Abstract
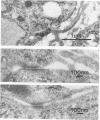
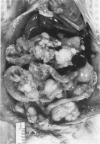
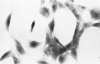
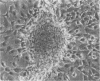
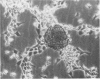
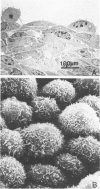
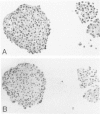
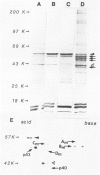
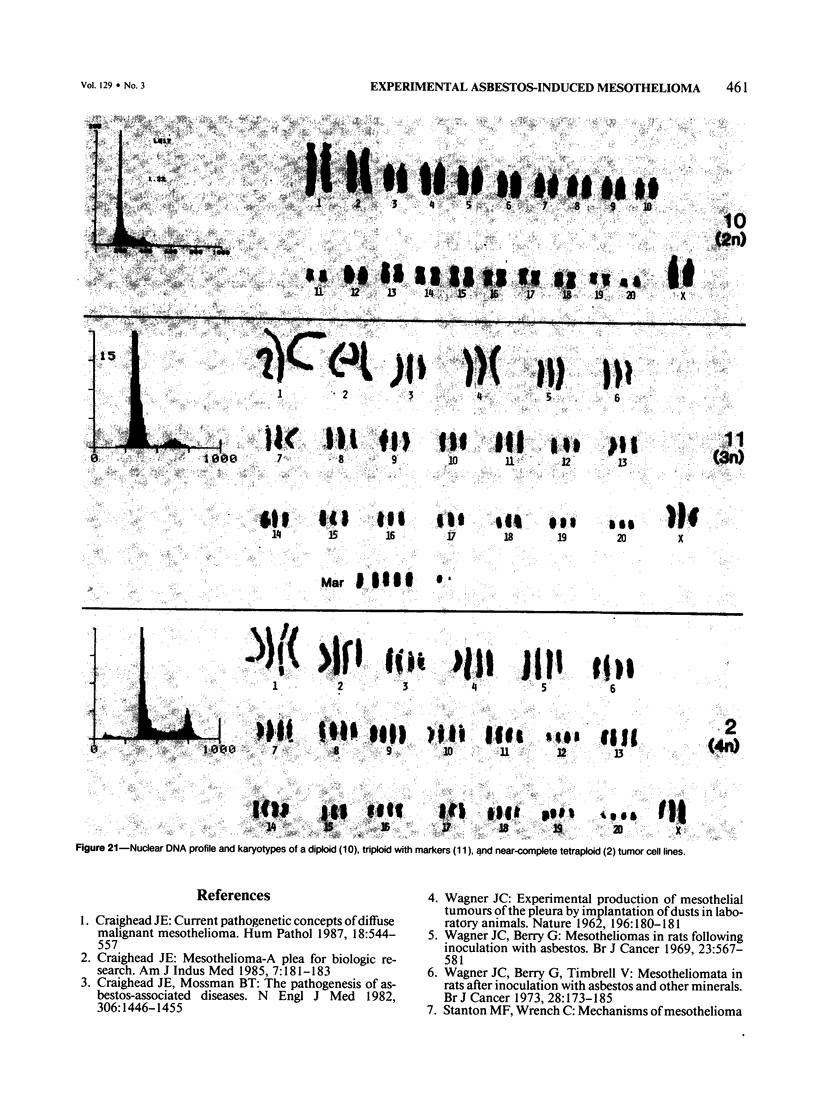
Mesotheliomas developed in rats in the abdominal cavity 6-23 months after peritoneal introduction of chrysotile and crocidolite asbestos. The tumors were strikingly similar to those occurring in man both with regard to histologic features and growth patterns. The authors have cultured cells from these tumors and established epithelial lines with a variety of karyologic features and doubling times shorter than those of normal mesothelial cells. Lines of diploid or near-diploid tumor cells required serum in the medium to replicate, whereas most aneuploid cell lines were maintained in a serum-free medium. In serum-free medium, the aneuploid line monolayers produced anchorage-independent excrescent masses of cells which "bud" and float free. These spheroids, which strikingly resemble the papillary structures in human mesotheliomas, are composed of mesothelial-like cells that produce hyaluronic acid and have a rich complement of intermediate filaments, predominantly cytokeratins. Aneuploid cells also replicated in soft agar with high efficiency, whereas the diploid and near-diploid cells did not. All but one cultured cell line, regardless of karyotype, produced tumors after subcutaneous or intraperitoneal inoculation (or both). Aneuploid cells caused lung tumors sporadically when introduced intravenously. Comparative analysis of the nuclear DNA of primary tumors and cultured cells demonstrated a high degree of chromosomal instability and selection among cells during early passage in vitro.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISCHOFF F., BRYSON G. CARCINOGENESIS THROUGH SOLID STATE SURFACES. Prog Exp Tumor Res. 1964;5:85–133. doi: 10.1159/000385997. [DOI] [PubMed] [Google Scholar]
- Brand K. G., Buoen L. C., Johnson K. H., Brand I. Etiological factors, stages, and the role of the foreign body in foreign body tumorigenesis: a review. Cancer Res. 1975 Feb;35(2):279–286. [PubMed] [Google Scholar]
- Craighead J. E. Current pathogenetic concepts of diffuse malignant mesothelioma. Hum Pathol. 1987 Jun;18(6):544–557. doi: 10.1016/s0046-8177(87)80354-4. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Mossman B. T. The pathogenesis of asbestos-associated diseases. N Engl J Med. 1982 Jun 17;306(24):1446–1455. doi: 10.1056/NEJM198206173062403. [DOI] [PubMed] [Google Scholar]
- Davis J. M. Epithelial outgrowths from lung tissue following intrapleural injection of asbestos dust in experimental animals. Int J Cancer. 1971 Mar 15;7(2):238–248. doi: 10.1002/ijc.2910070207. [DOI] [PubMed] [Google Scholar]
- Davis J. M. Histogenesis and fine structure of peritoneal tumors produced in animals by injections of asbestos. J Natl Cancer Inst. 1974 Jun;52(6):1823–1837. doi: 10.1093/jnci/52.6.1823. [DOI] [PubMed] [Google Scholar]
- Davis J. M. The histopathology and ultrastructure of pleural mesotheliomas produced in the rat by injections of crocidolite asbestos. Br J Exp Pathol. 1979 Dec;60(6):642–652. [PMC free article] [PubMed] [Google Scholar]
- LaRocca P. J., Rheinwald J. G. Coexpression of simple epithelial keratins and vimentin by human mesothelium and mesothelioma in vivo and in culture. Cancer Res. 1984 Jul;44(7):2991–2999. [PubMed] [Google Scholar]
- Mackay A. M., Tracy R. P., Craighead J. E. Intermediate filament proteins in asbestos-induced mesotheliomas of the rat. Cancer Res. 1987 Oct 15;47(20):5461–5468. [PubMed] [Google Scholar]
- Murakami H., Masui H. Hormonal control of human colon carcinoma cell growth in serum-free medium. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3464–3468. doi: 10.1073/pnas.77.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton M. F., Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst. 1972 Mar;48(3):797–821. [PubMed] [Google Scholar]
- Timbrell V., Gibson J. C., Webster I. UICC standard reference samples of asbestos. Int J Cancer. 1968 May 15;3(3):406–408. doi: 10.1002/ijc.2910030312. [DOI] [PubMed] [Google Scholar]
- Triol J. H., Conston A. S., Chandler S. V. Malignant mesothelioma. Cytopathology of 75 cases seen in a New Jersey community hospital. Acta Cytol. 1984 Jan-Feb;28(1):37–45. [PubMed] [Google Scholar]
- WAGNER J. C. Experimental production of mesothelial tumours of the pleura by implantation of dusts in laboratory animals. Nature. 1962 Oct 13;196:180–181. doi: 10.1038/196180a0. [DOI] [PubMed] [Google Scholar]
- Wagner J. C., Berry G. Mesotheliomas in rats following inoculation with asbestos. Br J Cancer. 1969 Sep;23(3):567–581. doi: 10.1038/bjc.1969.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Timbrell V. Mesotheliomata in rats after inoculation with asbestos and other materials. Br J Cancer. 1973 Aug;28(2):173–185. doi: 10.1038/bjc.1973.134. [DOI] [PMC free article] [PubMed] [Google Scholar]