine M., Skeggs L. T. Kinetic properties of pulmonary angiotensin-converting enzyme. Hydrolysis of hippurylglycylglycine. Biochim Biophys Acta. 1976 Mar 11;429(1):220–228. doi: 10.1016/0005-2744(76)90045-0. [DOI] [PubMed] [Google Scholar]
- Grond J., Beukers J. Y., Schilthuis M. S., Weening J. J., Elema J. D. Analysis of renal structural and functional features in two rat strains with a different susceptibility to glomerular sclerosis. Lab Invest. 1986 Jan;54(1):77–83. [PubMed] [Google Scholar]
- Grond J., Koudstaal J., Elema J. D. Mesangial function and glomerular sclerosis in rats with aminonucleoside nephrosis. Kidney Int. 1985 Feb;27(2):405–410. doi: 10.1038/ki.1985.24. [DOI] [PubMed] [Google Scholar]
- Grond J., Weening J. J., Elema J. D. Glomerular sclerosis in nephrotic rats. Comparison of the long-term effects of adriamycin and aminonucleoside. Lab Invest. 1984 Sep;51(3):277–285. [PubMed] [Google Scholar]
- Gross D. M., Sweet C. S., Ulm E. H., Backlund E. P., Morris A. A., Weitz D., Bohn D. L., Wenger H. C., Vassil T. C., Stone C. A. Effect of N-[(S)-1-carboxy-3-phenylpropyl]-L-Ala-L-Pro and its ethyl ester (MK-421) on angiotensin converting enzyme in vitro and angiotensin I pressor responses in vivo. J Pharmacol Exp Ther. 1981 Mar;216(3):552–557. [PubMed] [Google Scholar]
- Habib R. Editorial: Focal glomerular sclerosis. Kidney Int. 1973 Dec;4(6):355–361. doi: 10.1038/ki.1973.131. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H., Olson J. L., Rennke H. G., Venkatachalam M. A., Brenner B. M. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981 Jul;241(1):F85–F93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- Kashgarian M. Mesangium and glomerular disease. Lab Invest. 1985 Jun;52(6):569–571. [PubMed] [Google Scholar]
- Keane W. F., Raij L. Relationship among altered glomerular barrier permselectivity, angiotensin II, and mesangial uptake of macromolecules. Lab Invest. 1985 Jun;52(6):599–604. [PubMed] [Google Scholar]
- Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Converting enzyme inhibitor therapy limits progressive glomerular injury in rats with renal insufficiency. Am J Med. 1985 Sep 27;79(3C):31–36. doi: 10.1016/0002-9343(85)90077-4. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Keane W. F., Raij L., Vernier R. L., Mauer S. M. The glomerular mesangium. Kidney Int. 1980 Feb;17(2):141–154. doi: 10.1038/ki.1980.18. [DOI] [PubMed] [Google Scholar]
- Okuda S., Oh Y., Tsuruda H., Onoyama K., Fujimi S., Fujishima M. Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int. 1986 Feb;29(2):502–510. doi: 10.1038/ki.1986.28. [DOI] [PubMed] [Google Scholar]
- Olivetti G., Kithier K., Giacomelli F., Wiener J. Glomerular permeability to endogenous proteins in the rat: effects of acute hypertension. Lab Invest. 1981 Feb;44(2):127–137. [PubMed] [Google Scholar]
- Olson J. L., Hostetter T. H., Rennke H. G., Brenner B. M., Venkatachalam M. A. Altered glomerular permselectivity and progressive sclerosis following extreme ablation of renal mass. Kidney Int. 1982 Aug;22(2):112–126. doi: 10.1038/ki.1982.143. [DOI] [PubMed] [Google Scholar]
- Olson J. L., de Urdaneta A. G., Heptinstall R. H. Glomerular hyalinosis and its relation to hyperfiltration. Lab Invest. 1985 Apr;52(4):387–398. [PubMed] [Google Scholar]
- Raij L., Chiou X. C., Owens R., Wrigley B. Therapeutic implications of hypertension-induced glomerular injury. Comparison of enalapril and a combination of hydralazine, reserpine, and hydrochlorothiazide in an experimental model. Am J Med. 1985 Sep 27;79(3C):37–41. doi: 10.1016/0002-9343(85)90078-6. [DOI] [PubMed] [Google Scholar]
- Schor N., Ichikawa I., Brenner B. M. Mechanisms of action of various hormones and vasoactive substances on glomerular ultrafiltration in the rat. Kidney Int. 1981 Oct;20(4):442–451. doi: 10.1038/ki.1981.160. [DOI] [PubMed] [Google Scholar]
- Stein H. D., Feddergreen W., Kashgarian M., Sterzel R. B. Role of angiotensin II-induced renal functional changes in mesangial deposition of exogenous ferritin in rats. Lab Invest. 1983 Sep;49(3):270–280. [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Stein H. D., Kashgarian M. The mesangium and glomerulonephritis. Klin Wochenschr. 1982 Sep 15;60(18):1077–1094. doi: 10.1007/BF01715838. [DOI] [PubMed] [Google Scholar]
- Velosa J. A., Glasser R. J., Nevins T. E., Michael A. F. Experimental model of focal sclerosis. II. Correlation with immunopathologic changes, macromolecular kinetics, and polyanion loss. Lab Invest. 1977 May;36(5):527–534. [PubMed] [Google Scholar]
- Zimmerman B. G., Wong P. C., Kounenis G. K., Kraft E. J. No effect of intrarenal converting enzyme inhibition on canine renal blood flow. Am J Physiol. 1982 Aug;243(2):H277–H283. doi: 10.1152/ajpheart.1982.243.2.H277. [DOI] [PubMed] [Google Scholar]