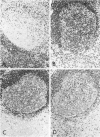
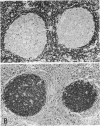
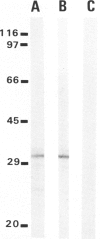
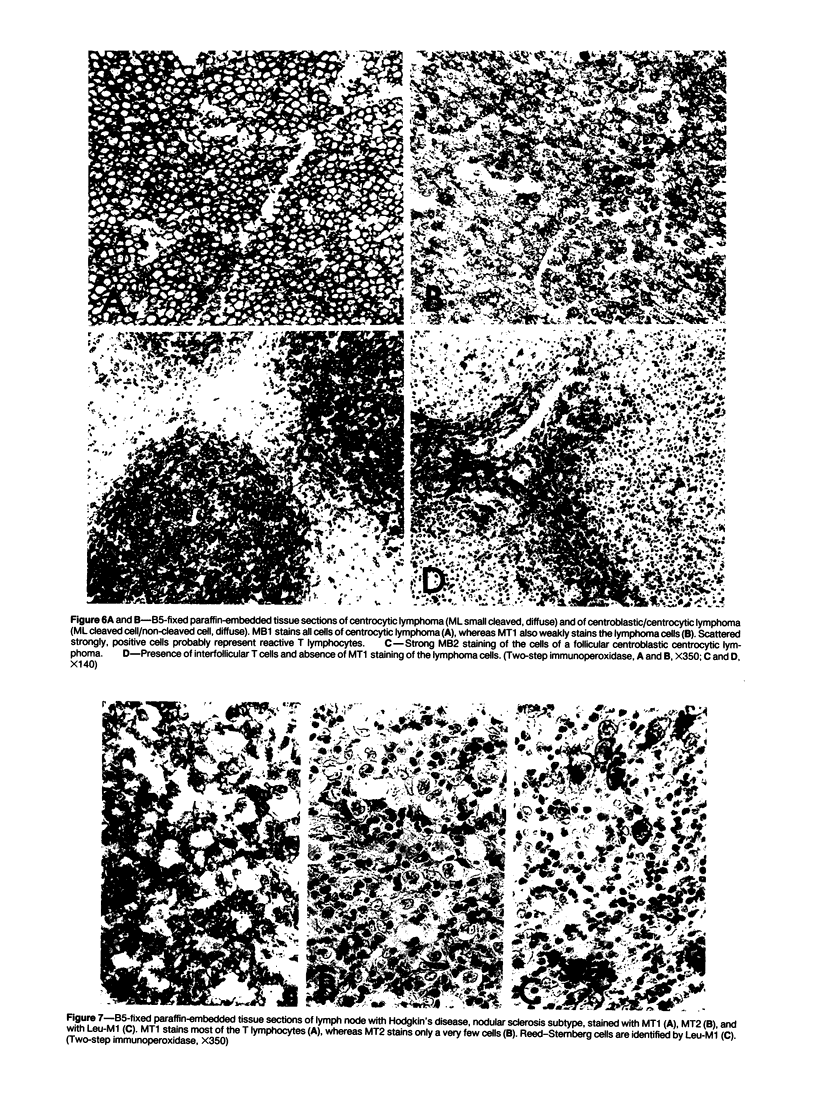
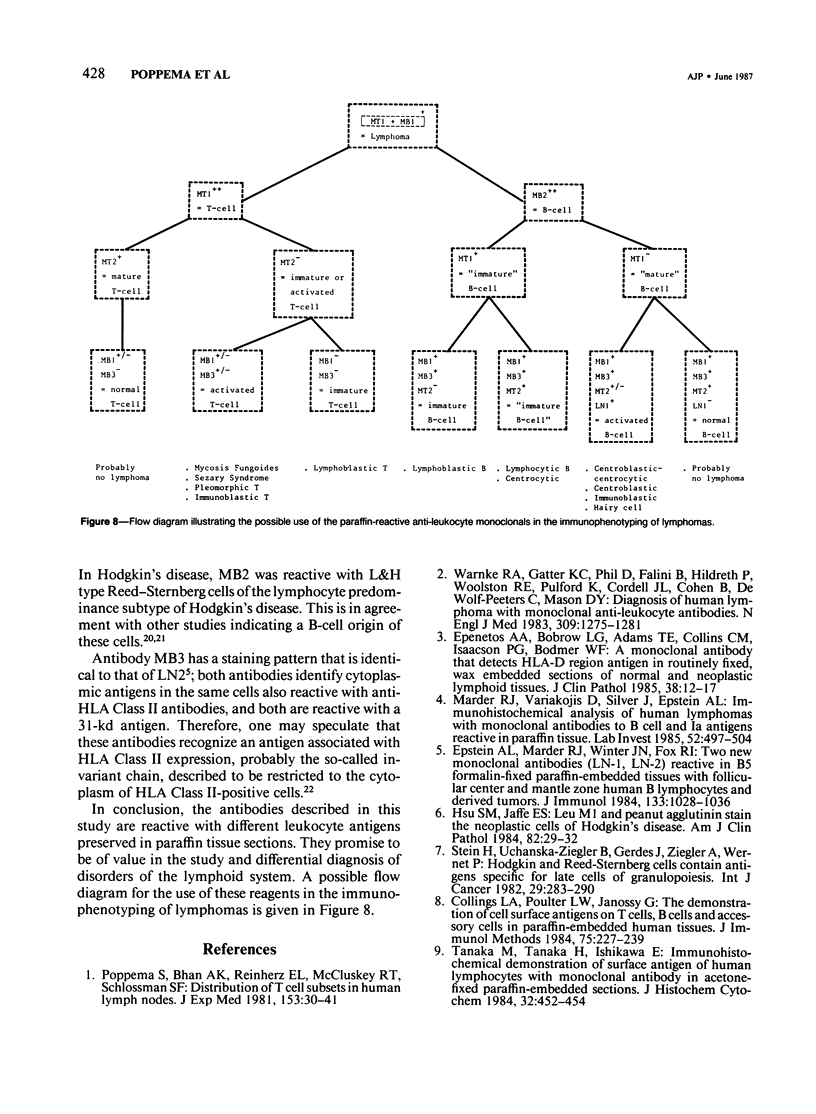
Abstract
The absence of reactivity on routinely prepared tissue sections has hampered the use of monoclonal antileukocyte antibodies in diagnostic histopathology. Here we describe five new antibodies reactive with leukocyte subsets in formaldehyde-fixed, paraffin-embedded tissue sections. Antibody MT1 is reactive with mature and immature T cells and not with mature B cells. MT2 is reactive with mature T cells and B cells, but not with immature T cells, activated T cells, and germinal center B cells. Antibody MB1 is reactive with all B cells, with about 50% of mature T cells, and not with immature T cells. MB2 is reactive with all B cells and not with T cells. However, MB2 also stains endothelial cells and several types of epithelial cells. MB3 is reactive with B cells and histiocytes, but not with T cells. The antibodies were tested on a series of lymphomas that were also immunophenotyped with a panel of well-established reagents on frozen tissue sections. The results indicate that the MB and MT antibodies are useful tools in the study of reactive and neoplastic disorders of the lymphoid system.
Full text
PDF


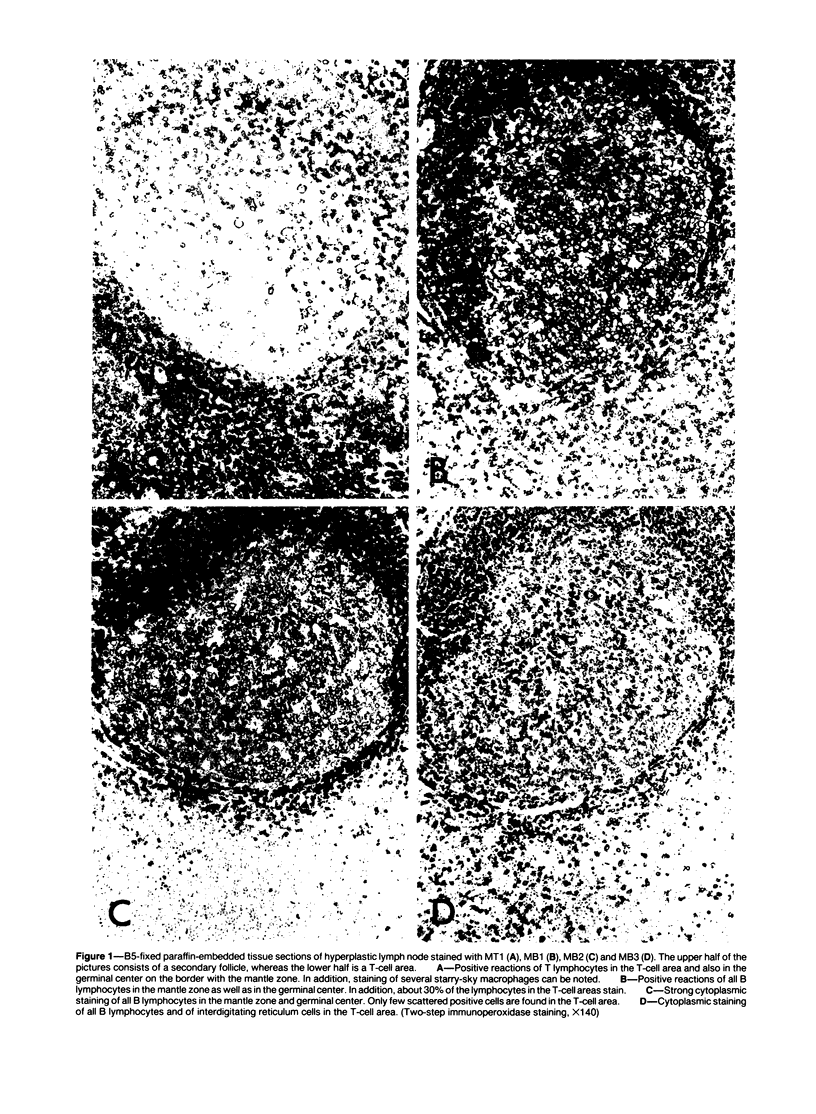
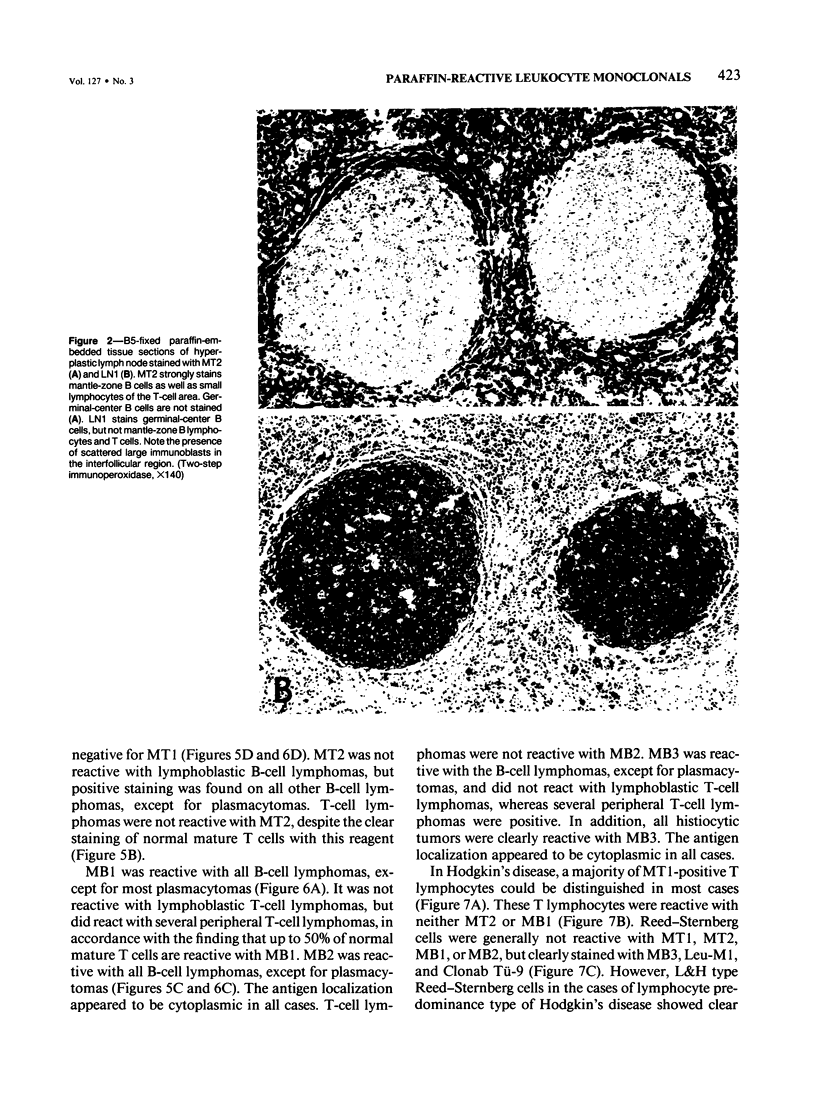
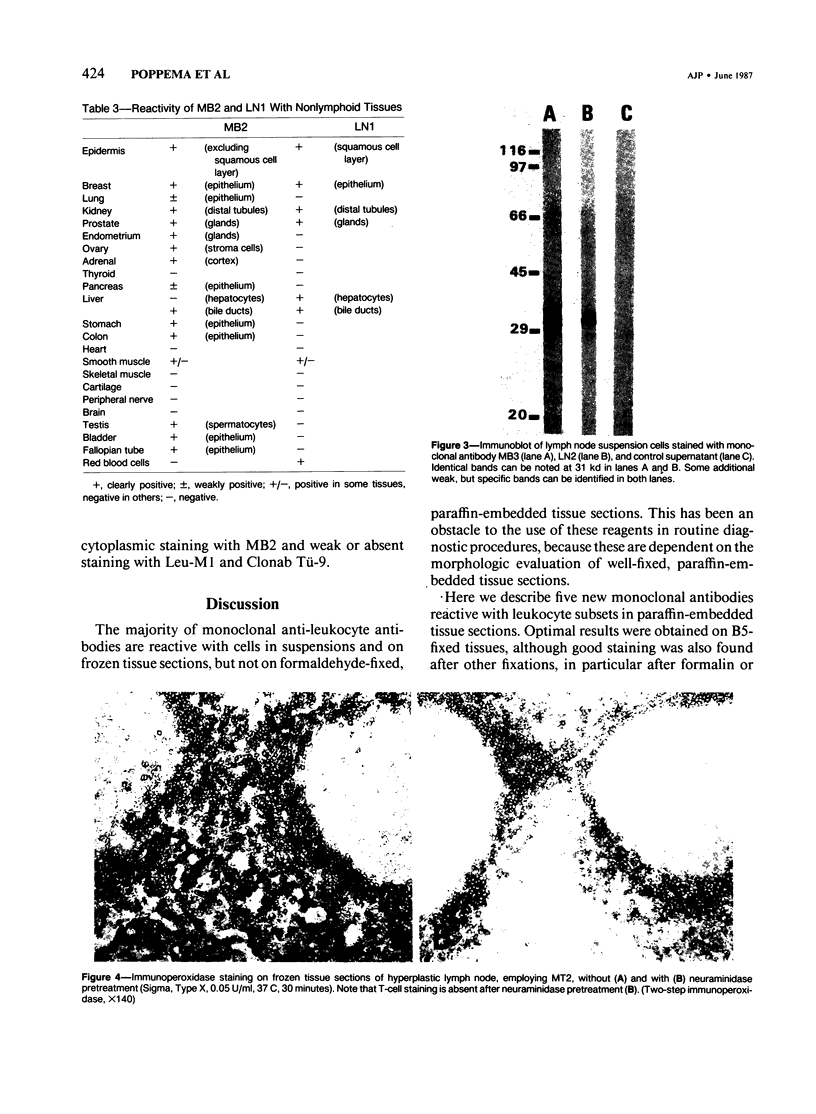

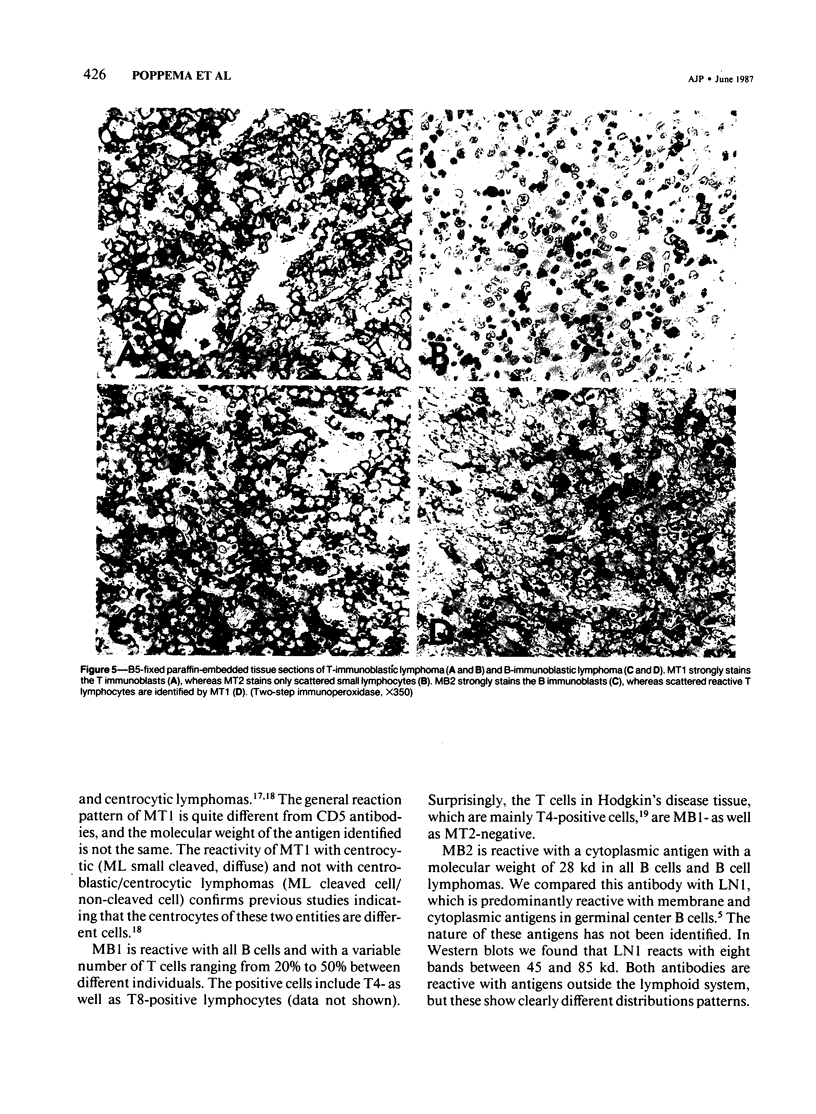

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collings L. A., Poulter L. W., Janossy G. The demonstration of cell surface antigens on T cells, B cells and accessory cells in paraffin-embedded human tissues. J Immunol Methods. 1984 Dec 31;75(2):227–239. doi: 10.1016/0022-1759(84)90106-6. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- De Leij L., Poppema S., The T. H. Cryopreservation of newly formed hybridomas. J Immunol Methods. 1983 Aug 12;62(1):69–72. doi: 10.1016/0022-1759(83)90111-4. [DOI] [PubMed] [Google Scholar]
- Epenetos A. A., Bobrow L. G., Adams T. E., Collins C. M., Isaacson P. G., Bodmer W. F. A monoclonal antibody that detects HLA-D region antigen in routinely fixed, wax embedded sections of normal and neoplastic lymphoid tissues. J Clin Pathol. 1985 Jan;38(1):12–17. doi: 10.1136/jcp.38.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L., Marder R. J., Winter J. N., Fox R. I. Two new monoclonal antibodies (LN-1, LN-2) reactive in B5 formalin-fixed, paraffin-embedded tissues with follicular center and mantle zone human B lymphocytes and derived tumors. J Immunol. 1984 Aug;133(2):1028–1036. [PubMed] [Google Scholar]
- Harris N. L., Nadler L. M., Bhan A. K. Immunohistologic characterization of two malignant lymphomas of germinal center type (centroblastic/centrocytic and centrocytic) with monoclonal antibodies. Follicular and diffuse lymphomas of small-cleaved-cell type are related but distinct entities. Am J Pathol. 1984 Nov;117(2):262–272. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marder R. J., Variakojis D., Silver J., Epstein A. L. Immunohistochemical analysis of human lymphomas with monoclonal antibodies to B cell and Ia antigens reactive in paraffin sections. Lab Invest. 1985 May;52(5):497–504. [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., McCluskey R. T., Schlossman S. F. Distribution of T cell subsets in human lymph nodes. J Exp Med. 1981 Jan 1;153(1):30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., Posner M. R., Schlossman S. F. In situ immunologic characterization of cellular constituents in lymph nodes and spleens involved by Hodgkin's disease. Blood. 1982 Feb;59(2):226–232. [PubMed] [Google Scholar]
- Poppema S., De Jong B., Atmosoerodjo J., Idenburg V., Visser L., De Ley L. Morphologic, immunologic, enzymehistochemical and chromosomal analysis of a cell line derived from Hodgkin's disease. Evidence for a B-cell origin of Sternberg-Reed cells. Cancer. 1985 Feb 15;55(4):683–690. doi: 10.1002/1097-0142(19850215)55:4<683::aid-cncr2820550402>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Poppema S. The diversity of the immunohistological staining pattern of Sternberg-Reed cells. J Histochem Cytochem. 1980 Aug;28(8):788–791. doi: 10.1177/28.8.6777426. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Majdic O., Stingl G., Liszka K., Honigsmann H., Knapp W. A human Ia cytoplasmic determinant located on multiple forms of invariant chain (gamma, gamma 2, gamma 3). J Immunol. 1984 Apr;132(4):1900–1905. [PubMed] [Google Scholar]
- Royston I., Majda J. A., Baird S. M., Meserve B. L., Griffiths J. C. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J Immunol. 1980 Aug;125(2):725–731. [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Stein H., Uchánska-Ziegler B., Gerdes J., Ziegler A., Wernet P. Hodgkin and Sternberg-Reed cells contain antigens specific to late cells of granulopoiesis. Int J Cancer. 1982 Mar 15;29(3):283–290. doi: 10.1002/ijc.2910290310. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Tanaka H., Ishikawa E. Immunohistochemical demonstration of surface antigen of human lymphocytes with monoclonal antibody in acetone-fixed paraffin-embedded sections. J Histochem Cytochem. 1984 Apr;32(4):452–454. doi: 10.1177/32.4.6368682. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke R. A., Gatter K. C., Falini B., Hildreth P., Woolston R. E., Pulford K., Cordell J. L., Cohen B., De Wolf-Peeters C., Mason D. Y. Diagnosis of human lymphoma with monoclonal antileukocyte antibodies. N Engl J Med. 1983 Nov 24;309(21):1275–1281. doi: 10.1056/NEJM198311243092102. [DOI] [PubMed] [Google Scholar]
- de Leij L., Poppema S., Nulend J. K., Ter Haar J. G., Schwander E., The T. H. Immunoperoxidase staining on frozen tissue sections as a first screening assay in the preparation of monoclonal antibodies directed against small cell carcinoma of the lung. Eur J Cancer Clin Oncol. 1984 Jan;20(1):123–128. doi: 10.1016/0277-5379(84)90043-9. [DOI] [PubMed] [Google Scholar]