Abstract
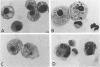
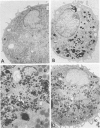
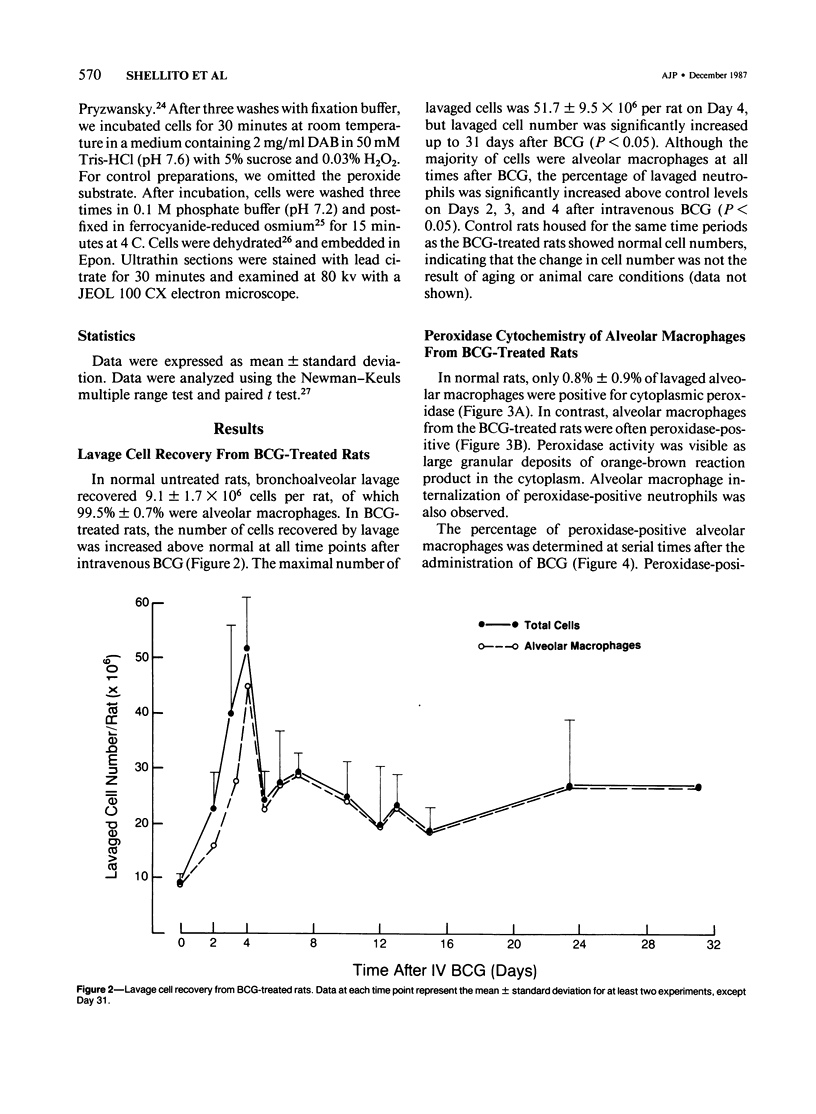
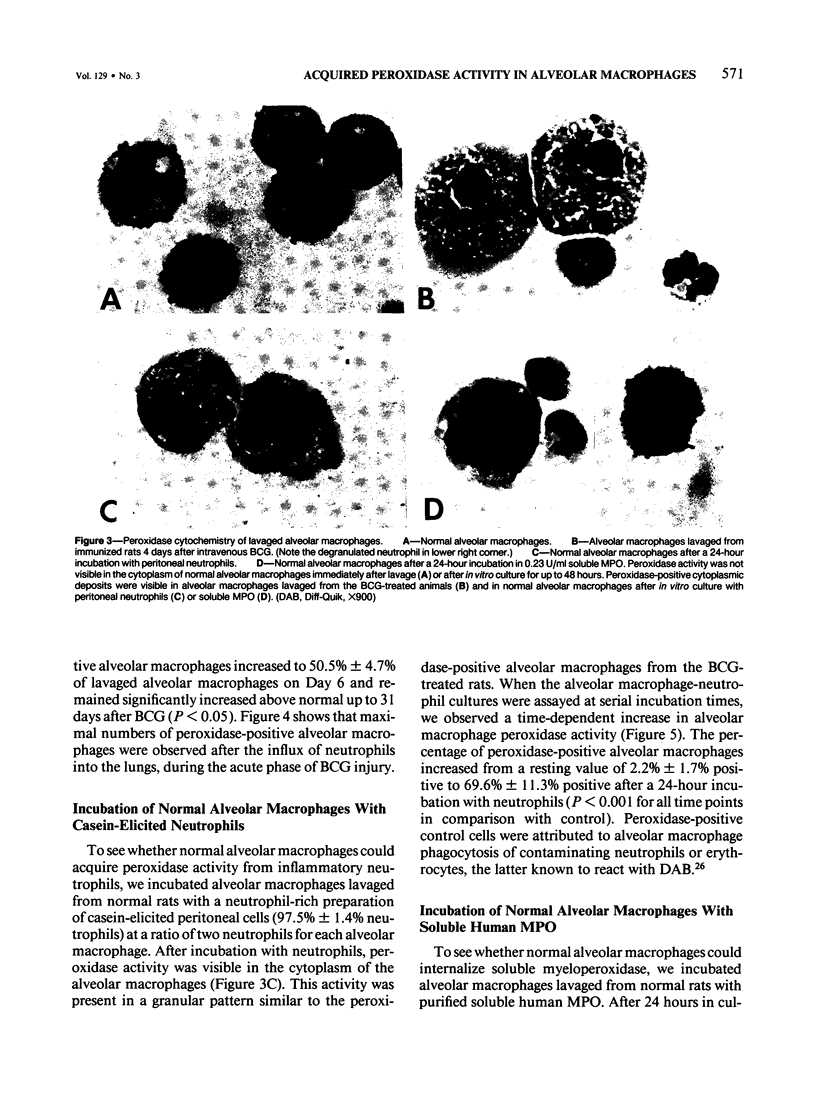
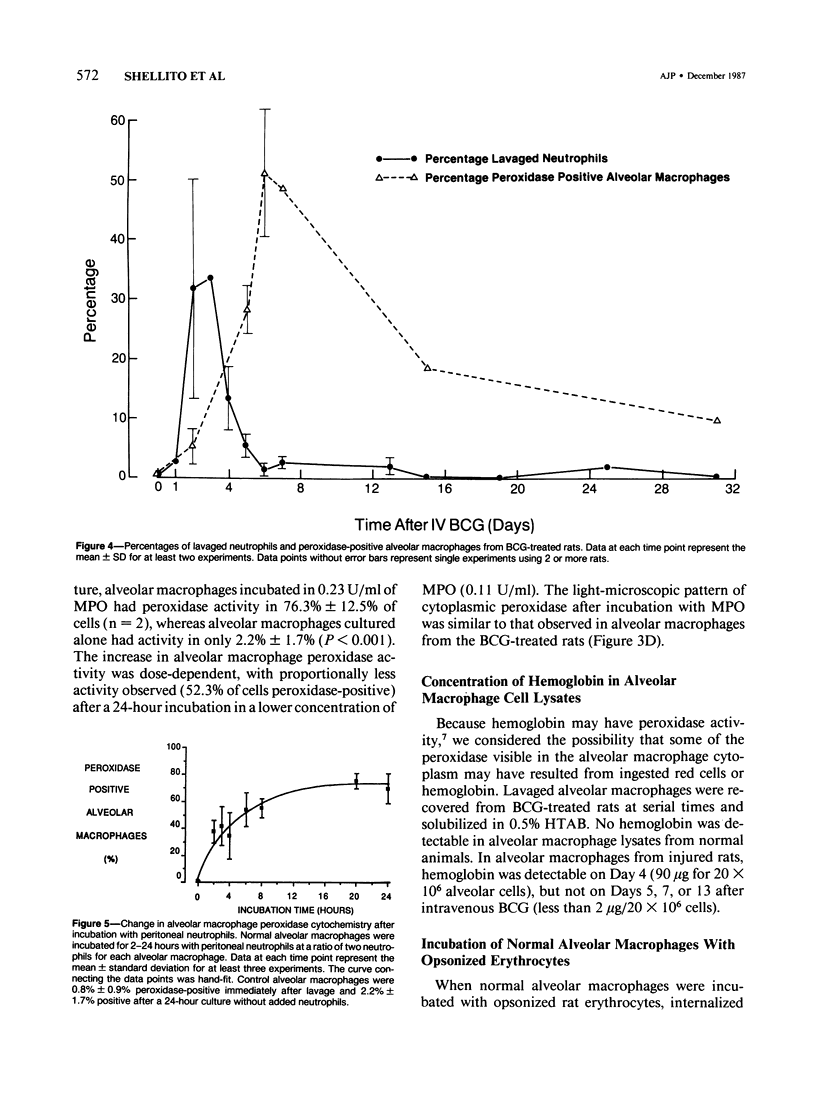
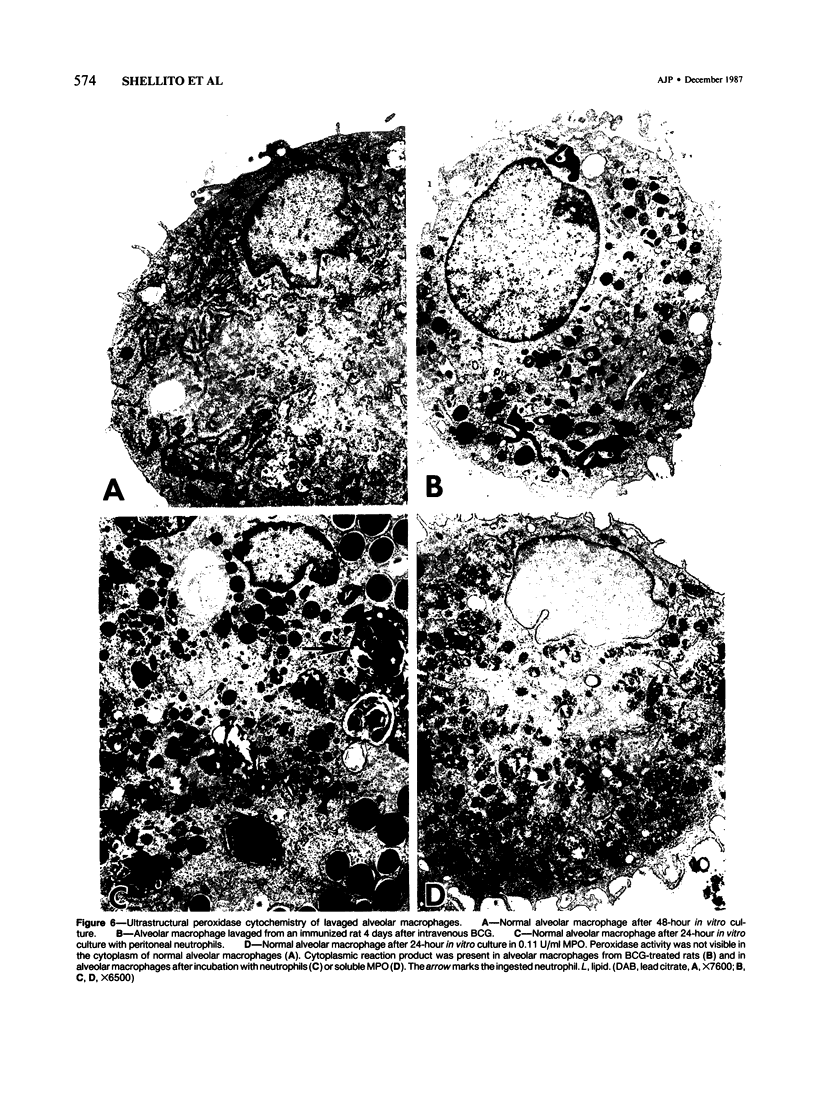
The authors investigated the ability of rat alveolar macrophages to acquire peroxidase activity in the course of pulmonary inflammation. Granulomatous pulmonary inflammation was induced in bacille Calmette-Guérin (BCG)-immunized rats by intravenous injection of BCG in mineral oil. In contrast to normal alveolar macrophages, which are peroxidase-negative, alveolar macrophages lavaged from the BCG-treated rats showed significant peroxidase activity in large cytoplasmic inclusions compatible with internalized exogenous material. Alveolar macrophage uptake of intact peroxidase-positive neutrophils was also observed. Maximal numbers of peroxidase-positive alveolar macrophages were observed after the initial influx of neutrophils into the lungs, and peroxidase activity could be demonstrated in cell-free lavage fluid during the acute phase of lung injury. Normal alveolar macrophages acquired peroxidase activity after incubation with peritoneal exudate neutrophils, with purified soluble human myeloperoxidase, and with opsonized erythrocytes. It is concluded that alveolar macrophages acquire peroxidase activity from multiple sources during pulmonary inflammation. Internalization of peroxidase by the alveolar macrophage may serve to clear a potentially toxic enzyme(s) from the alveolar space and contribute to the resolution of pulmonary inflammation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwal O. S. Cytoenzymological behavior of peritoneal exudate cells of rat in vivo. I. Histochemical study of enzymatic function of peroxidase. J Reticuloendothel Soc. 1971 Aug;10(2):163–172. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R. H., Walker W. S. Dynamics of cytochemically distinct subpopulations of macrophages in elicited rat peritoneal exudates. Cell Immunol. 1983 Dec;82(2):246–257. doi: 10.1016/0008-8749(83)90159-4. [DOI] [PubMed] [Google Scholar]
- Biggar W. D., Sturgess J. M. Peroxidase activity of alveolar macrophages. Lab Invest. 1976 Jan;34(1):31–42. [PubMed] [Google Scholar]
- Bradley P. P., Christensen R. D., Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982 Sep;60(3):618–622. [PubMed] [Google Scholar]
- Campbell E. J., Wald M. S. Hypoxic injury to human alveolar macrophages accelerates release of previously bound neutrophil elastase. Implications for lung connective tissue injury including pulmonary emphysema. Am Rev Respir Dis. 1983 May;127(5):631–635. doi: 10.1164/arrd.1983.127.5.631. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., White R. R., Senior R. M., Rodriguez R. J., Kuhn C. Receptor-mediated binding and internalization of leukocyte elastase by alveolar macrophages in vitro. J Clin Invest. 1979 Sep;64(3):824–833. doi: 10.1172/JCI109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979 Oct;64(4):913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- De Bakker J. M., De Wit A. W., Woelders H., Ginsel L. A., Daems W. T. On the origin of peritoneal resident macrophages. II. Recovery of the resident macrophage population in the peritoneal cavity and in the milky spots after peritoneal cell depletion. J Submicrosc Cytol. 1985 Apr;17(2):141–151. [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med. 1973 Jul 1;138(1):318–323. doi: 10.1084/jem.138.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Heifets L., Imai K., Goren M. B. Expression of peroxidase-dependent iodination by macrophages ingesting neutrophil debris. J Reticuloendothel Soc. 1980 Oct;28(4):391–404. [PubMed] [Google Scholar]
- Jong E. C., Henderson W. R., Klebanoff S. J. Bactericidal activity of eosinophil peroxidase. J Immunol. 1980 Mar;124(3):1378–1382. [PubMed] [Google Scholar]
- Jong E. C., Klebanoff S. J. Eosinophil-mediated mammalian tumor cell cytotoxicity: role of the peroxidase system. J Immunol. 1980 Apr;124(4):1949–1953. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975 May;45(5):699–707. [PubMed] [Google Scholar]
- Koeffler H. P., Ranyard J., Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985 Feb;65(2):484–491. [PubMed] [Google Scholar]
- Lee C. W., Lewis R. A., Tauber A. I., Mehrotra M., Corey E. J., Austen K. F. The myeloperoxidase-dependent metabolism of leukotrienes C4, D4, and E4 to 6-trans-leukotriene B4 diastereoisomers and the subclass-specific S-diastereoisomeric sulfoxides. J Biol Chem. 1983 Dec 25;258(24):15004–15010. [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUFELD H. A., LEVAY A. N., LUCAS F. V., MARTIN A. P., STOTZ E. Peroxidase and cytochrome oxidase in rat tissues. J Biol Chem. 1958 Jul;233(1):209–211. [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. A., Bainton D. F., Farquhar M. G. Differentiation of monocytes. Origin, nature, and fate of their azurophil granules. J Cell Biol. 1971 Aug;50(2):498–515. doi: 10.1083/jcb.50.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J. M., Weiss S. J. Human neutrophils transform prostaglandins by a myeloperoxidase-dependent mechanism. J Biol Chem. 1982 Mar 25;257(6):2738–2740. [PubMed] [Google Scholar]
- Pryzwansky K. B., Schliwa M., Porter K. R. Comparison of the three-dimensional organization of unextracted and Triton-extracted human neutrophilic polymorphonuclear leukocytes. Eur J Cell Biol. 1983 Mar;30(1):112–125. [PubMed] [Google Scholar]
- Ramsey P. G., Martin T., Chi E., Klebanoff S. J. Arming of mononuclear phagocytes by eosinophil peroxidase bound to Staphylococcus aureus. J Immunol. 1982 Jan;128(1):415–420. [PubMed] [Google Scholar]
- Roels F., Wisse E., De Prest B., van der Meulen J. Cytochemical discrimination between catalases and peroxidases using diaminobenzidine. Histochemistry. 1975;41(4):281–312. doi: 10.1007/BF00490073. [DOI] [PubMed] [Google Scholar]
- Shellito J., Kaltreider H. B. Heterogeneity of immunologic function among subfractions of normal rat alveolar macrophages. Am Rev Respir Dis. 1984 May;129(5):747–753. doi: 10.1164/arrd.1984.129.5.747. [DOI] [PubMed] [Google Scholar]
- Soranzo M. R., Koerten H. K., Daems W. T. Peroxidatic activity and morphometric analysis of alveolar macrophages in the guinea pig. J Reticuloendothel Soc. 1978 May;23(5):343–359. [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984 Feb;98(2):748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Garcia M. L. Enhancement of neutrophils function as a result of prior exposure to chemotactic factor. J Clin Invest. 1980 Aug;66(2):167–175. doi: 10.1172/JCI109841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Slivka A. Monocyte and granulocyte-mediated tumor cell destruction. A role for the hydrogen peroxide-myeloperoxidase-chloride system. J Clin Invest. 1982 Feb;69(2):255–262. doi: 10.1172/JCI110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]