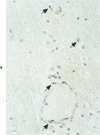
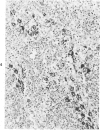
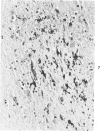
Abstract
Antigens of human (HIV) or simian immunodeficiency viruses (SIV) were identified with polyclonal or monoclonal antibodies and avidin-biotin complex (ABC) immunohistochemistry in fixed surgical pathology and autopsy specimens of humans or monkeys with the acquired immunodeficiency syndrome. With B-5 fixative, viral antigens were readily detected in lymph nodes of 8 of 13 patients with follicular hyperplasia, but in only 1 of 12 patients with follicular atrophy. Antigen was detected in follicular dendritic reticular cells and rare blastlike cells, extracellularly, and in postcapillary venules, medullary lymphocytes, sinus histiocytes, and macrophages in some lymph nodes. In the brain at autopsy, antigen could be found in gliomesenchymal-cell nodules, astrocytes, vascular endothelial cells, multinucleated cells, and astrocytes and macrophages associated with demyelination. In contrast, 4 rhesus monkeys with experimental SIV infection had abundant antigen in sinus histiocytes, macrophages, and multinucleated giant cells of lymph nodes and spleen and in thymic epithelial cells. Brain lesions of monkeys resembled those of humans, with antigen found in macrophages and multinucleated giant cells. Antibodies to HIV also were immunoreactive in formalin-fixed tissue sections of monkeys containing SIV antigens. The ABC technique provided a fast and efficient method for localizing HIV and SIV antigens in fixed surgical and autopsy specimens. These findings are consistent with those found with in situ hybridization, ultrastructural studies, frozen sections of lymph nodes, and permanent sections of brain.
Full text
PDF

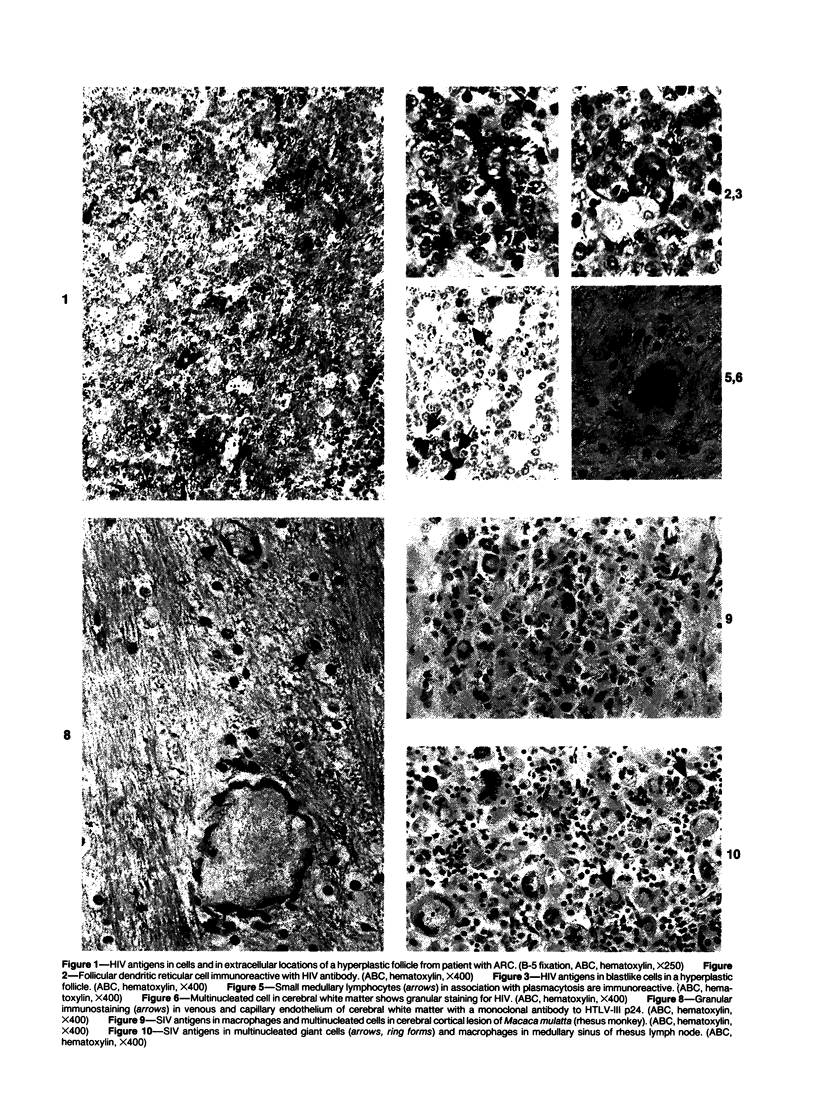
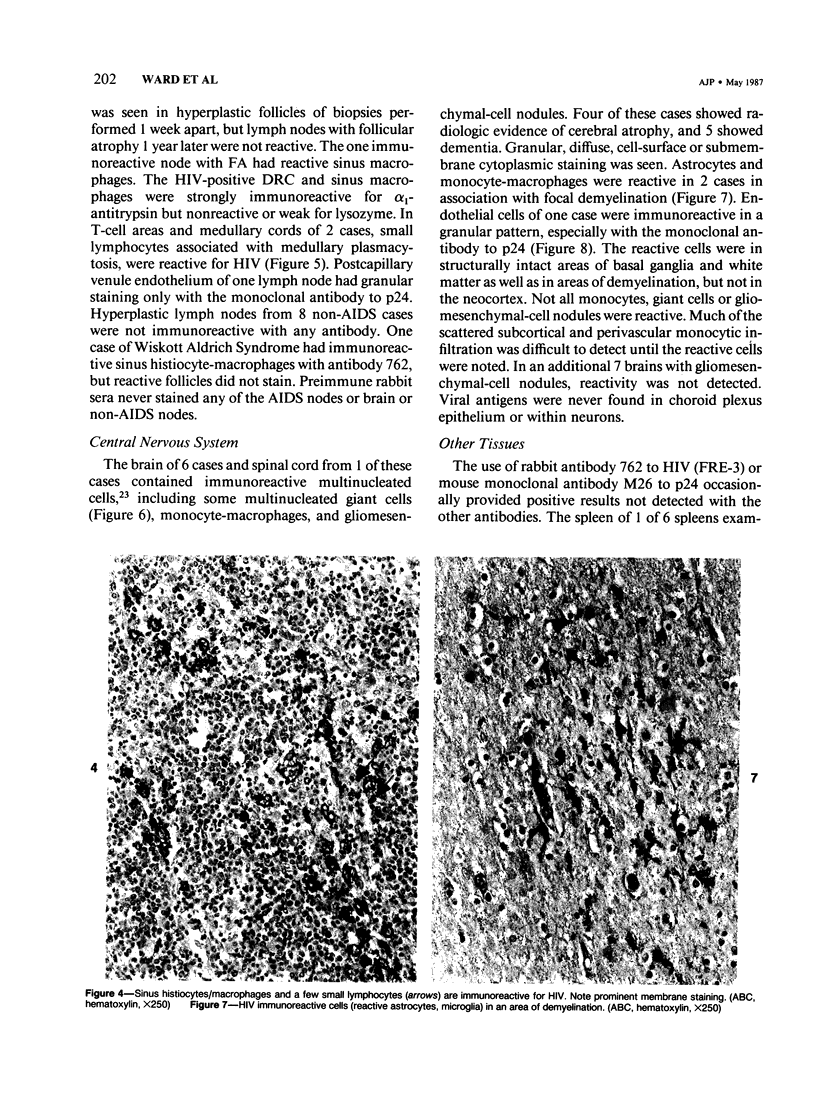



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Horne R. Follicular dendritic cells and virus-like particles in AIDS-related lymphadenopathy. Lancet. 1984 Aug 18;2(8399):370–372. doi: 10.1016/s0140-6736(84)90540-3. [DOI] [PubMed] [Google Scholar]
- Baroni C. D., Pezzella F., Mirolo M., Ruco L. P., Rossi G. B. Immunohistochemical demonstration of p24 HTLV III major core protein in different cell types within lymph nodes from patients with lymphadenopathy syndrome (LAS). Histopathology. 1986 Jan;10(1):5–13. doi: 10.1111/j.1365-2559.1986.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Baskin G. B., Martin L. N., Rangan S. R., Gormus B. J., Murphey-Corb M., Wolf R. H., Soike K. F. Transmissible lymphoma and simian acquired immunodeficiency syndrome in rhesus monkeys. J Natl Cancer Inst. 1986 Jul;77(1):127–139. [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P., Chayt K. J., Marselle L. M., Biberfeld G., Gallo R. C., Harper M. E. HTLV-III expression in infected lymph nodes and relevance to pathogenesis of lymphadenopathy. Am J Pathol. 1986 Dec;125(3):436–442. [PMC free article] [PubMed] [Google Scholar]
- Budka H. Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS). Acta Neuropathol. 1986;69(3-4):253–258. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- Chalifoux L. V., King N. W., Daniel M. D., Kannagi M., Desrosiers R. C., Sehgal P. K., Waldron L. M., Hunt R. D., Letvin N. L. Lymphoproliferative syndrome in an immunodeficient rhesus monkey naturally infected with an HTLV-III-like virus (STLV-III). Lab Invest. 1986 Jul;55(1):43–50. [PubMed] [Google Scholar]
- Chayt K. J., Harper M. E., Marselle L. M., Lewin E. B., Rose R. M., Oleske J. M., Epstein L. G., Wong-Staal F., Gallo R. C. Detection of HTLV-III RNA in lungs of patients with AIDS and pulmonary involvement. JAMA. 1986 Nov 7;256(17):2356–2359. [PubMed] [Google Scholar]
- Coffin J., Haase A., Levy J. A., Montagnier L., Oroszlan S., Teich N., Temin H., Toyoshima K., Varmus H., Vogt P. Human immunodeficiency viruses. Science. 1986 May 9;232(4751):697–697. doi: 10.1126/science.3008335. [DOI] [PubMed] [Google Scholar]
- Diebold J., Marche C., Audouin J., Aubert J. P., Le Tourneau A., Bouton C., Reynes M., Wizniak J., Capron F., Tricottet V. Lymph node modification in patients with the acquired immunodeficiency syndrome (AIDS) or with AIDS related complex (ARC). A histological, immuno-histopathological and ultrastructural study of 45 cases. Pathol Res Pract. 1985 Dec;180(6):590–611. doi: 10.1016/S0344-0338(85)80037-6. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Sharer L. R., Cho E. S., Myenhofer M., Navia B., Price R. W. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res. 1984;1(6):447–454. doi: 10.1089/aid.1.1983.1.447. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Sarngadharan M. G., Popovic M., Shaw G. M., Hahn B., Wong-Staal F., Robert-Guroff M., Zaki Salahuddin S., Markham P. D. HTLV-III and the etiology of AIDS. Prog Allergy. 1986;37:1–45. [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Sinkovics J. G., Gyorkey P. Retrovirus resembling HTLV in macrophages of patients with AIDS. Lancet. 1985 Jan 12;1(8420):106–106. doi: 10.1016/s0140-6736(85)91995-6. [DOI] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Szakal A. K., Tyndall R. L. Histoproliferative effect of Rauscher leukemia virus on lymphatic tissue: histological and ultrastructural studies of germinal centers and their relation to leukemogenesis. Cancer Res. 1970 Jun;30(6):1748–1763. [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki P. J., Barin F., M'Boup S., Allan J. S., Romet-Lemonne J. L., Marlink R., McLane M. F., Lee T. H., Arbeille B., Denis F. New human T-lymphotropic retrovirus related to simian T-lymphotropic virus type III (STLV-IIIAGM). Science. 1986 Apr 11;232(4747):238–243. doi: 10.1126/science.3006256. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Le Tourneau A., Audouin J., Diebold J., Marche C., Tricottet V., Reynes M. LAV-like viral particles in lymph node germinal centers in patients with the persistent lymphadenopathy syndrome and the acquired immunodeficiency syndrome-related complex: an ultrastructural study of 30 cases. Hum Pathol. 1986 Oct;17(10):1047–1053. doi: 10.1016/s0046-8177(86)80089-2. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Reichert C. M., O'Leary T. J., Levens D. L., Simrell C. R., Macher A. M. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983 Sep;112(3):357–382. [PMC free article] [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Kahl C., Feller A. C., Kern P., Dietrich M. Spectrum of morphologic changes of lymph nodes from patients with AIDS or AIDS-related complexes. Prog Allergy. 1986;37:81–181. doi: 10.1159/000318442. [DOI] [PubMed] [Google Scholar]
- Sharer L. R., Epstein L. G., Cho E. S., Joshi V. V., Meyenhofer M. F., Rankin L. F., Petito C. K. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986 Mar;17(3):271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Harper M. E., Hahn B. H., Epstein L. G., Gajdusek D. C., Price R. W., Navia B. A., Petito C. K., O'Hara C. J., Groopman J. E. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. doi: 10.1126/science.2981429. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Eskin T. A., Benn S., Angerer R. C., Angerer L. M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986 Nov 7;256(17):2360–2364. [PubMed] [Google Scholar]
- Tenner-Racz K., Racz P., Bofill M., Schulz-Meyer A., Dietrich M., Kern P., Weber J., Pinching A. J., Veronese-Dimarzo F., Popovic M. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986 Apr;123(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Tenner-Rácz K., Rácz P., Dietrich M., Kern P. Altered follicular dendritic cells and virus-like particles in AIDS and AIDS-related lymphadenopathy. Lancet. 1985 Jan 12;1(8420):105–106. doi: 10.1016/s0140-6736(85)91994-4. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Pardue R. L., Junker J. L., Takahashi K., Shih T. Y., Weislow O. S. Immunocytochemical localization of RasHa p21 in normal and neoplastic cells in fixed tissue sections from Harvey sarcoma virus-infected mice. Carcinogenesis. 1986 Apr;7(4):645–651. doi: 10.1093/carcin/7.4.645. [DOI] [PubMed] [Google Scholar]
- Warner T. F., Crass B., Gabel C., Maki D., Hafez G. R., Golubjatnikov R. Diagnosis of HTLV-III infection by ultrastructural examination of germinal centers in lymph node--a case report. AIDS Res. 1986 Feb;2(1):43–50. doi: 10.1089/aid.1.1986.2.43. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Marzo Veronese F., Sarngadharan M. G., Rahman R., Markham P. D., Popovic M., Bodner A. J., Gallo R. C. Monoclonal antibodies specific for p24, the major core protein of human T-cell leukemia virus type III. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5199–5202. doi: 10.1073/pnas.82.15.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]